Mr Quinn Ms Tom March 24 2014 Aim



- Slides: 13

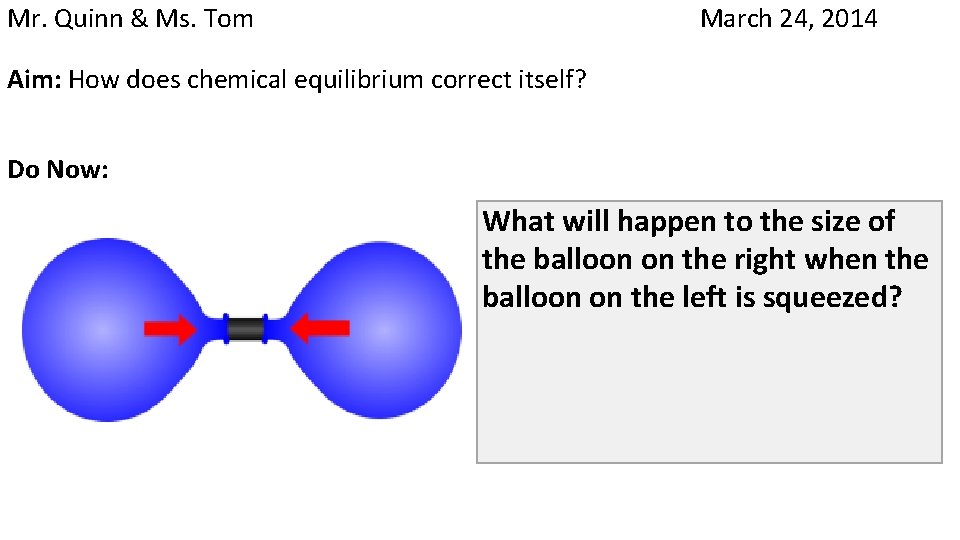
Mr. Quinn & Ms. Tom March 24, 2014 Aim: How does chemical equilibrium correct itself? Do Now: What will happen to the size of the balloon on the right when the balloon on the left is squeezed?

Introduction Le Châtlier’s Principle: When we ADD to one side of an equilibrium, it shifts to the other side. When we REMOVE from one side of an equilibrium, it shifts to that side.

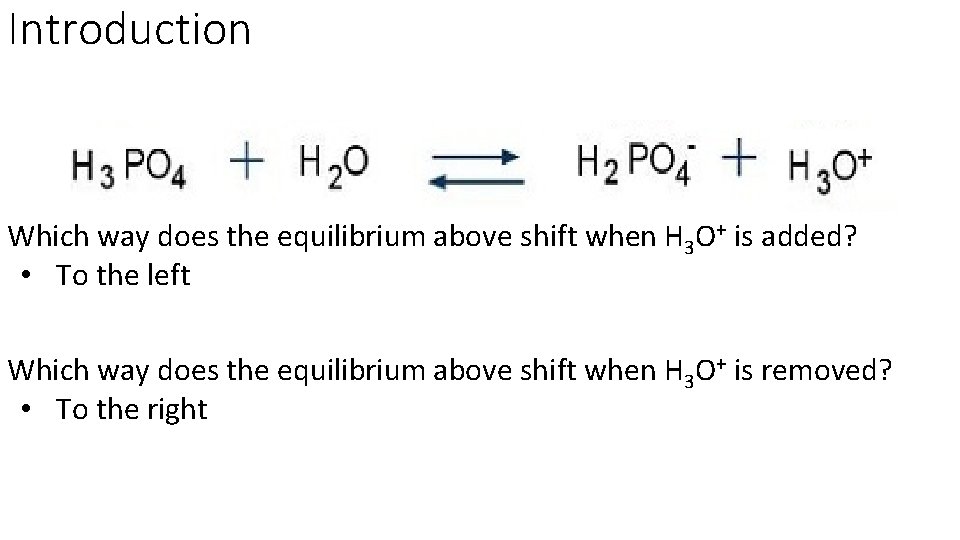
Introduction Which way does the equilibrium above shift when H 3 O+ is added? • To the left Which way does the equilibrium above shift when H 3 O+ is removed? • To the right

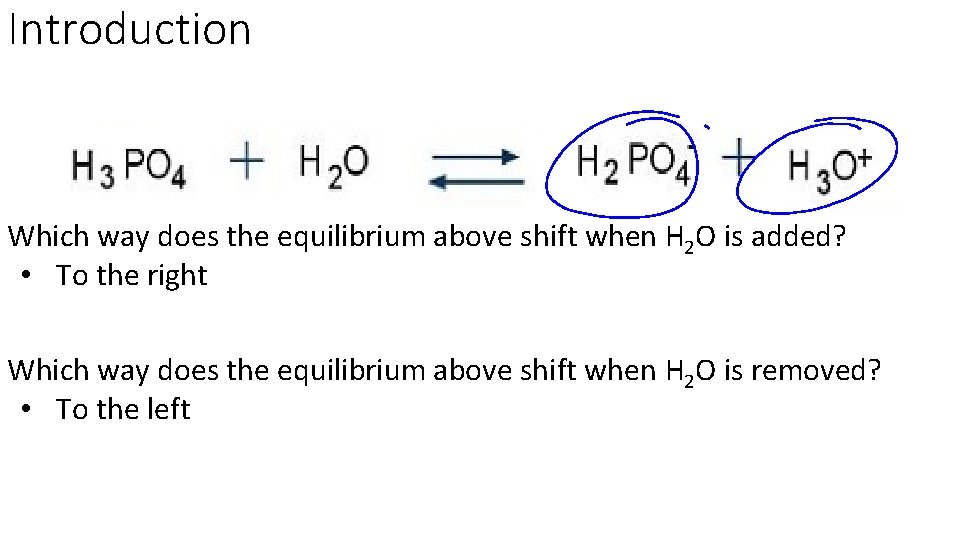
Introduction Which way does the equilibrium above shift when H 2 O is added? • To the right Which way does the equilibrium above shift when H 2 O is removed? • To the left

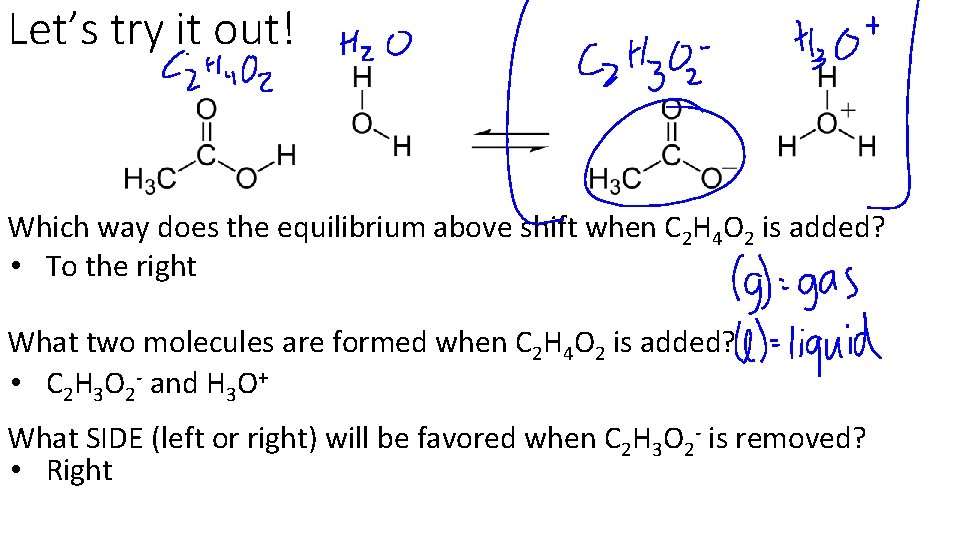
Let’s try it out! Which way does the equilibrium above shift when C 2 H 4 O 2 is added? • To the right What two molecules are formed when C 2 H 4 O 2 is added? • C 2 H 3 O 2 - and H 3 O+ What SIDE (left or right) will be favored when C 2 H 3 O 2 - is removed? • Right

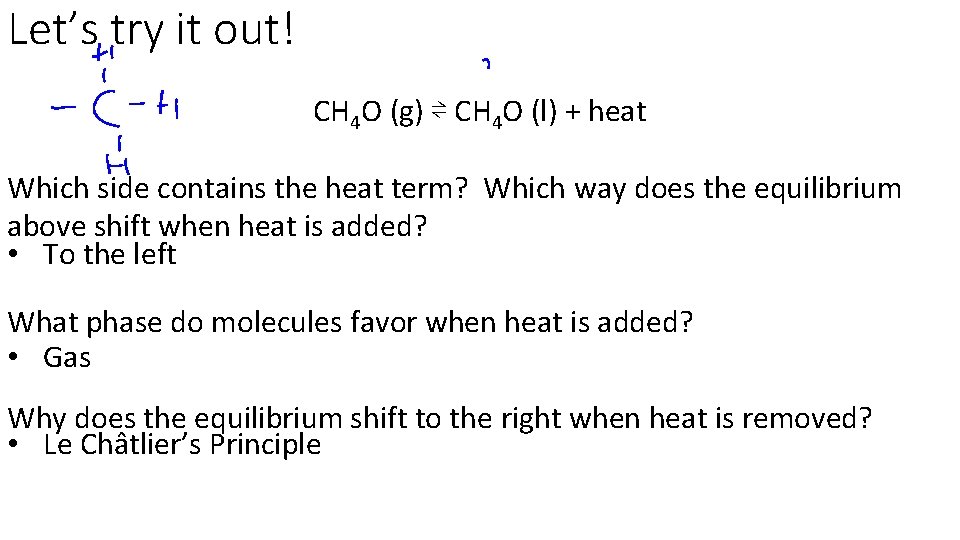
Let’s try it out! CH 4 O (g) ⇌ CH 4 O (l) + heat Which side contains the heat term? Which way does the equilibrium above shift when heat is added? • To the left What phase do molecules favor when heat is added? • Gas Why does the equilibrium shift to the right when heat is removed? • Le Châtlier’s Principle

Your Turn!

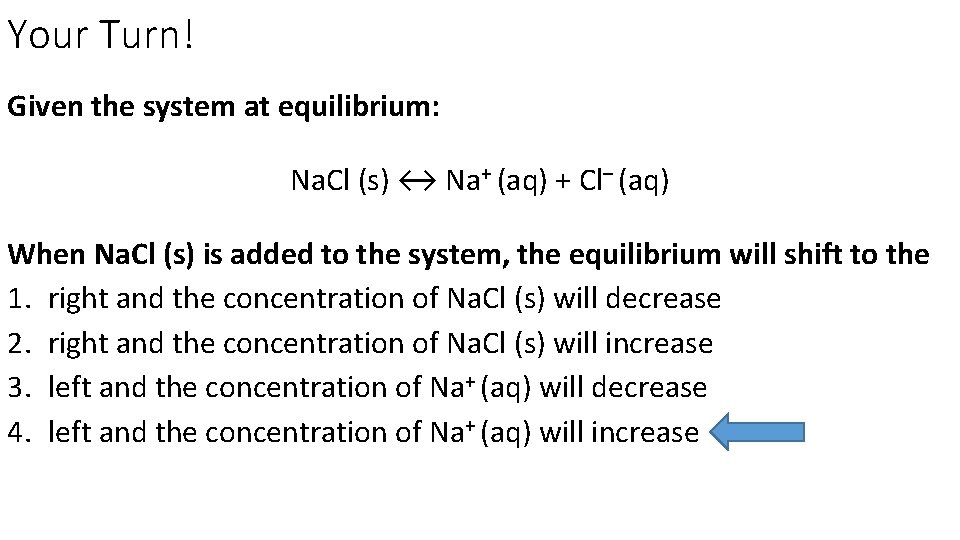
Your Turn! Given the system at equilibrium: Na. Cl (s) ↔ Na+ (aq) + Cl– (aq) When Na. Cl (s) is added to the system, the equilibrium will shift to the 1. right and the concentration of Na. Cl (s) will decrease 2. right and the concentration of Na. Cl (s) will increase 3. left and the concentration of Na+ (aq) will decrease 4. left and the concentration of Na+ (aq) will increase

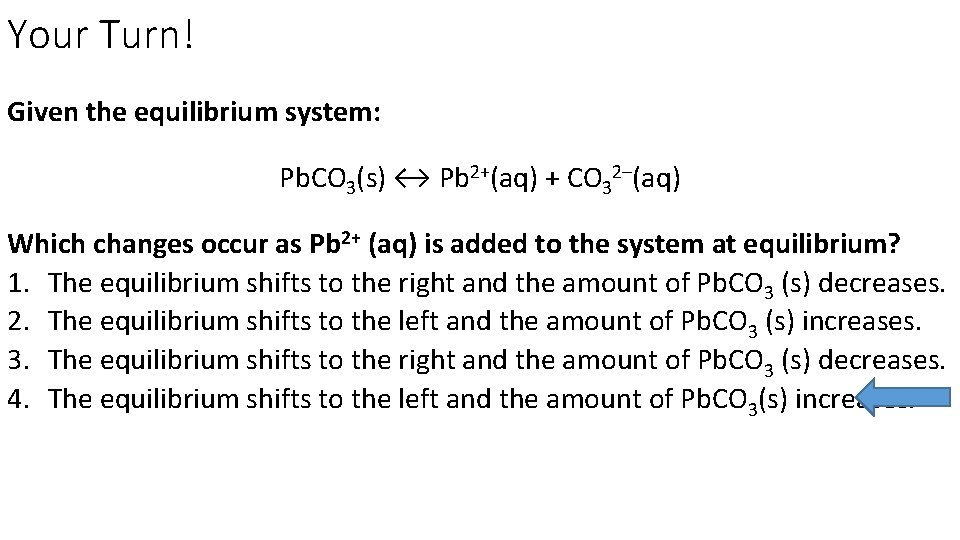
Your Turn! Given the equilibrium system: Pb. CO 3(s) ↔ Pb 2+(aq) + CO 32–(aq) Which changes occur as Pb 2+ (aq) is added to the system at equilibrium? 1. The equilibrium shifts to the right and the amount of Pb. CO 3 (s) decreases. 2. The equilibrium shifts to the left and the amount of Pb. CO 3 (s) increases. 3. The equilibrium shifts to the right and the amount of Pb. CO 3 (s) decreases. 4. The equilibrium shifts to the left and the amount of Pb. CO 3(s) increases.

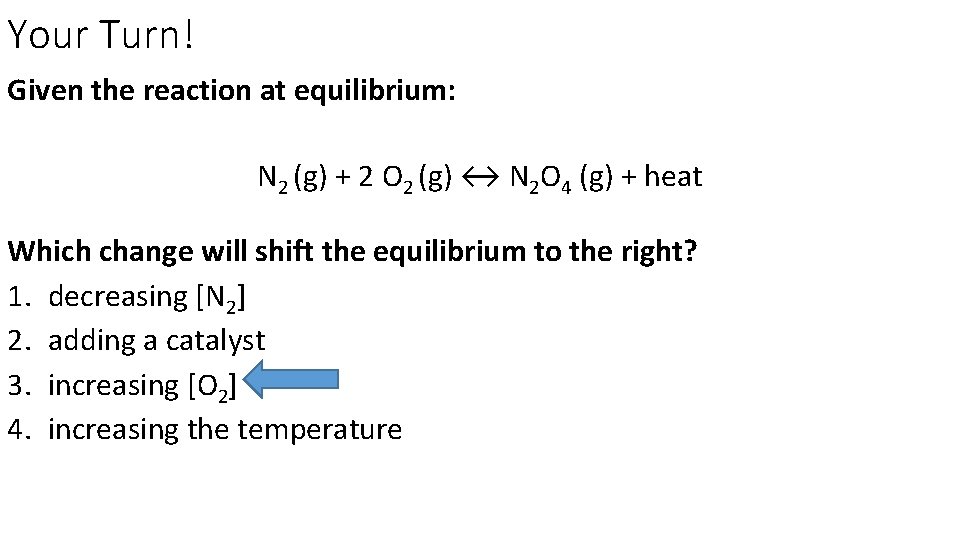
Your Turn! Given the reaction at equilibrium: N 2 (g) + 2 O 2 (g) ↔ N 2 O 4 (g) + heat Which change will shift the equilibrium to the right? 1. decreasing [N 2] 2. adding a catalyst 3. increasing [O 2] 4. increasing the temperature

Your Turn! Given the equilibrium reaction in a closed system: 2 HI(g)⇌ H 2(g) + I 2(g) + heat What will be the result of an increase in temperature? 1. The equilibrium will shift to the left and [H 2] will increase. 2. The equilibrium will shift to the left and [H 2] will decrease. 3. The equilibrium will shift to the right and [I 2] will increase. 4. The equilibrium will shift to the right and [HI] will increase.

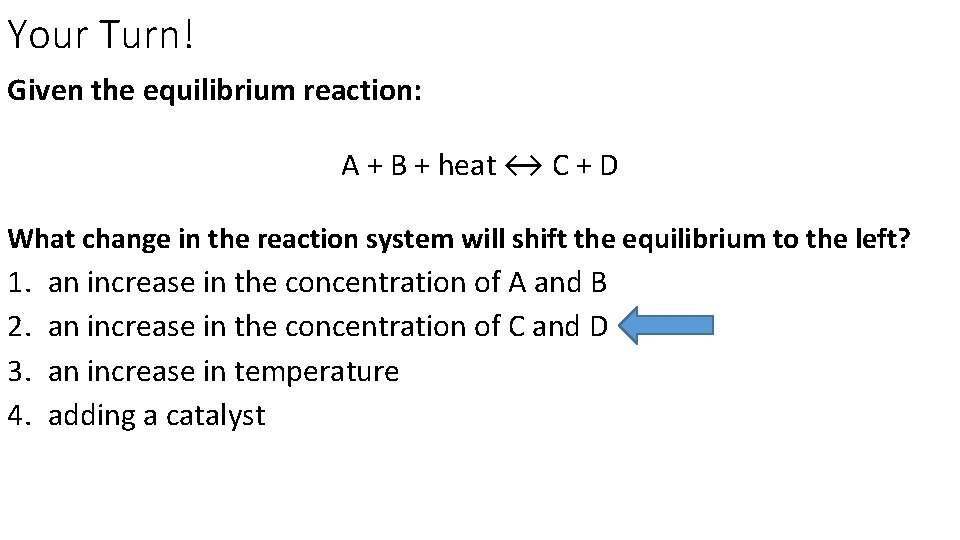
Your Turn! Given the equilibrium reaction: A + B + heat ↔ C + D What change in the reaction system will shift the equilibrium to the left? 1. 2. 3. 4. an increase in the concentration of A and B an increase in the concentration of C and D an increase in temperature adding a catalyst

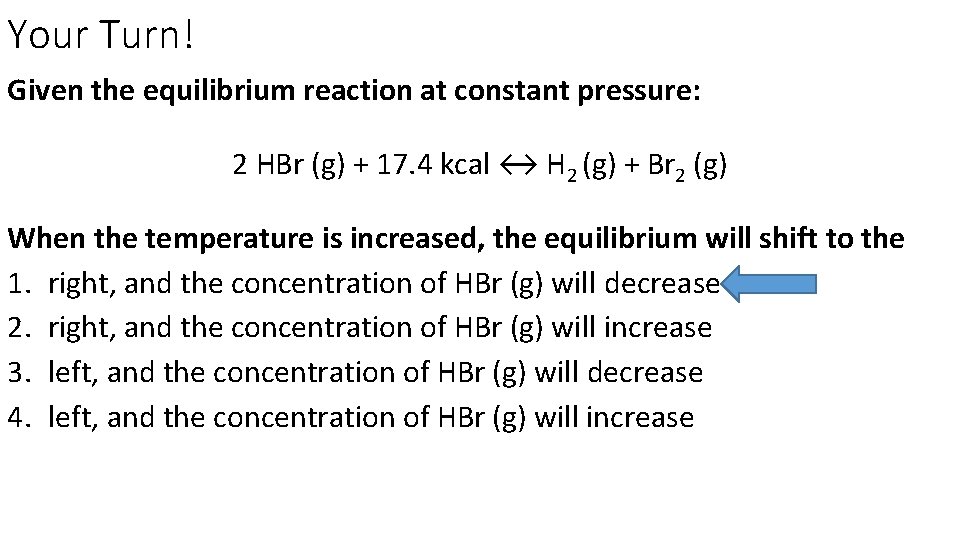
Your Turn! Given the equilibrium reaction at constant pressure: 2 HBr (g) + 17. 4 kcal ↔ H 2 (g) + Br 2 (g) When the temperature is increased, the equilibrium will shift to the 1. right, and the concentration of HBr (g) will decrease 2. right, and the concentration of HBr (g) will increase 3. left, and the concentration of HBr (g) will decrease 4. left, and the concentration of HBr (g) will increase