Mr Quinn Ms Tom March 17 2014 Aim







- Slides: 15

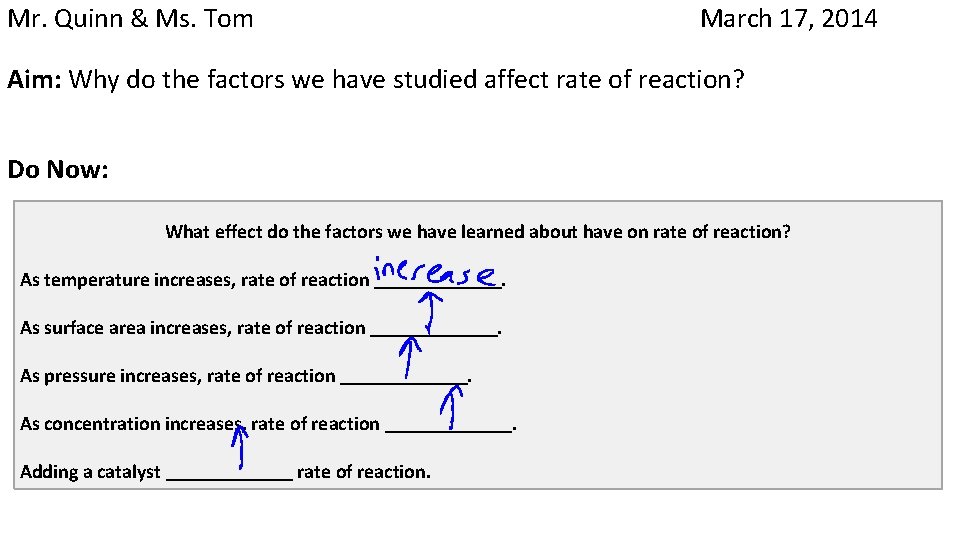
Mr. Quinn & Ms. Tom March 17, 2014 Aim: Why do the factors we have studied affect rate of reaction? Do Now: What effect do the factors we have learned about have on rate of reaction? As temperature increases, rate of reaction . As surface area increases, rate of reaction . As pressure increases, rate of reaction As concentration increases, rate of reaction Adding a catalyst rate of reaction. . .

Introduction: How does each factor work? Temperature increases rate of reaction because Surface area increases rate of reaction because 1) The number of collisions increases 2) Each molecule has more energy to react 3) The orientation of the molecules is better 4) The activation energy decreases Note: The highest temperature means the greatest rate 1) The number of collisions increases 2) Each molecule has more energy to react 3) The orientation of the molecules is better 4) The activation energy decreases Note: A powder had greater surface area than a sheet.

Introduction: How does each factor work? Pressure increases rate of reaction because Concentration increases rate of reaction because 1) The number of collisions increases 2) Each molecule has more energy to react 3) The orientation of the molecules is better 4) The activation energy decreases Note: The highest pressure means the greatest rate 1) The number of collisions increases 2) Each molecule has more energy to react 3) The orientation of the molecules is better 4) The activation energy decreases Note: The highest concentration means the greatest rate.

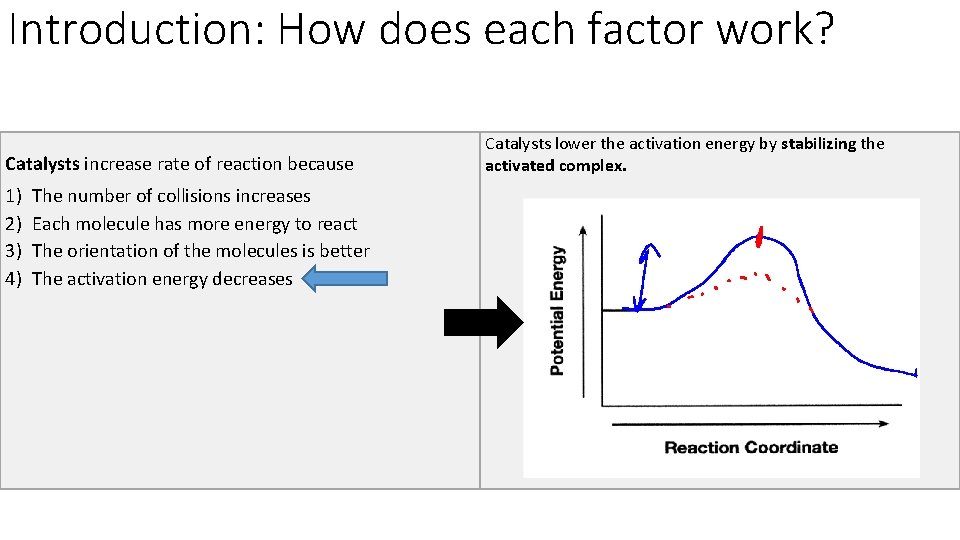
Introduction: How does each factor work? Catalysts increase rate of reaction because 1) 2) 3) 4) The number of collisions increases Each molecule has more energy to react The orientation of the molecules is better The activation energy decreases Catalysts lower the activation energy by stabilizing the activated complex.

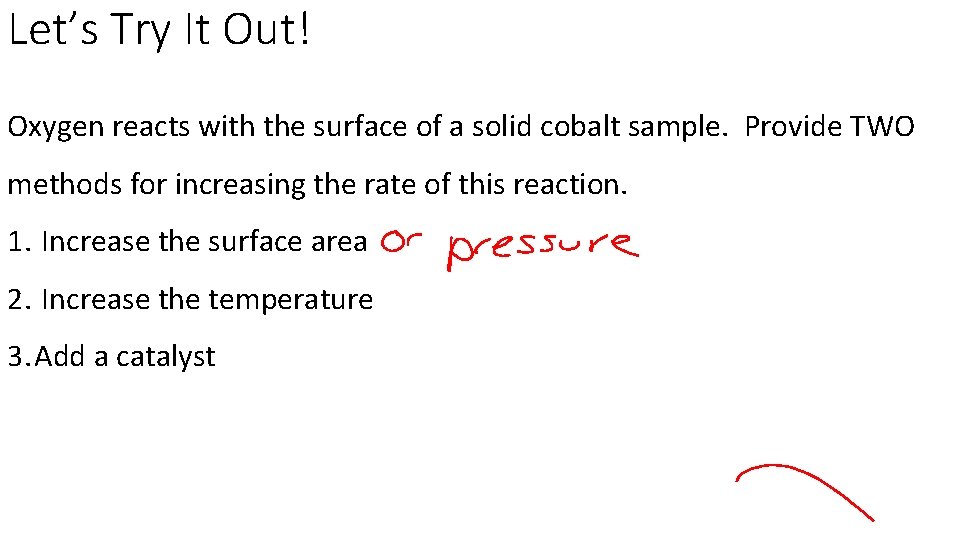
Let’s Try It Out! Oxygen reacts with the surface of a solid cobalt sample. Provide TWO methods for increasing the rate of this reaction. 1. Increase the surface area 2. Increase the temperature 3. Add a catalyst

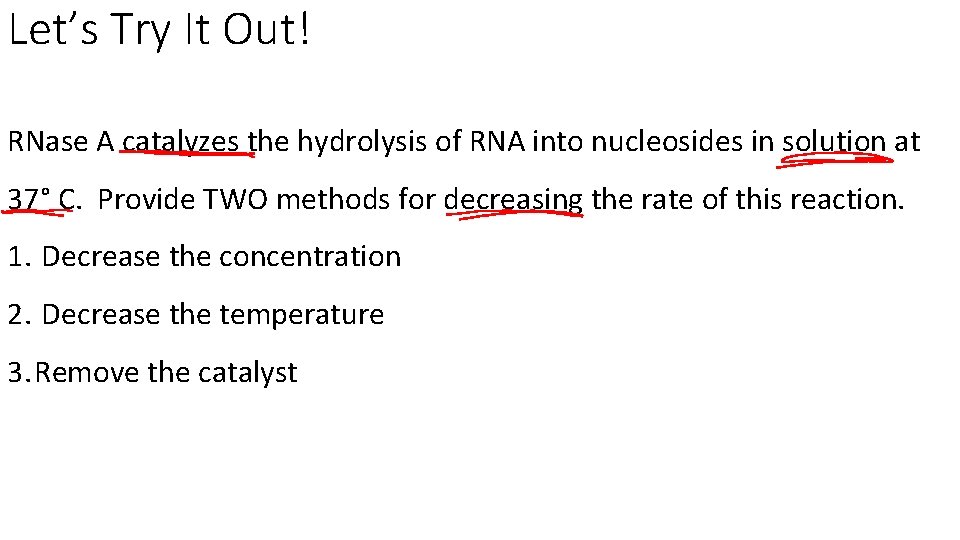
Let’s Try It Out! RNase A catalyzes the hydrolysis of RNA into nucleosides in solution at 37° C. Provide TWO methods for decreasing the rate of this reaction. 1. Decrease the concentration 2. Decrease the temperature 3. Remove the catalyst

Your Turn!

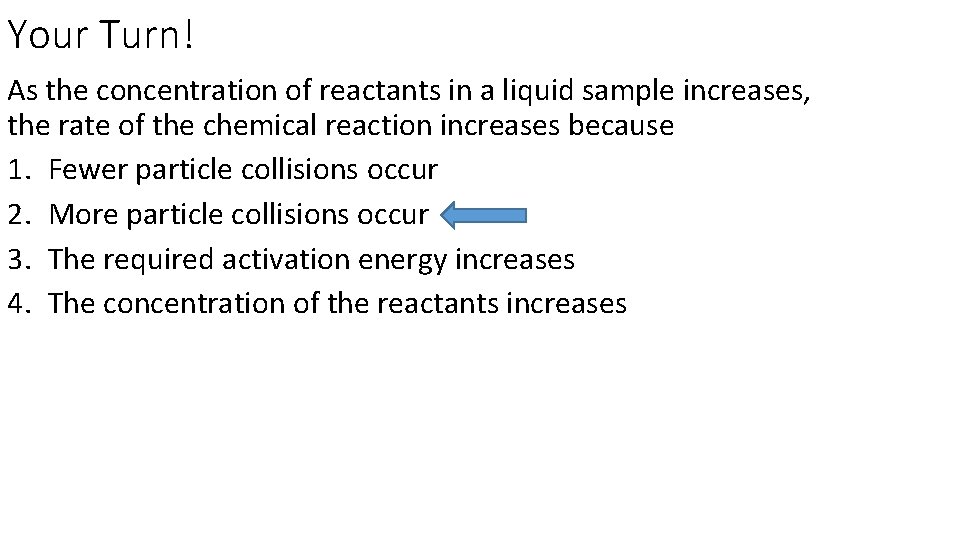
Your Turn! As the concentration of reactants in a liquid sample increases, the rate of the chemical reaction increases because 1. Fewer particle collisions occur 2. More particle collisions occur 3. The required activation energy increases 4. The concentration of the reactants increases

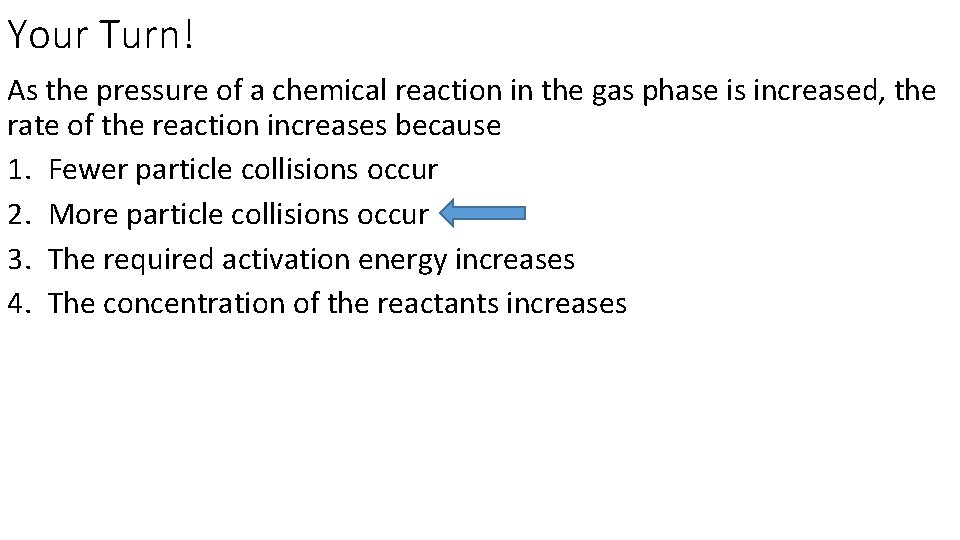
Your Turn! As the pressure of a chemical reaction in the gas phase is increased, the rate of the reaction increases because 1. Fewer particle collisions occur 2. More particle collisions occur 3. The required activation energy increases 4. The concentration of the reactants increases

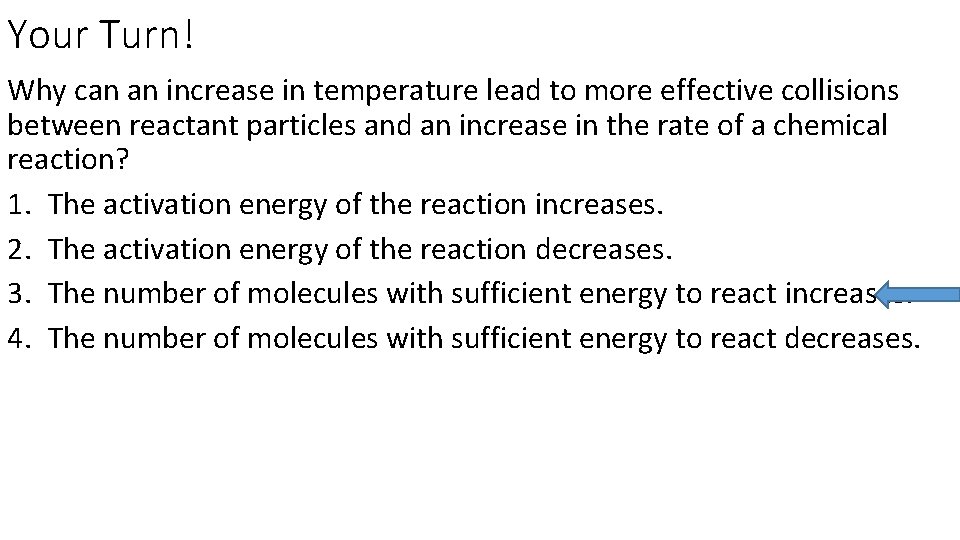
Your Turn! Why can an increase in temperature lead to more effective collisions between reactant particles and an increase in the rate of a chemical reaction? 1. The activation energy of the reaction increases. 2. The activation energy of the reaction decreases. 3. The number of molecules with sufficient energy to react increases. 4. The number of molecules with sufficient energy to react decreases.

Your Turn! At what concentration will Na. OH and HCl react the most quickly? 1. 0. 1 % 2. 1 % 3. 5 % 4. 15 %

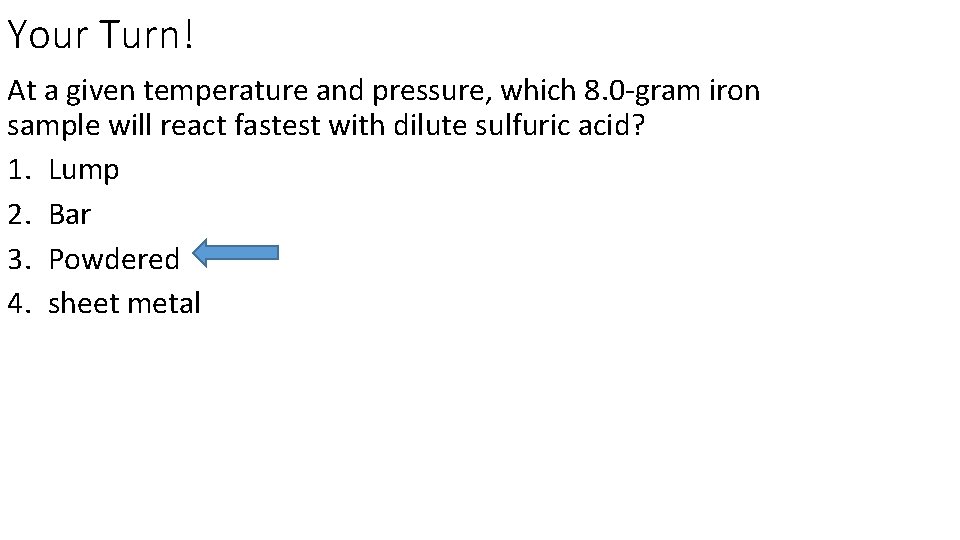
Your Turn! At a given temperature and pressure, which 8. 0 -gram iron sample will react fastest with dilute sulfuric acid? 1. Lump 2. Bar 3. Powdered 4. sheet metal

Your Turn! Given the reaction: Na + 2 H 2 O → 2 Na. OH + H 2 At which temperature will the reaction occur at the greatest rate? 1. -5°C 2. 0°C 3. 45°C 4. 90°C

Your Turn! Given the reaction: N 2 (g) + 2 O 2 (g) N 2 O 4 (g) At what pressure will the reaction occur at the greatest rate? (Note: “atm” is a measure of pressure) 1. 0. 1 atm 2. 1 atm 3. 2 atm 4. 5 atm

Your Turn! Proteins naturally break down in water, but each bond has a half-life of 1000 years. Trypsin is an enzyme that catalyzes the breakdown of proteins, reducing the half-life to 15 minutes. The rate of reaction increases because 1. The concentration of protein increases 2. The activation energy decreases 3. The temperature of the reaction increases 4. The pressure of the reaction increases