NON FERROUS METALS AND ALLOYS INTRODUCTION Nonferrous metals















































- Slides: 55

NON FERROUS METALS AND ALLOYS

INTRODUCTION • Nonferrous metals and their alloys do not contain iron as a principle ingredient, although they may contain small percentages.

• Non- ferrous metal and alloys cover a wide range of material, from the more common metal, such as 1. Copper 2. Aluminium 3. Zinc 4. Tin 5. Lead

Other Non ferrous metal • Cobalt • Gold • Silver • Platinum • Titanium • Manganese • Refractory Metals

Properties of non ferrous-metal and alloys • Good electrical and thermal conductivity. • Resistance to corrosion. • Good weldability • Low density • Good colour and colour imparting properties • Good modulus of elasticity • They can be cast easily

COPPER

History of copper • Copper is one of the oldest metals of mankind. It’s history traces back over 10, 000 years. • It was mined mainly in Cyprus in the Mediterranean in the Ancient times. • Copper was the first metal to be mined and crafted by man. It was used for tools, weapons, art objects and ornaments.

Properties of Copper • Copper is a reddish coloured metallic metal. • Malleability - the ability to be beaten or rolled into shape. • Ductility - The ability to be drawn into shape as in the manufacture of copper wire. • Copper is a good conductor of heat and electricity. • It’s atomic number is 29. • It is easily mixed with other metals to form alloys • High corrosion resistance.

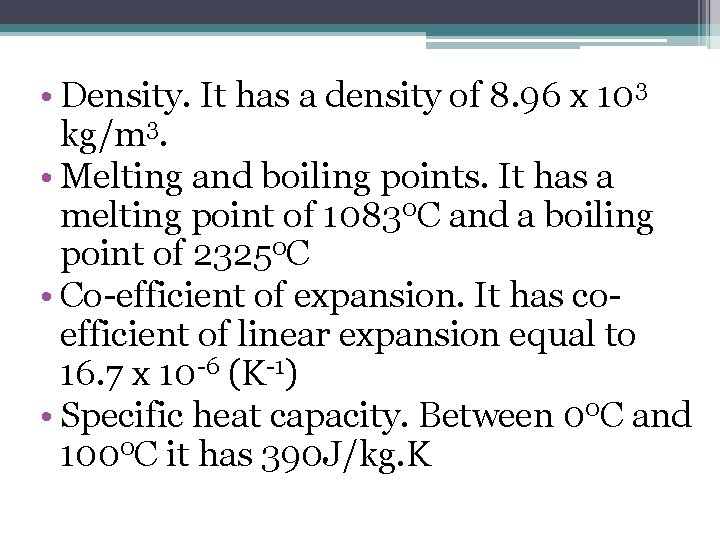
• Density. It has a density of 8. 96 x 103 kg/m 3. • Melting and boiling points. It has a melting point of 10830 C and a boiling point of 23250 C • Co-efficient of expansion. It has coefficient of linear expansion equal to 16. 7 x 10 -6 (K-1) • Specific heat capacity. Between 00 C and 1000 C it has 390 J/kg. K

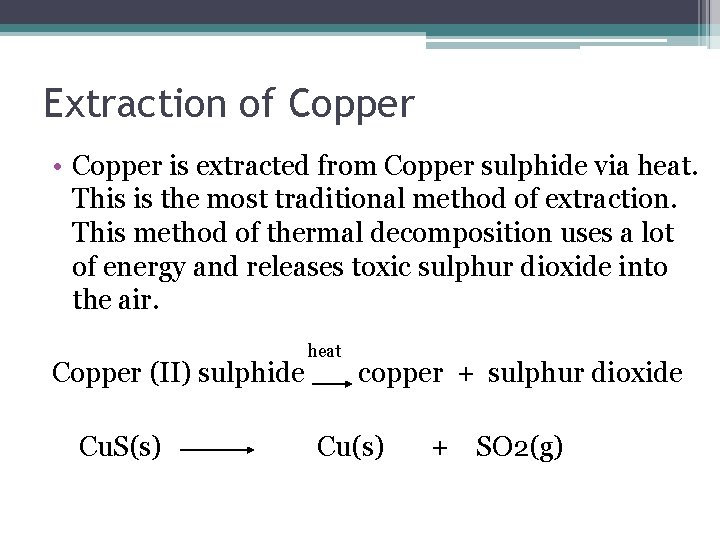
Extraction of Copper • Copper is extracted from Copper sulphide via heat. This is the most traditional method of extraction. This method of thermal decomposition uses a lot of energy and releases toxic sulphur dioxide into the air. heat Copper (II) sulphide copper + sulphur dioxide Cu. S(s) Cu(s) + SO 2(g)

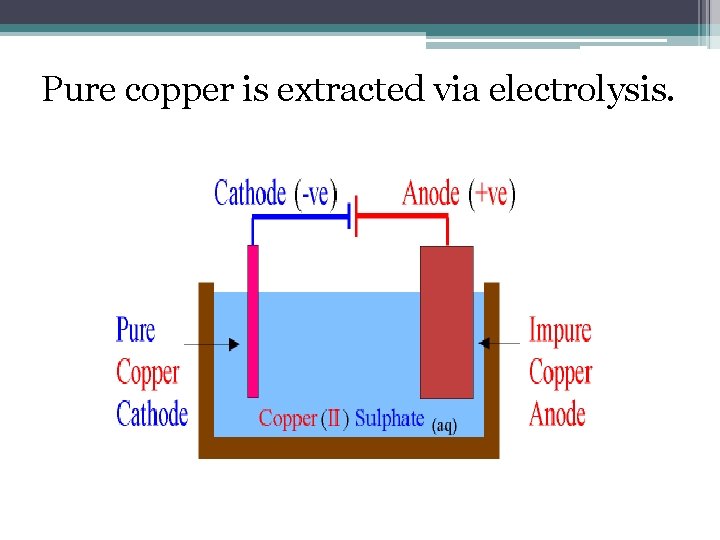
Pure copper is extracted via electrolysis.

• When electricity is passed through the cell copper is dissolved at the anode by oxidation, Cu 2+ ions go into the solution. At the cathode, copper is deposited by reduction. • As copper ions move from the anode to the cathode the anode gets smaller as the cathode gets bigger. This is called a redox reaction.

Use of copper • Copper is mostly used in the manufacturing of electric wires, cables and conductors. • Its other uses are water and central heating pipes and cylinders, Printed circuit boards and manufacturing of special roofs.

ALUMINIUM • Aluminium (Al) - atomic number 13 • Whitish with bluish cast • The third most abundant element (after oxygen and silicon), and the most abundant metal in the Earth’s crust

Production of Aluminum • Aluminum is primarily made by the electrolytic reduction of aluminum oxide. • The main ore of aluminum is bauxite. • Bauxite is in the form of mainly aluminum hydroxide Al(OH)3, and other impurities such as iron(III) oxide and titanium hydroxide, and other few oxides.

Properties of Aluminum • It is light weight – 1/3 weight of steel and cooper • Excellent corrosion characteristics • Good reflector of heat and light • Easy to weld • Aluminum has a high strength to weight ratio, • High thermal and electrical conductivity • Ease of formability and of machinability and it is non magnetic

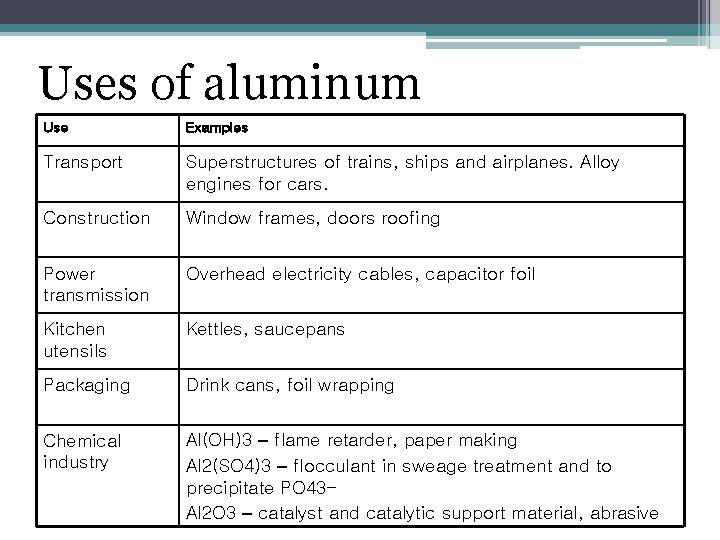
Uses of Aluminium • Construction of aircraft, lightweight vehicles, and ladders. • Drink cans and roofing materials. • Window frames. • boilers, cookers and cookware. • High reflectivity makes it ideal for mirrors, reflectors and heat resistant clothing for fire fighting.

Uses of aluminum Use Examples Transport Superstructures of trains, ships and airplanes. Alloy engines for cars. Construction Window frames, doors roofing Power transmission Overhead electricity cables, capacitor foil Kitchen utensils Kettles, saucepans Packaging Drink cans, foil wrapping Chemical industry Al(OH)3 – flame retarder, paper making Al 2(SO 4)3 – flocculant in sweage treatment and to precipitate PO 43 Al 2 O 3 – catalyst and catalytic support material, abrasive

Aluminum Alloys • Small amounts of manganese increase the strength of aluminum • Silicon or magnesium or both together produces good corrosion resistance much improved strength • Copper zinc produce alloys high strength to weight ratios

Tin �Tin (Sn) - atomic number 50 �White, lustrous, soft, malleable, ductile, resistant to corrosion

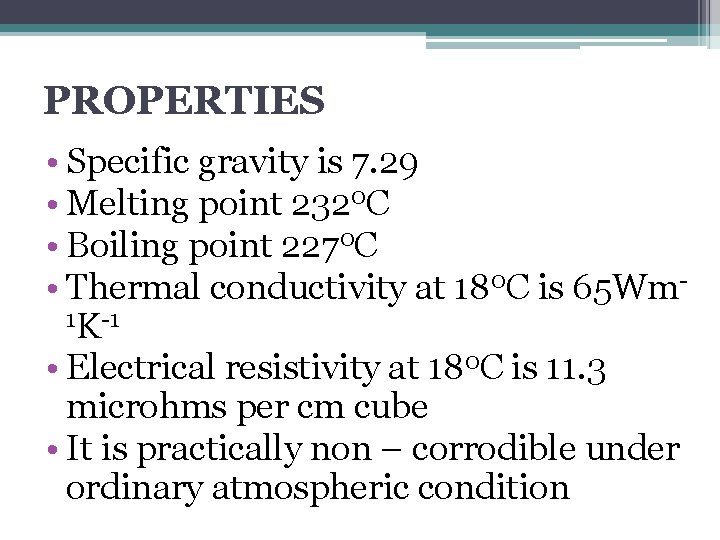
PROPERTIES • Specific gravity is 7. 29 • Melting point 2320 C • Boiling point 2270 C • Thermal conductivity at 180 C is 65 Wm 1 K-1 • Electrical resistivity at 180 C is 11. 3 microhms per cm cube • It is practically non – corrodible under ordinary atmospheric condition

Tin (Continued) • Uses ▫ Tin Plating ▫ Tin Cans

Lead • Lead (Pb) - atomic number 82 • Metallic lead has a bluish-white colour after being freshly cut, but it soon tarnishes to a dull grayish color when exposed to air

• Has a shiny chrome-silver luster when it is melted into a liquid soft, malleable, has little ductility • Specific gravity 11. 3 to 11. 4 and silvery – white in colour when freshly cut • Melting point 3270 C • Boiling point at atmospheric pressure 15250 C • Specific heat capacity 128 J/Kg-K between 200 C to 1000 C

Lead • Uses ▫ Plates for storage batteries, covering for electrical cables, X-Rays, Shielding Radioactive Material

SILVER • Element, Ag • Melts At 962 o. F • Boils At 2212 o. F • Specific Gravity = 10. 5

Silver (Continued) • Properties ▫ Lustrous (High Polish) ▫ Most Malleable & Ductile ▫ Excellent Electrical Conductivity • Uses ▫ Jewelry ▫ Electrical Components

ZINC �Zinc (Zn), atomic number 30 �Bluish white �Corrosion resistant in air due to a thin oxide film forming on its surface

Zinc (Continued) • Properties ▫ Brittle ▫ Insoluble In Water ▫ Soluble In Alcohol, Acids, Alkalies • Uses ▫ Protective Coating ▫ Galvanizing ▫ Alloying With Copper ▫ Die Castings

Nickel • General ▫ Element, Ni ▫ Melts At 2651 o. F ▫ Boils At 2730 o. F ▫ Specific Gravity = 8. 9 (Same As Copper) ▫ Silver-White Color

Nickel (Continued) • Properties ▫ Tough and ductile ▫ Good high and low temperature strength ▫ High oxidation resistance ▫ Good corrosion resistance

Nickel (Continued) • Uses ▫ Coating - Protective & Ornamental �Iron & Steel �Electrolysis In Nickel Solution ▫ Alloy �Steel - Hardness & Strength �Automobile Parts - Axles, Crankshafts, Etc. ▫ Batteries �Nickel-Cadmium

Nickel (Continued) • Processing ▫ Ores Are Smelted In Blast Furnace � Ingot Of Copper & Nickel Sulfide ▫ Electrolytic Process � Copper & Nickel Are Separated � Different Voltage To Different Electrolyte

Magnesium • General ▫ Element, Mg ▫ Melts At 1200 o. F ▫ Boils At 2025 o. F ▫ Specific Gravity = 1. 74 ▫ Lightest Stable Metal ▫ Silver-White

Magnesium (Continued) • Properties ▫ Malleable & Ductile When Heated ▫ Reactive With Acids ▫ Reacts With Oxygen Above 1472 o. F

Magnesium (Continued) • Uses ▫ Textiles - Refractory & Insulating Material ▫ Cosmetics ▫ Alloys � Castings, Artificial Limbs, ▫ Pure � Flash Powders, Incendiary Bombs.

Titanium • General ▫ Element, Ti ▫ Melts At 3020 o. F ▫ Specific Gravity = 4. 5 • Properties ▫ High strength to weight ratio ▫ Moderate-high temperature properties ▫ Corrosion resistance

• Uses ▫ Pure Titanium Is Very Brittle When Cold ▫ Aerospace Applications

Cobalt • General ▫ Element, Co, Melts At 1495 o. F ▫ Specific Gravity = 8. 9 ▫ Low Strength, Low Ductility, Hardness • Uses ▫ Permanent Magnets - Cobalt Steel ▫ Tool Bits - Tungsten Carbide

Chromium • General ▫ Element, Cr ▫ Melts At 3375 o. F ▫ Specific Gravity = 7. 2 • Uses ▫ Corrosion Resistance, Compatibility, & Reactivity � Alloy - Hardness, Strength, Corrosion Resistance. Stainless Steel

Platinum • General ▫ Element, Pt ▫ Melts At 3222 o. F ▫ Specific Gravity = 21. 45 ▫ Weight & Hardness ▫ Powder Metallurgy • Uses ▫ Chemically Inert - Surgical & Dental ▫ Jewelry


ALLOYS (NON-FERROUS) • An alloy is the product of mixing metal elements (and also some chemicals) to create metals with varying properties.

COPPER ALLOYS • Copper alloys are mainly of two types ▫ Brasses ▫ Bronzes

BRASS • Brass for industrial purposes is an alloy of Copper (60%) Zinc (37%) and Lead (3%). • This yellowish colored alloy is used to produce such articles as meter fitting (taps), wood screws, machine screws, electrical components etc.

Types of brasses • Brasses can be classified as follows ▫ α-brasses ▫ α-β brasses ▫ Delta metal

α-brasses • All brasses obtained between o to 39% of zinc content known as α-brasses, because the resulting alloy consists of crystals of a uniform solid solution of zinc dissolved in copper which is known as alpha solution

• The alpha brasses possess good tensile strength combined with considerably ductile when cold, making them suitable for producing sheet, strips, wire etc. • These brasses retain high strength in compression at elevated temperatures as compared to alpha – beta brass, and there fore require greater power to hot them.

α-β brasses • All brasses obtainable between 39% to 46. 6% zinc content are known as α-β brasses, because every resulting alloys consists of a second zinc- rich constituent, namely the beta solid solution, in additional to α-solid solution. • The alpha-beta brasses when cold contain hard beta crystal which increased tensile strength but reduce the ductility

DELTA METAL • This is a high tensile brass produced by adding 3% Fe to a composition of the brass, thus overall composition being Cu 60%, Zn 37% and Fe 3%. This brass resist corrosion. Too much iron reduces machinability. • At a temperature of 5500 C this metal can be worked by forging, rolling, stamping or pressing into desired shape.

BRONZES • The term bronze is normally applied to the alloys of copper and tin, but more generally with the additional of other elements such as zinc, nickel, phosphorus, aluminum, lead etc • The composition of Bronze is typically Copper (88%), Tin (10%) and Zinc (2%). • Typical examples of these are phosphor bronze, gunmetal, lead bronze, plastic bronze and nickel bronze.

• The physical appearance of Bronze is a dark reddish-yellow colour • The copper-tin alloys usually contain 5 to 20%, although most of the commercial bronze contain from 10 to 12% tin, other cast gun metal have 5 to 10% of tin.

Phosphor bronze • Phosphor bronze are copper-tin containing 5 to 20% of tin, 0. 1 to 1. 5% of phosphorus and rest copper. Phosphorus results in the formation of every hard copper phosphide constituent which improves hardness and wear resistance of the metal.

GUNMETALS • These are alloys of copper, tin and zinc, with the zinc in small proportions up to 6%(max) Usually, however, the percentage of zinc is limited to 2. 5 owing to the tendency toward brittleness with greater proportions • The zinc improves the casting properties and enable sounder casting to be made. It is also a much cheaper metal than tin so that it uses enables an economy to be affected.

• A typical composition of a gum metal is admiralty gum metal which its composition are Cu 88%, Sn 10% and Zn 2%. • Gunmetals are used for less expensive sand castings than the alloy bronzes where harder and better wearing qualities are required than for brass parts. Typical application of gunmetals include gearwheel, pump casting, bearing bushing and marine fitting.