Moderator Disclosure SPEAKER NAME Speaker Credentials Relationships With



![Accreditation Statement • [To be completed] Accreditation Statement • [To be completed]](https://slidetodoc.com/presentation_image_h2/fbf736b601292ef6a4627525ee79fdf8/image-5.jpg)





















- Slides: 73


Moderator Disclosure SPEAKER NAME Speaker Credentials Relationships With Financial Sponsors CONSULTING FEES/ ADVISORY BOARD MEMBER: SPEAKERS BUREAU/HONORARIA: GRANTS/RESEARCH SUPPORT: OTHER: 2

Disclosure of Financial Support This program has received • Financial support from Servier Canada in the form of an educational grant • In-kind support from Servier Canada in the form of logistical support Potential for conflict of interest • The speaker has received honoraria from the CPD Network Association • Servier Canada benefits from the sale of products that may be discussed in this program

Disclosure of Financial Support Mitigation of potential bias • The CPD Network is a not-for-profit physician organization who received an educational grant to develop this program. The CPD Network engaged the scientific planning committee and participated in the content and format of this program. • The steering committee was solely and fully responsible for developing all content and was involved at all stages of CME development to achieve scientific integrity, objectivity and balance. • Servier Canada provided funding for the content development and this CME event but were not involved in any aspect of the program development process. • Speakers have received instructions on the Conflict of Interest disclosure requirements and are required to complete all necessary documents as mandated by the CFPC. Should any conflict arise, it will be brought to the attention of the CPD Network and the subsequent course of action will be dependent on the nature of the conflict. Every effort will be made to mitigate any perceived conflicts as well. • Speakers must inform the audience if unapproved or off-label uses of a product are discussed. If any discussions represent the personal opinions of the speakers, and unsolicited questions should be directed to the speakers.
![Accreditation Statement To be completed Accreditation Statement • [To be completed]](https://slidetodoc.com/presentation_image_h2/fbf736b601292ef6a4627525ee79fdf8/image-5.jpg)
Accreditation Statement • [To be completed]

Scientific Planning Committee Jeff Healey, MD, FRCPC (chair) Professor, Division of Cardiology, Department of Medicine Mc. Master University Hamilton, ON Jeff Habert MD, CCFP, FCFP Assistant Professor Department of Family and Community Medicine University of Toronto, ON Dominique Dion, MD, MSc Assistant Professor Department of Family Medicine and Emergency Medicine University of Montreal, QC Jaqueline Joza, MD, FRCPC Assistant Professor Department of Medicine Mc. Gill University Montreal, QC Carl Fournier, MD, CFPC Assistant Clinical Professor Department of Family Medicine and Emergency Medicine University of Montreal, QC Kevin Saunders, MD, CCFP Family Physician Rivergrove Medical Clinic Winnipeg, MB

Learning Objectives At the conclusion of this program, participants will be able to: Apply best practices regarding the use of NOACs for stroke prevention in AF patients, with a focus on appropriate dosing, drug-drug interactions and treatment selection Discuss the efficacy and safety of NOACs in the general AF population and in selected sub-groups Integrate in clinical practice the recent realworld data on the use of NOACs for stroke prevention

Why is this topic so important?

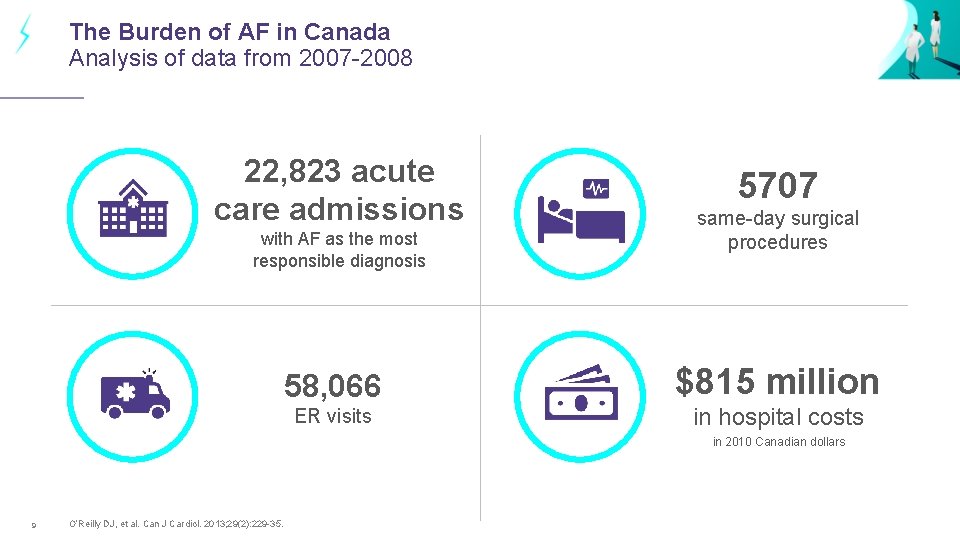
The Burden of AF in Canada Analysis of data from 2007 -2008 22, 823 acute care admissions with AF as the most responsible diagnosis 5707 same-day surgical procedures 58, 066 $815 million ER visits in hospital costs in 2010 Canadian dollars 9 O’Reilly DJ, et al. Can J Cardiol. 2013; 29(2): 229 -35.

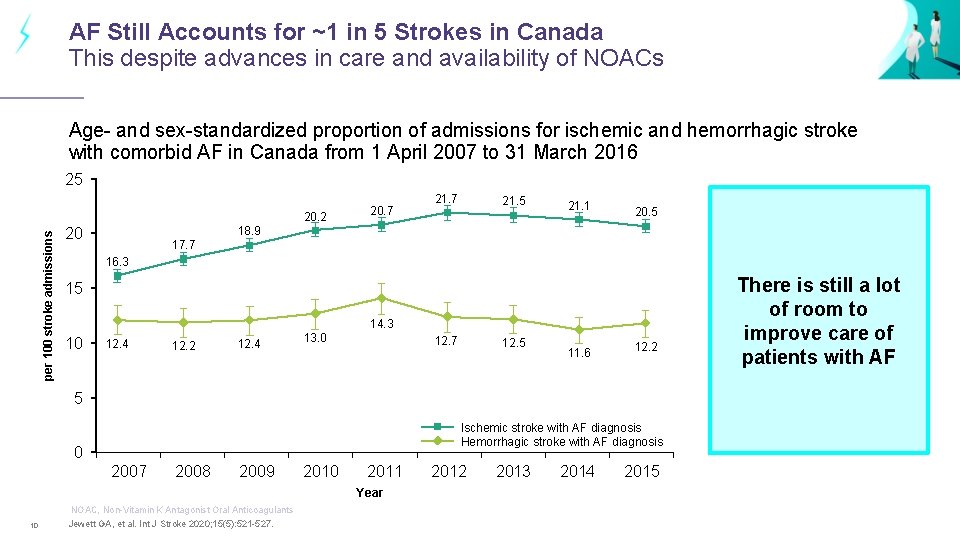
AF Still Accounts for ~1 in 5 Strokes in Canada This despite advances in care and availability of NOACs Age- and sex-standardized proportion of admissions for ischemic and hemorrhagic stroke with comorbid AF in Canada from 1 April 2007 to 31 March 2016 25 per 100 stroke admissions 20. 2 20. 7 21. 5 21. 1 20. 5 18. 9 20 17. 7 16. 3 15 14. 3 10 12. 4 12. 2 12. 4 13. 0 12. 7 12. 5 11. 6 12. 2 5 Ischemic stroke with AF diagnosis Hemorrhagic stroke with AF diagnosis 0 2007 2008 2009 2010 2011 Year NOAC, Non-Vitamin K Antagonist Oral Anticoagulants 10 Jewett GA, et al. Int J Stroke 2020; 15(5): 521 -527. 2012 2013 2014 2015 There is still a lot of room to improve care of patients with AF

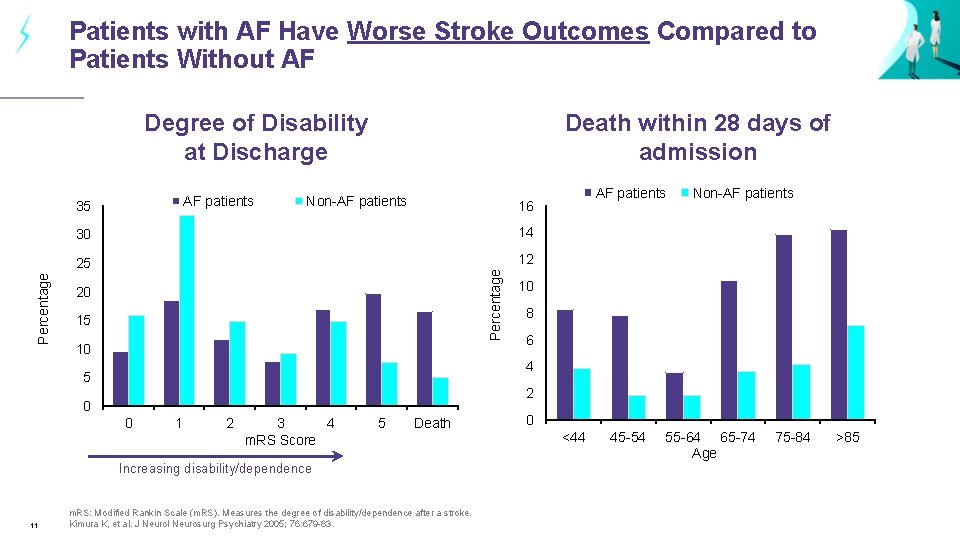
Patients with AF Have Worse Stroke Outcomes Compared to Patients Without AF Degree of Disability at Discharge AF patients Non-AF patients 30 14 25 12 20 15 10 Non-AF patients 10 8 6 4 5 2 0 0 1 2 3 4 m. RS Score 5 Death Increasing disability/dependence 11 AF patients 16 Percentage 35 Death within 28 days of admission m. RS: Modified Rankin Scale (m. RS). Measures the degree of disability/dependence after a stroke. Kimura K, et al. J Neurol Neurosurg Psychiatry 2005; 76: 679 -83. 0 <44 45 -54 55 -64 65 -74 Age 75 -84 >85

What is the clinical trial evidence behind these guidelines?

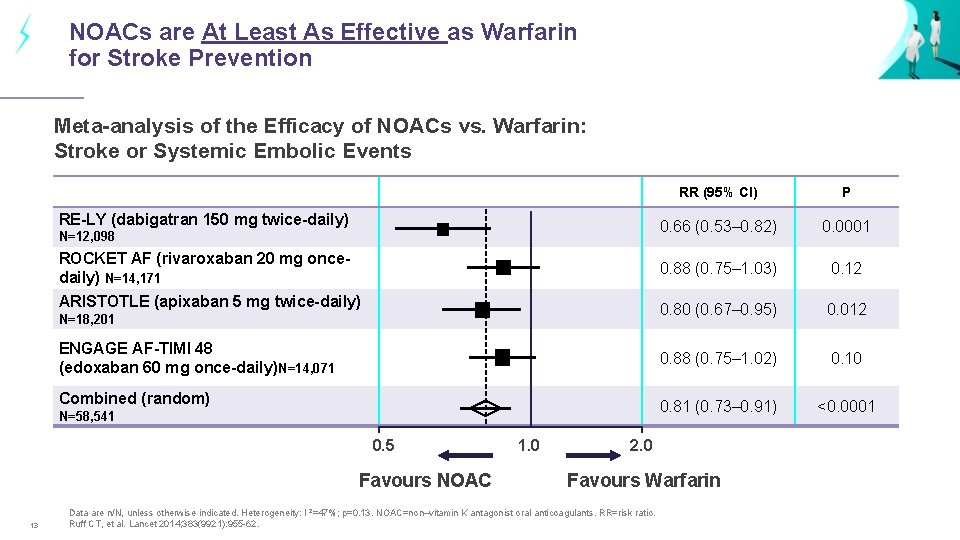
NOACs are At Least As Effective as Warfarin for Stroke Prevention Meta-analysis of the Efficacy of NOACs vs. Warfarin: Stroke or Systemic Embolic Events RR (95% CI) P 0. 66 (0. 53– 0. 82) 0. 0001 0. 88 (0. 75– 1. 03) 0. 12 0. 80 (0. 67– 0. 95) 0. 012 ENGAGE AF-TIMI 48 (edoxaban 60 mg once-daily)N=14, 071 0. 88 (0. 75– 1. 02) 0. 10 Combined (random) 0. 81 (0. 73– 0. 91) <0. 0001 RE-LY (dabigatran 150 mg twice-daily) N=12, 098 ROCKET AF (rivaroxaban 20 mg oncedaily) N=14, 171 ARISTOTLE (apixaban 5 mg twice-daily) N=18, 201 N=58, 541 0. 5 Favours NOAC 13 1. 0 2. 0 Favours Warfarin Data are n/N, unless otherwise indicated. Heterogeneity: I 2=47%; p=0. 13. NOAC=non–vitamin K antagonist oral anticoagulants. RR=risk ratio. Ruff CT, et al. Lancet 2014; 383(9921): 955 -62.

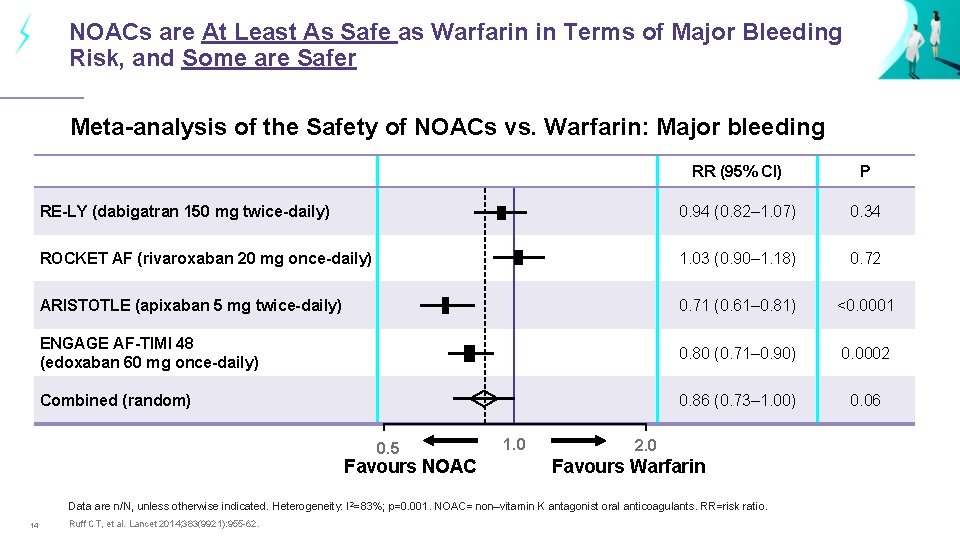
NOACs are At Least As Safe as Warfarin in Terms of Major Bleeding Risk, and Some are Safer Meta-analysis of the Safety of NOACs vs. Warfarin: Major bleeding RR (95% CI) P RE-LY (dabigatran 150 mg twice-daily) 0. 94 (0. 82– 1. 07) 0. 34 ROCKET AF (rivaroxaban 20 mg once-daily) 1. 03 (0. 90– 1. 18) 0. 72 ARISTOTLE (apixaban 5 mg twice-daily) 0. 71 (0. 61– 0. 81) <0. 0001 ENGAGE AF-TIMI 48 (edoxaban 60 mg once-daily) 0. 80 (0. 71– 0. 90) 0. 0002 Combined (random) 0. 86 (0. 73– 1. 00) 0. 06 0. 5 Favours NOAC 1. 0 2. 0 Favours Warfarin Data are n/N, unless otherwise indicated. Heterogeneity: I 2=83%; p=0. 001. NOAC= non–vitamin K antagonist oral anticoagulants. RR=risk ratio. 14 Ruff CT, et al. Lancet 2014; 383(9921): 955 -62.

What more can we learn from real-world evidence? Review of RWD studies: ARISTOPHANES, ORBIT-AF, ETNA-AF, etc. Impact of RCT and RWD on clinical practice

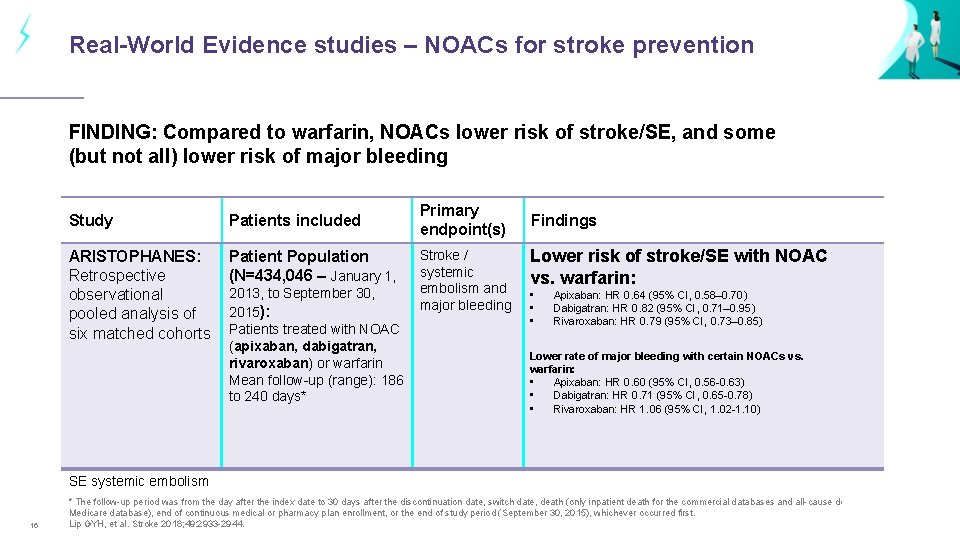
Real-World Evidence studies – NOACs for stroke prevention FINDING: Compared to warfarin, NOACs lower risk of stroke/SE, and some (but not all) lower risk of major bleeding Study Patients included ARISTOPHANES: Retrospective observational pooled analysis of six matched cohorts Patient Population (N=434, 046 – January 1, 2013, to September 30, 2015): Patients treated with NOAC (apixaban, dabigatran, rivaroxaban) or warfarin Mean follow-up (range): 186 to 240 days* Primary endpoint(s) Stroke / systemic embolism and major bleeding Findings Lower risk of stroke/SE with NOAC vs. warfarin: • • • Apixaban: HR 0. 64 (95% CI, 0. 58– 0. 70) Dabigatran: HR 0. 82 (95% CI, 0. 71– 0. 95) Rivaroxaban: HR 0. 79 (95% CI, 0. 73– 0. 85) Lower rate of major bleeding with certain NOACs vs. warfarin: • Apixaban: HR 0. 60 (95% CI, 0. 56 -0. 63) • Dabigatran: HR 0. 71 (95% CI, 0. 65 -0. 78) • Rivaroxaban: HR 1. 06 (95% CI, 1. 02 -1. 10) SE systemic embolism 16 * The follow-up period was from the day after the index date to 30 days after the discontinuation date, switch date, death (only inpatient death for the commercial databases and all-cause death for Medicare database), end of continuous medical or pharmacy plan enrollment, or the end of study period (September 30, 2015), whichever occurred first. Lip GYH, et al. Stroke 2018; 49: 2933 -2944.

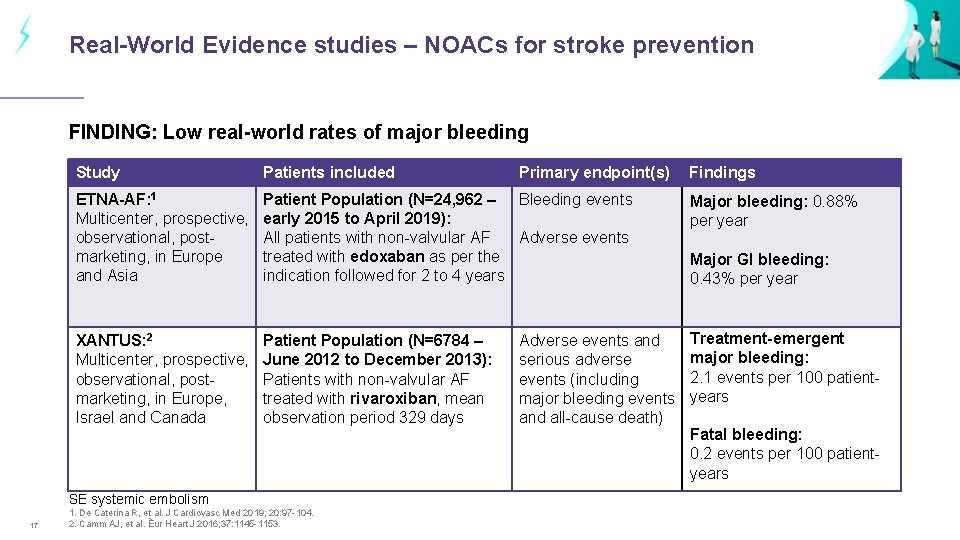
Real-World Evidence studies – NOACs for stroke prevention FINDING: Low real-world rates of major bleeding Study Patients included ETNA-AF: 1 Multicenter, prospective, observational, postmarketing, in Europe and Asia Patient Population (N=24, 962 – Bleeding events early 2015 to April 2019): All patients with non-valvular AF Adverse events treated with edoxaban as per the indication followed for 2 to 4 years Major bleeding: 0. 88% per year XANTUS: 2 Multicenter, prospective, observational, postmarketing, in Europe, Israel and Canada Patient Population (N=6784 – June 2012 to December 2013): Patients with non-valvular AF treated with rivaroxiban, mean observation period 329 days Treatment-emergent major bleeding: 2. 1 events per 100 patientyears SE systemic embolism 17 1. De Caterina R, et al. J Cardiovasc Med 2019; 20: 97 -104. 2. Camm AJ, et al. Êur Heart J 2016; 37: 1145 -1153. Primary endpoint(s) Adverse events and serious adverse events (including major bleeding events and all-cause death) Findings Major GI bleeding: 0. 43% per year Fatal bleeding: 0. 2 events per 100 patientyears

How do you know if a patient needs Stroke Prevention for AF?

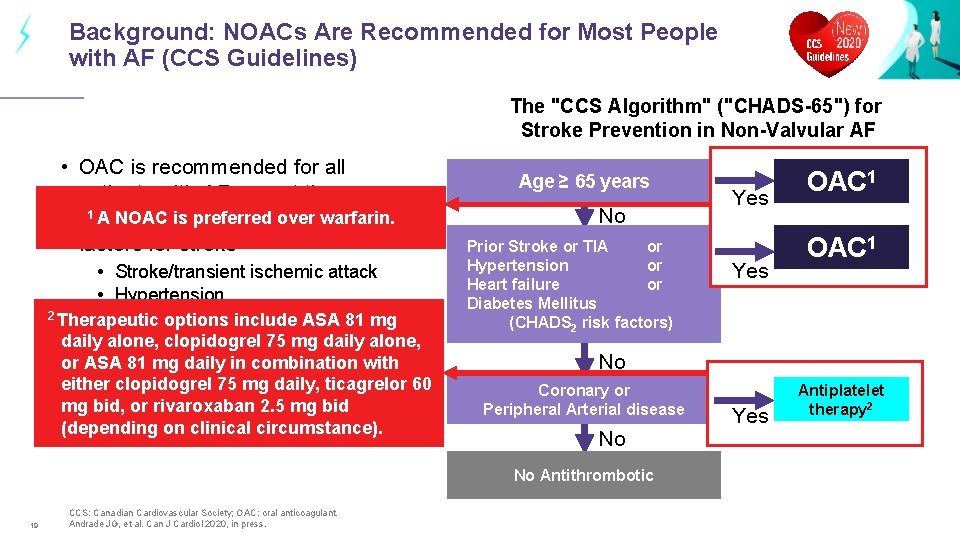
Background: NOACs Are Recommended for Most People with AF (CCS Guidelines) The "CCS Algorithm" ("CHADS-65") for Stroke Prevention in Non-Valvular AF • OAC is recommended for all patients with AF except those 1 A NOAC is preferred over warfarin. <65 years & no additional risk factors for stroke • Stroke/transient ischemic attack • Hypertension 2 Therapeutic • Heart options failure include ASA 81 mg Diabetes Mellitus 75 mg daily alone, daily • alone, clopidogrel or ASA 81 mg daily in combination with • either A NOAC is recommended in clopidogrel 75 mg daily, ticagrelor 60 preference to a VKA 2. 5 for nonmg bid, or rivaroxaban mg bid valvular AF (NVAF)circumstance). (depending on clinical Age ≥ 65 years No Prior Stroke or TIA or Hypertension or Heart failure or Diabetes Mellitus (CHADS 2 risk factors) CCS: Canadian Cardiovascular Society; OAC: oral anticoagulant. Andrade JG, et al. Can J Cardiol 2020, in press. Yes OAC 1 No Coronary or Peripheral Arterial disease No No Antithrombotic 19 Yes OAC 1 Yes Antiplatelet therapy 2

Review of therapeutic Agents

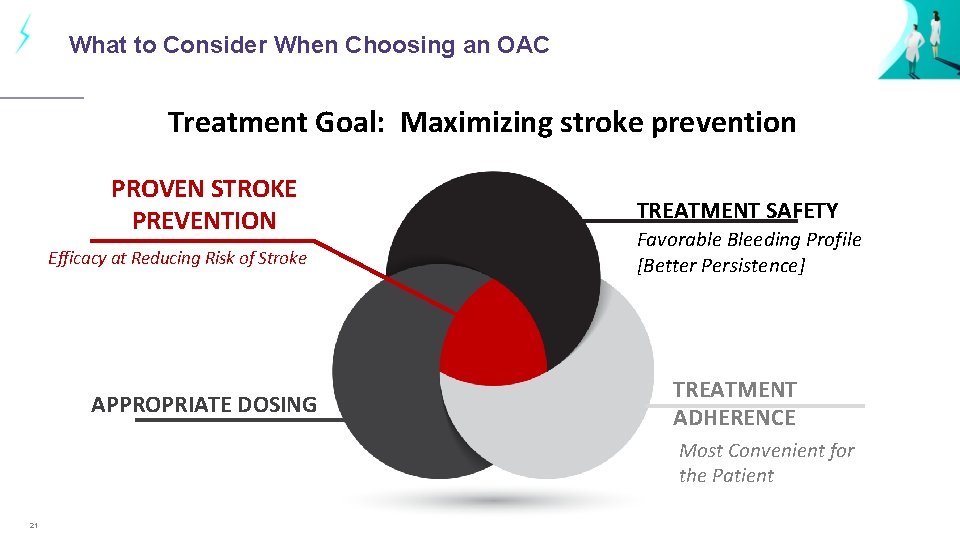
What to Consider When Choosing an OAC Treatment Goal: Maximizing stroke prevention PROVEN STROKE PREVENTION Efficacy at Reducing Risk of Stroke APPROPRIATE DOSING TREATMENT SAFETY Favorable Bleeding Profile [Better Persistence] TREATMENT ADHERENCE Most Convenient for the Patient 21

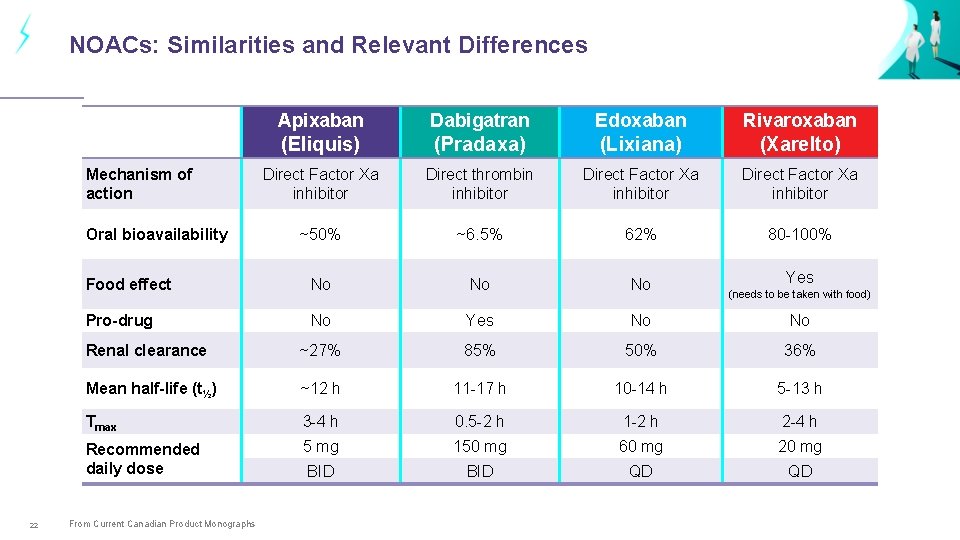
NOACs: Similarities and Relevant Differences Apixaban Dabigatran Edoxaban Rivaroxaban (Eliquis) (Pradaxa) (Lixiana) (Xarelto) Direct Factor Xa inhibitor Direct thrombin inhibitor Direct Factor Xa inhibitor ~50% ~6. 5% 62% 80 -100% Food effect No No No Yes Pro-drug No Yes No No Renal clearance ~27% 85% 50% 36% Mean half-life (t½) ~12 h 11 -17 h 10 -14 h 5 -13 h Tmax 3 -4 h 0. 5 -2 h 1 -2 h 2 -4 h Recommended daily dose 5 mg 150 mg 60 mg 20 mg BID QD QD Mechanism of action Oral bioavailability 22 From Current Canadian Product Monographs (needs to be taken with food)

Case #1: 70 -year-old man presents to the ER with a stroke. You discover that he was underdosed.

Polling Question How frequently do you think patients are underdosed on their NOACs? 24 A. Very often B. Sometimes C. Rarely D. Never

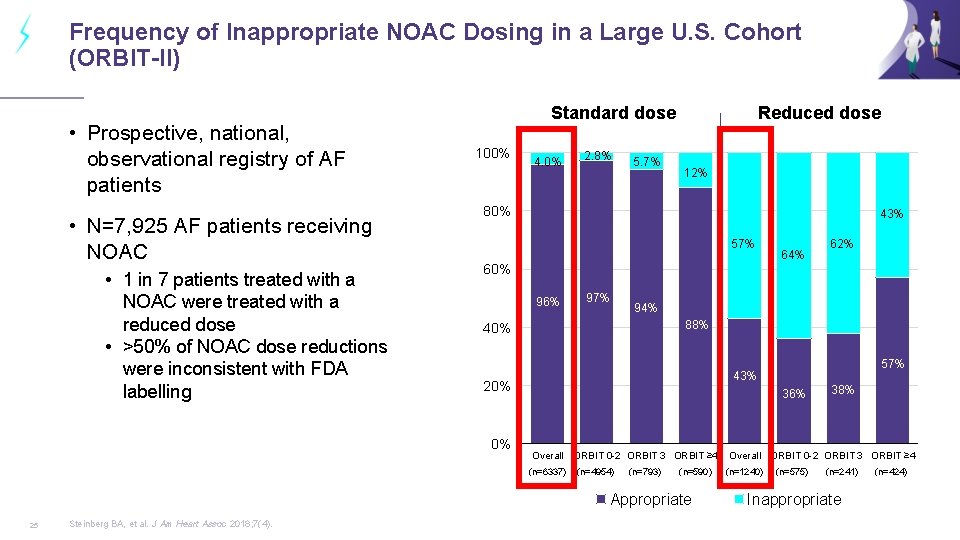
Frequency of Inappropriate NOAC Dosing in a Large U. S. Cohort (ORBIT-II) • Prospective, national, observational registry of AF patients • N=7, 925 AF patients receiving NOAC • 1 in 7 patients treated with a NOAC were treated with a reduced dose • >50% of NOAC dose reductions were inconsistent with FDA labelling Standard dose 100% 4. 0% 2. 8% 5. 7% Reduced dose 12% 80% 43% 57% 96% 97% 94% 88% 40% 57% 43% 20% 36% Overall ORBIT 0 -2 ORBIT 3 ORBIT ≥ 4 (n=6337) (n=4954) (n=793) (n=590) Appropriate Steinberg BA, et al. J Am Heart Assoc 2018; 7(4). 62% 60% 0% 25 64% 38% Overall ORBIT 0 -2 ORBIT 3 ORBIT ≥ 4 (n=1240) (n=575) (n=241) Inappropriate (n=424)

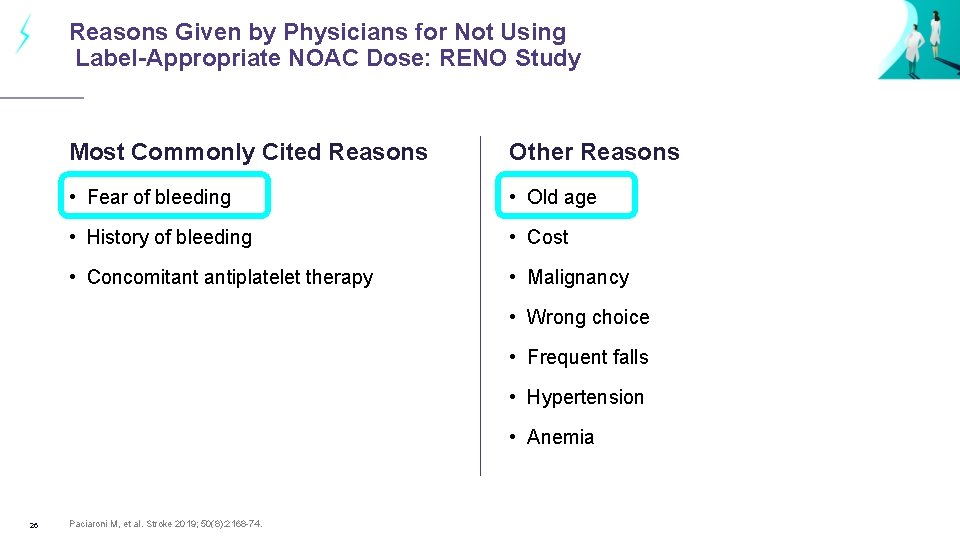
Reasons Given by Physicians for Not Using Label-Appropriate NOAC Dose: RENO Study Most Commonly Cited Reasons Other Reasons • Fear of bleeding • Old age • History of bleeding • Cost • Concomitant antiplatelet therapy • Malignancy • Wrong choice • Frequent falls • Hypertension • Anemia 26 Paciaroni M, et al. Stroke 2019; 50(8): 2168 -74.

True or False? Reducing the NOAC dose significantly lowers the risk of serious bleeds. 27 True False

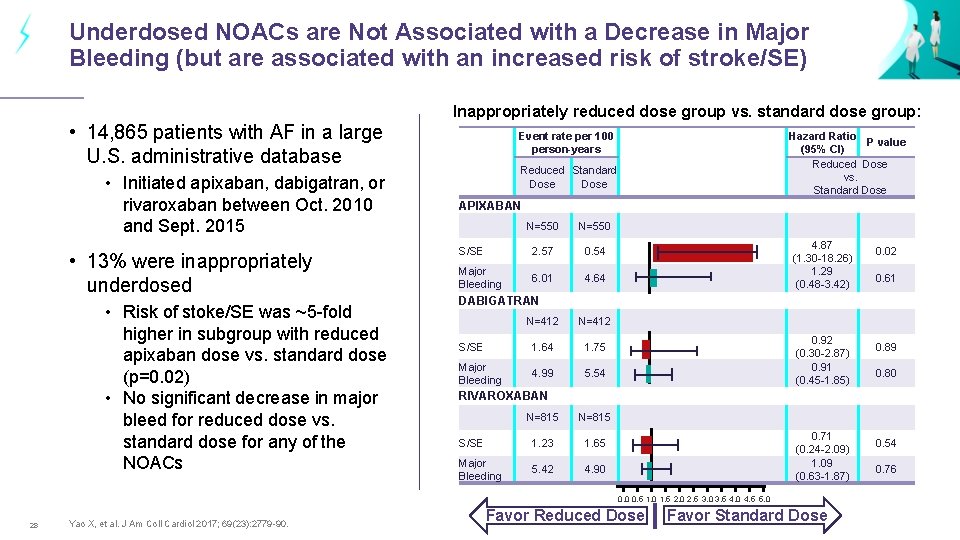
Underdosed NOACs are Not Associated with a Decrease in Major Bleeding (but are associated with an increased risk of stroke/SE) Inappropriately reduced dose group vs. standard dose group: • 14, 865 patients with AF in a large U. S. administrative database • Initiated apixaban, dabigatran, or rivaroxaban between Oct. 2010 and Sept. 2015 • 13% were inappropriately underdosed • Risk of stoke/SE was ~5 -fold higher in subgroup with reduced apixaban dose vs. standard dose (p=0. 02) • No significant decrease in major bleed for reduced dose vs. standard dose for any of the NOACs Event rate per 100 person-years Hazard Ratio P value (95% CI) Reduced Dose vs. Standard Dose Reduced Standard Dose APIXABAN N=550 S/SE 2. 57 0. 54 Major Bleeding 6. 01 4. 64 4. 87 (1. 30 -18. 26) 1. 29 (0. 48 -3. 42) Yao X, et al. J Am Coll Cardiol 2017; 69(23): 2779 -90. 0. 61 DABIGATRAN N=412 S/SE 1. 64 1. 75 Major Bleeding 4. 99 5. 54 0. 92 (0. 30 -2. 87) 0. 91 (0. 45 -1. 85) 0. 89 0. 80 RIVAROXABAN N=815 S/SE 1. 23 1. 65 Major Bleeding 5. 42 4. 90 0. 71 (0. 24 -2. 09) 1. 09 (0. 63 -1. 87) 0. 0 0. 5 1. 0 1. 5 2. 0 2. 5 3. 0 3. 5 4. 0 4. 5 5. 0 28 0. 02 Favor Reduced Dose Favor Standard Dose 0. 54 0. 76

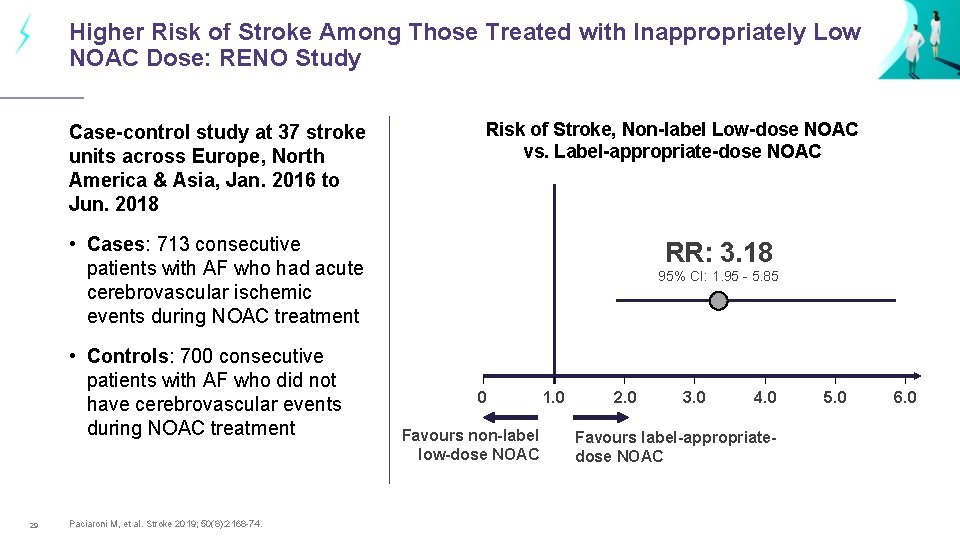
Higher Risk of Stroke Among Those Treated with Inappropriately Low NOAC Dose: RENO Study Case-control study at 37 stroke units across Europe, North America & Asia, Jan. 2016 to Jun. 2018 Risk of Stroke, Non-label Low-dose NOAC vs. Label-appropriate-dose NOAC • Cases: 713 consecutive patients with AF who had acute cerebrovascular ischemic events during NOAC treatment • Controls: 700 consecutive patients with AF who did not have cerebrovascular events during NOAC treatment 29 Paciaroni M, et al. Stroke 2019; 50(8): 2168 -74. RR: 3. 18 95% CI: 1. 95 - 5. 85 0 Favours non-label low-dose NOAC 1. 0 2. 0 3. 0 4. 0 Favours label-appropriatedose NOAC 5. 0 6. 0

Changing Case #1… What if the patient were 80 years old? Would a dose reduction be warranted? Would the risk of bleeding be higher? Would treatment be as effective overall?

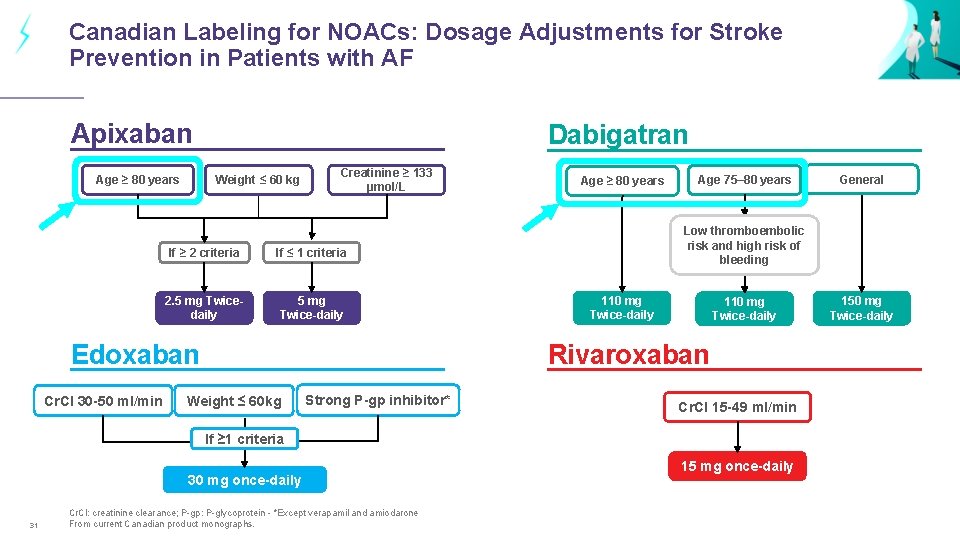
Canadian Labeling for NOACs: Dosage Adjustments for Stroke Prevention in Patients with AF Apixaban Age ≥ 80 years Dabigatran Weight ≤ 60 kg Creatinine ≥ 133 µmol/L If ≥ 2 criteria If ≤ 1 criteria 2. 5 mg Twicedaily 5 mg Twice-daily Edoxaban Cr. Cl 30 -50 ml/min Age ≥ 80 years Age 75– 80 years Low thromboembolic risk and high risk of bleeding 110 mg Twice-daily Rivaroxaban Weight ≤ 60 kg Strong P-gp inhibitor* Cr. Cl 15 -49 ml/min If ≥ 1 criteria 30 mg once-daily 31 Cr. Cl: creatinine clearance; P-gp: P-glycoprotein - *Except verapamil and amiodarone From current Canadian product monographs. General 15 mg once-daily 150 mg Twice-daily

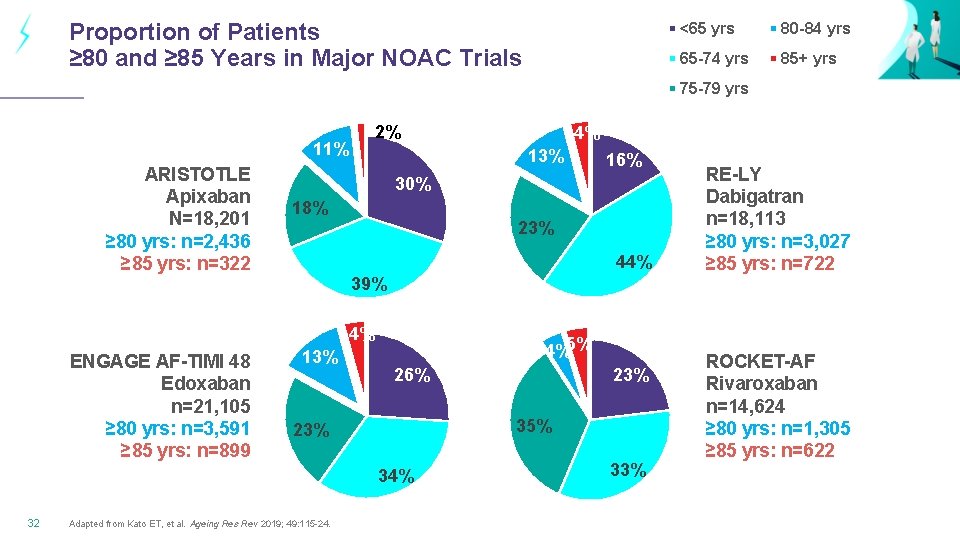
Proportion of Patients ≥ 80 and ≥ 85 Years in Major NOAC Trials <65 yrs 80 -84 yrs 65 -74 yrs 85+ yrs 75 -79 yrs 2% 11% ARISTOTLE Apixaban N=18, 201 ≥ 80 yrs: n=2, 436 ≥ 85 yrs: n=322 13% 18% 23% 44% 39% 13% 4%5% 26% Adapted from Kato ET, et al. Ageing Res Rev 2019; 49: 115 -24. 23% 35% 23% 34% 32 16% 30% 4% ENGAGE AF-TIMI 48 Edoxaban n=21, 105 ≥ 80 yrs: n=3, 591 ≥ 85 yrs: n=899 4% 33% RE-LY Dabigatran n=18, 113 ≥ 80 yrs: n=3, 027 ≥ 85 yrs: n=722 ROCKET-AF Rivaroxaban n=14, 624 ≥ 80 yrs: n=1, 305 ≥ 85 yrs: n=622

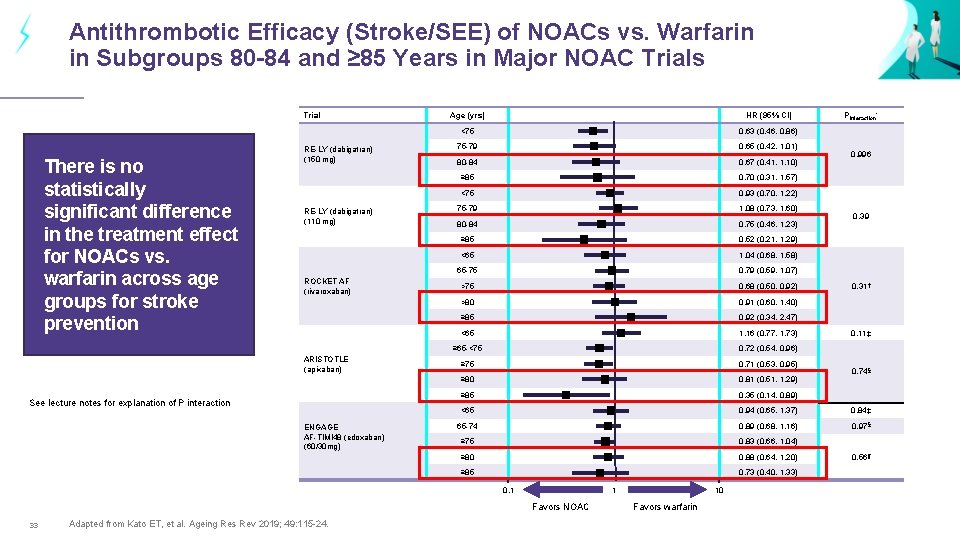
Antithrombotic Efficacy (Stroke/SEE) of NOACs vs. Warfarin in Subgroups 80 -84 and ≥ 85 Years in Major NOAC Trials Trial There is no statistically significant difference in the treatment effect for NOACs vs. warfarin across age groups for stroke prevention RE-LY (dabigatran) (150 mg) RE-LY (dabigatran) (110 mg) ROCKET AF (rivaroxaban) ARISTOTLE (apixaban) See lecture notes for explanation of P interaction ENGAGE AF-TIMI 48 (edoxaban) (60/30 mg) Age (yrs) HR (95% CI) <75 0. 63 (0. 46, 0. 86) 75 -79 0. 65 (0. 42, 1. 01) 80 -84 0. 67 (0. 41, 1. 10) ≥ 85 0. 70 (0. 31, 1. 57) <75 0. 93 (0. 70, 1. 22) 75 -79 1. 08 (0. 73, 1. 60) 80 -84 0. 75 (0. 46, 1. 23) ≥ 85 0. 52 (0. 21, 1. 29) <65 1. 04 (0. 68, 1. 58) 65 -75 0. 79 (0. 59, 1. 07) >75 0. 68 (0. 50, 0. 92) >80 0. 91 (0. 60, 1. 40) 0. 39 0. 31† ≥ 85 0. 92 (0. 34, 2. 47) 1. 16 (0. 77, 1. 73) ≥ 65 -<75 0. 72 (0. 54, 0. 96) ≥ 75 0. 71 (0. 53, 0. 95) ≥ 80 0. 81 (0. 51, 1. 29) ≥ 85 0. 35 (0. 14, 0. 89) <65 0. 94 (0. 65, 1. 37) 0. 84‡ 65 -74 0. 89 (0. 68, 1. 16) 0. 97§ ≥ 75 0. 83 (0. 66, 1. 04) ≥ 80 0. 88 (0. 64, 1. 20) ≥ 85 0. 73 (0. 40, 1. 33) 1 Favors NOAC Adapted from Kato ET, et al. Ageing Res Rev 2019; 49: 115 -24. 0. 996 <65 0. 1 33 Pinteraction* 10 Favors warfarin 0. 11‡ 0. 74§ 0. 56¶

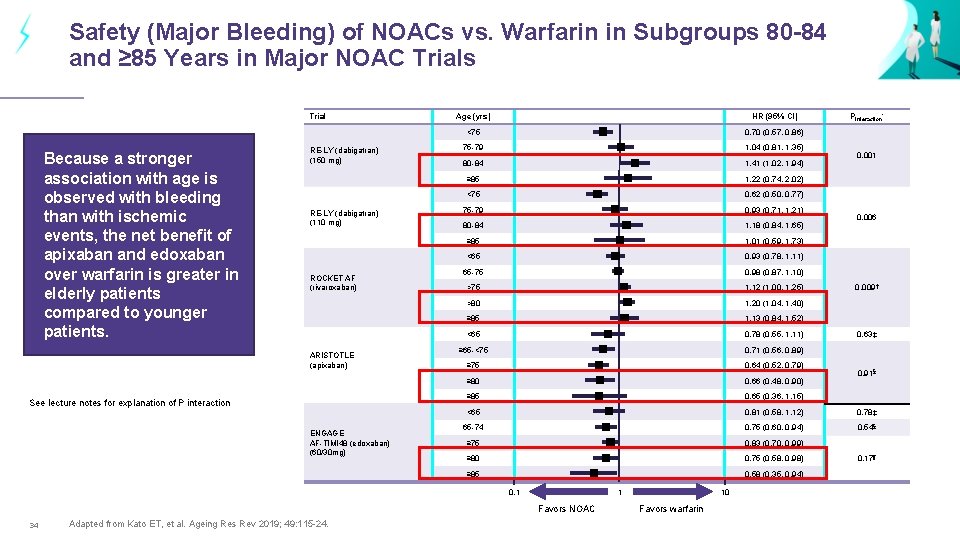
Safety (Major Bleeding) of NOACs vs. Warfarin in Subgroups 80 -84 and ≥ 85 Years in Major NOAC Trials Trial Because a stronger association with age is observed with bleeding than with ischemic events, the net benefit of apixaban and edoxaban over warfarin is greater in elderly patients compared to younger patients. RE-LY (dabigatran) (150 mg) RE-LY (dabigatran) (110 mg) ROCKET AF (rivaroxaban) ARISTOTLE (apixaban) See lecture notes for explanation of P interaction ENGAGE AF-TIMI 48 (edoxaban) (60/30 mg) Age (yrs) HR (95% CI) <75 0. 70 (0. 57, 0. 86) 75 -79 1. 04 (0. 81, 1. 35) 80 -84 1. 41 (1. 02, 1. 94) ≥ 85 1. 22 (0. 74, 2. 02) <75 0. 62 (0. 50, 0. 77) 75 -79 0. 93 (0. 71, 1. 21) 80 -84 1. 18 (0. 84, 1. 65) ≥ 85 1. 01 (0. 59, 1. 73) <65 0. 93 (0. 78, 1. 11) 65 -75 0. 98 (0. 87, 1. 10) >75 1. 12 (1. 00, 1. 25) >80 1. 20 (1. 04, 1. 40) ≥ 85 1. 13 (0. 84, 1. 52) <65 0. 78 (0. 55, 1. 11) ≥ 65 -<75 0. 71 (0. 56, 0. 89) ≥ 75 0. 64 (0. 52, 0. 79) ≥ 80 0. 66 (0. 48, 0. 90) ≥ 85 0. 65 (0. 36, 1. 15) <65 0. 81 (0. 58, 1. 12) 0. 78‡ 65 -74 0. 75 (0. 60, 0. 94) 0. 54§ ≥ 75 0. 83 (0. 70, 0. 99) ≥ 80 0. 75 (0. 58, 0. 98) ≥ 85 0. 58 (0. 35, 0. 94) 0. 1 1 Favors NOAC 34 Adapted from Kato ET, et al. Ageing Res Rev 2019; 49: 115 -24. 10 Favors warfarin Pinteraction* 0. 001 0. 006 0. 009 † 0. 63‡ 0. 91§ 0. 17¶

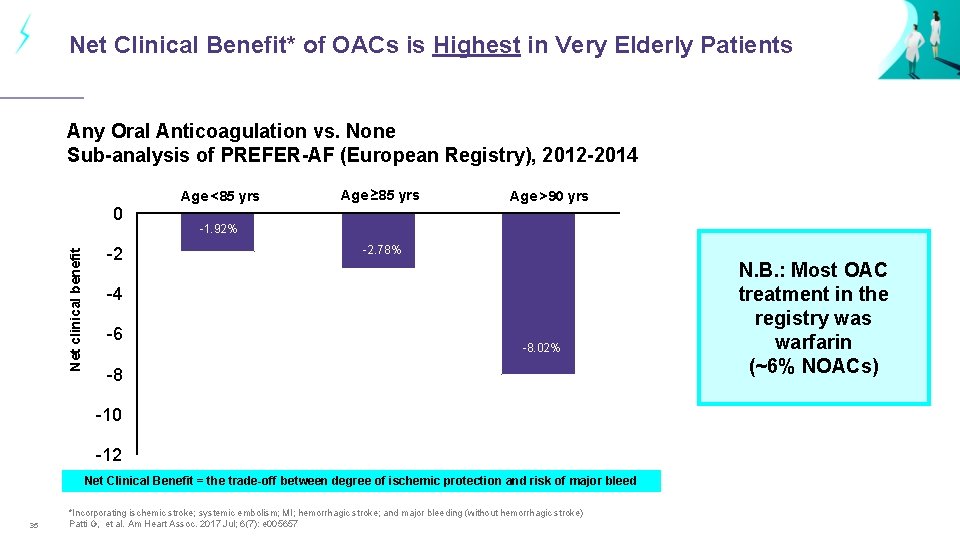
Net Clinical Benefit* of OACs is Highest in Very Elderly Patients Any Oral Anticoagulation vs. None Sub-analysis of PREFER-AF (European Registry), 2012 -2014 Net clinical benefit 0 -2 Age <85 yrs Age ≥ 85 yrs -1. 92% Category 1 Category 2 -2. 78% Age >90 yrs Category 3 -4 -6 -8. 02% -8 -10 -12 Net Clinical Benefit = the trade-off between degree of ischemic protection and risk of major bleed 35 *Incorporating ischemic stroke; systemic embolism; MI; hemorrhagic stroke; and major bleeding (without hemorrhagic stroke) Patti G, et al. Am Heart Assoc. 2017 Jul; 6(7): e 005657 N. B. : Most OAC treatment in the registry was warfarin (~6% NOACs)

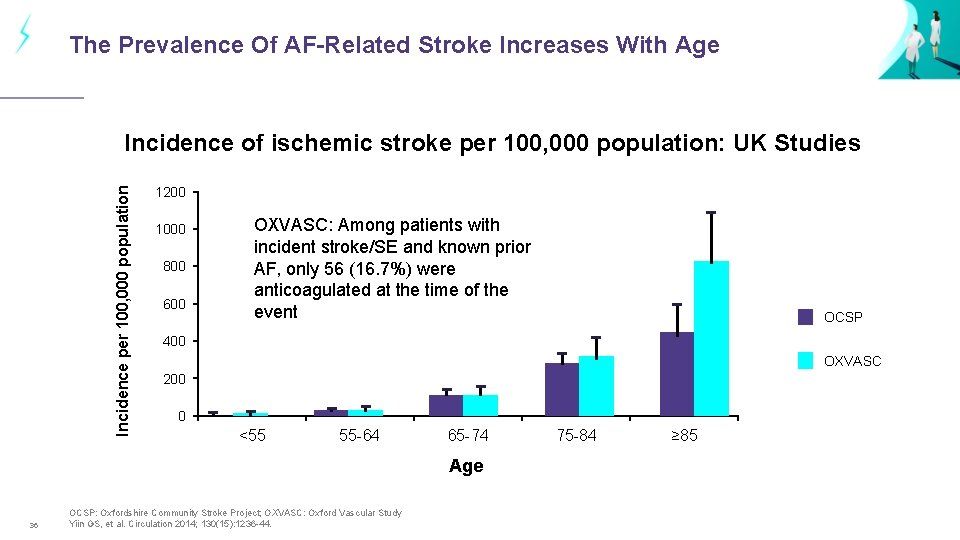
The Prevalence Of AF-Related Stroke Increases With Age Incidence per 100, 000 population Incidence of ischemic stroke per 100, 000 population: UK Studies 1200 1000 800 600 OXVASC: Among patients with incident stroke/SE and known prior AF, only 56 (16. 7%) were anticoagulated at the time of the event 400 OXVASC 200 0 <55 55 -64 65 -74 Age 36 OCSP: Oxfordshire Community Stroke Project; OXVASC: Oxford Vascular Study Yiin GS, et al. Circulation 2014; 130(15): 1236 -44. 75 -84 ≥ 85

Real-world Experience with NOACs: Major Bleeding in the ARISTOPHANES Registry with Apixaban, Dabigatran or Rivaroxaban (N=466, 991) Apixaban Dabigatran 10 Rivaroxaban 10 10 Rate / 100 patient-years 8, 16 8 8 6 6 5, 94 6 4, 84 3, 59 4 4, 31 3, 63 4 2, 8 2 4 2, 72 1, 73 2 0 2, 24 2 1, 2 0 <65 37 8 65 -74 75 -79 Age group Lip GY, et al. Stroke 2018; 49(12): 2933 -44. 80+ 0 <65 65 -74 75 -79 Age group 80+

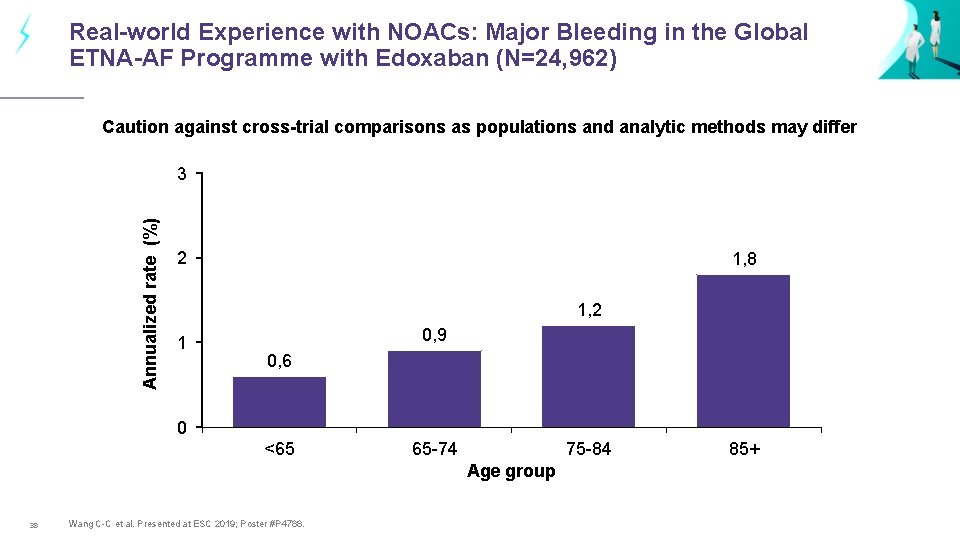
Real-world Experience with NOACs: Major Bleeding in the Global ETNA-AF Programme with Edoxaban (N=24, 962) Caution against cross-trial comparisons as populations and analytic methods may differ Annualized rate (%) 3 2 1, 8 1, 2 1 0, 9 0, 6 0 <65 65 -74 75 -84 Age group 38 Wang C-C et al. Presented at ESC 2019; Poster #P 4788. 85+

Conclusion: NOACs are efficacious and safe even in very elderly patients • There is no statistically significant difference in the treatment effect for NOACs vs. warfarin across age groups for stroke prevention 1 • The net benefit of apixaban and edoxaban over warfarin was greater in elderly patients compared to younger patients in the major RCTs 1 • Although event rates increase with age, 2 -4 there is also evidence of greater treatment effect of OACs among older age groups relative to younger 2 39 1. Kato ET, et al. Ageing Res Rev 2019; 49: 115 -24. 2. Patti G, et al. Am Heart Assoc. 2017 Jul; 6(7): e 005657 3. Wang CC et al. Presented at ESC 2019; Poster #P 4788. 4. Lip GY, et al. Stroke 2018; 49(12): 2933 -44.

Case #2: 70 -year-old woman, presents for routine follow -up. In discussion, she mentions that she has trouble remembering to take her meds

True or False? Research indicates that patient adherence is better with once-daily medication (vs. twice daily). 41 True False

True or False? Twice daily NOACs are more “forgiving” than once-daily NOACs for patients with suboptimal adherence. 42 True False

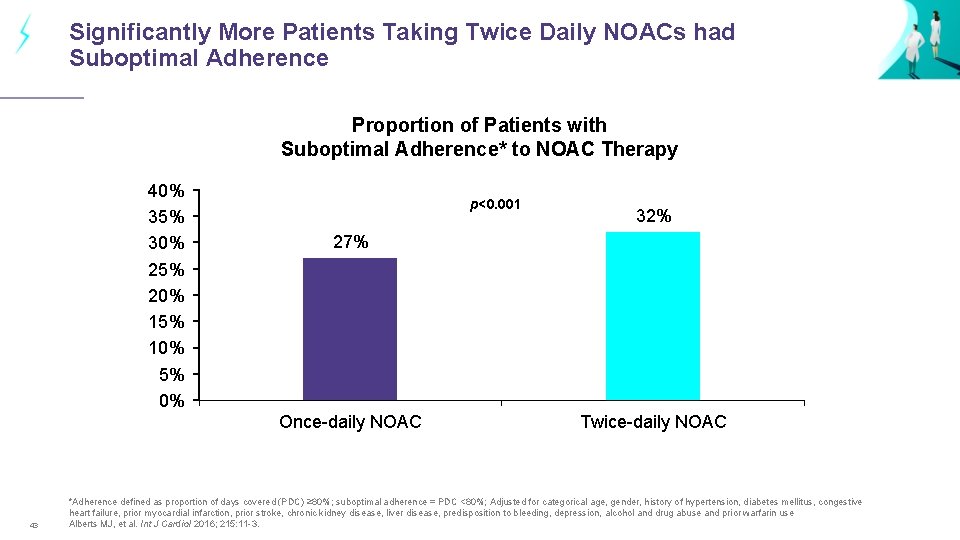
Significantly More Patients Taking Twice Daily NOACs had Suboptimal Adherence Proportion of Patients with Suboptimal Adherence* to NOAC Therapy 40% 35% 30% 25% 20% 15% 10% 5% 0% p<0. 001 27% Once-daily NOAC 43 32% Twice-daily NOAC *Adherence defined as proportion of days covered (PDC) ≥ 80%; suboptimal adherence = PDC <80%; Adjusted for categorical age, gender, history of hypertension, diabetes mellitus, congestive heart failure, prior myocardial infarction, prior stroke, chronic kidney disease, liver disease, predisposition to bleeding, depression, alcohol and drug abuse and prior warfarin use Alberts MJ, et al. Int J Cardiol 2016; 215: 11 -3.

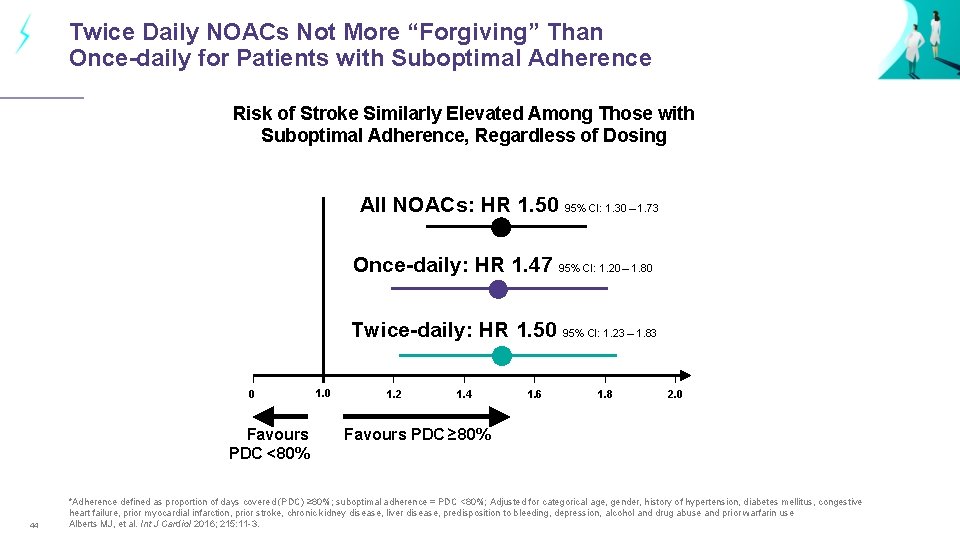
Twice Daily NOACs Not More “Forgiving” Than Once-daily for Patients with Suboptimal Adherence Risk of Stroke Similarly Elevated Among Those with Suboptimal Adherence, Regardless of Dosing All NOACs: HR 1. 50 95% CI: 1. 30 – 1. 73 Once-daily: HR 1. 47 95% CI: 1. 20 – 1. 80 Twice-daily: HR 1. 50 95% CI: 1. 23 – 1. 83 0 Favours PDC <80% 44 1. 0 1. 2 1. 4 1. 6 1. 8 2. 0 Favours PDC ≥ 80% *Adherence defined as proportion of days covered (PDC) ≥ 80%; suboptimal adherence = PDC <80%; Adjusted for categorical age, gender, history of hypertension, diabetes mellitus, congestive heart failure, prior myocardial infarction, prior stroke, chronic kidney disease, liver disease, predisposition to bleeding, depression, alcohol and drug abuse and prior warfarin use Alberts MJ, et al. Int J Cardiol 2016; 215: 11 -3.

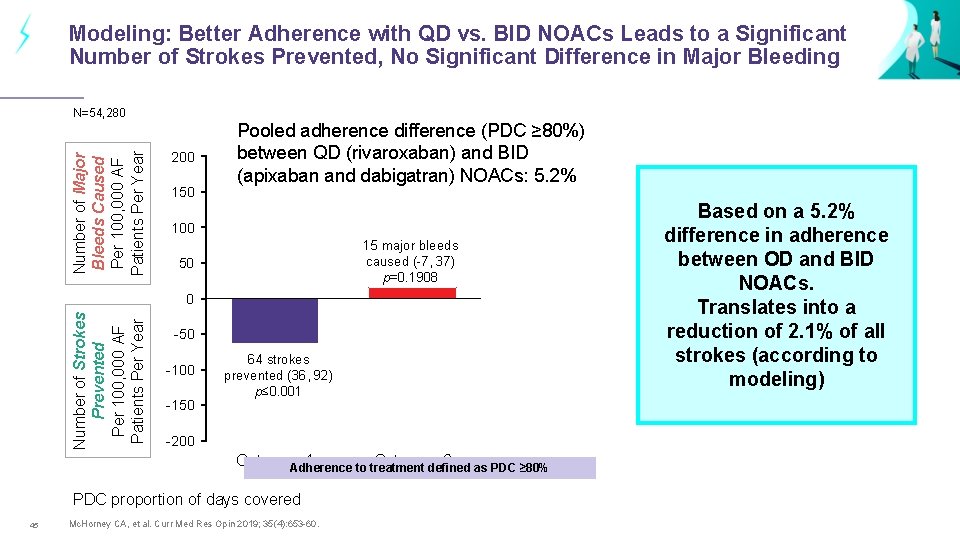
Modeling: Better Adherence with QD vs. BID NOACs Leads to a Significant Number of Strokes Prevented, No Significant Difference in Major Bleeding Number of Major Bleeds Caused Per 100, 000 AF Patients Per Year N=54, 280 200 150 Pooled adherence difference (PDC ≥ 80%) between QD (rivaroxaban) and BID (apixaban and dabigatran) NOACs: 5. 2% 100 15 major bleeds caused (-7, 37) p=0. 1908 50 Number of Strokes Prevented Per 100, 000 AF Patients Per Year 0 -50 -100 -150 64 strokes prevented (36, 92) p≤ 0. 001 -200 Category 1 Category 2 Adherence to treatment defined as PDC ≥ 80% PDC proportion of days covered 45 Mc. Horney CA, et al. Curr Med Res Opin 2019; 35(4): 653 -60. Based on a 5. 2% difference in adherence between OD and BID NOACs. Translates into a reduction of 2. 1% of all strokes (according to modeling)

Key message Once daily dosing may improve adherence in some patients. 46

Changing Case #2… What if there were a risk of drug-drug interaction? How would you proceed?

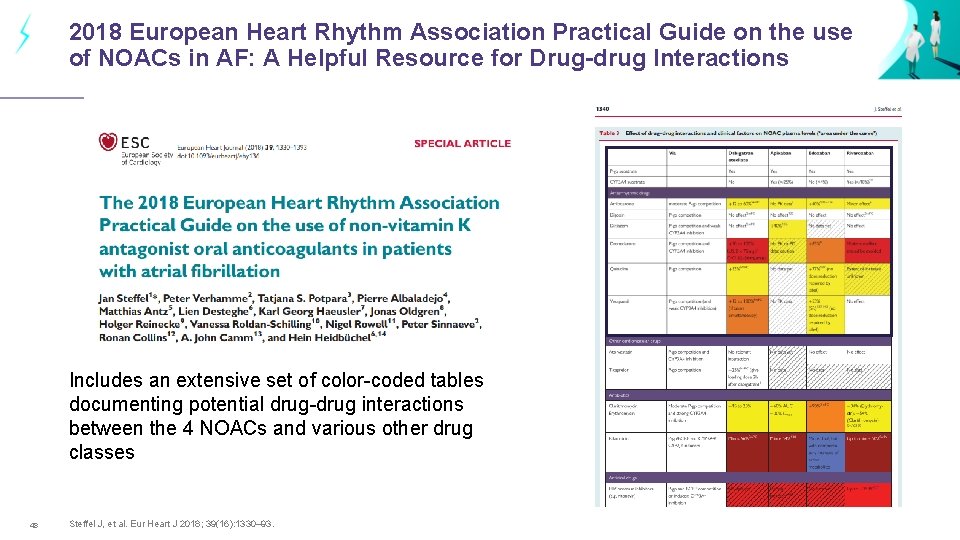
2018 European Heart Rhythm Association Practical Guide on the use of NOACs in AF: A Helpful Resource for Drug-drug Interactions Includes an extensive set of color-coded tables documenting potential drug-drug interactions between the 4 NOACs and various other drug classes 48 Steffel J, et al. Eur Heart J 2018; 39(16): 1330– 93.

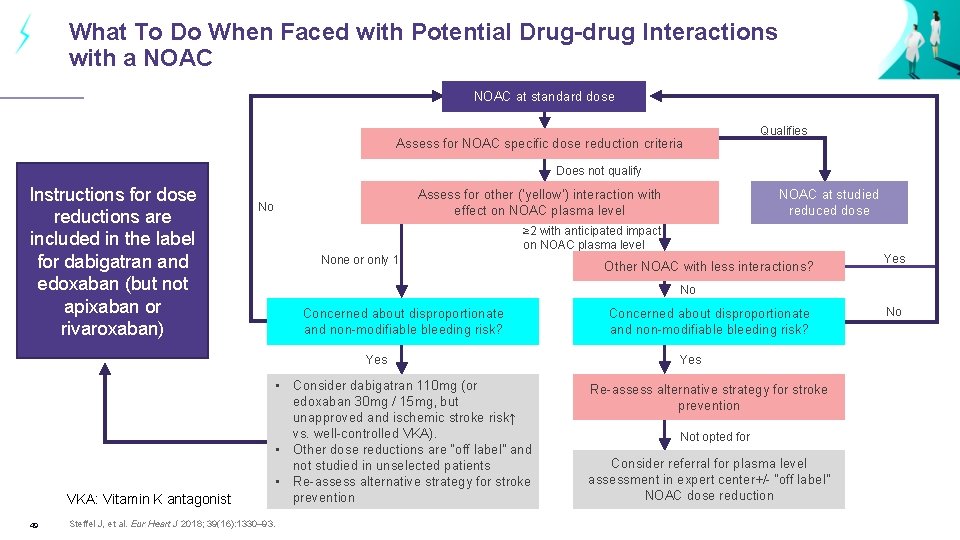
What To Do When Faced with Potential Drug-drug Interactions with a NOAC at standard dose Assess for NOAC specific dose reduction criteria Qualifies Does not qualify Instructions for dose reductions are included in the label for dabigatran and edoxaban (but not apixaban or rivaroxaban) Assess for other (‘yellow’) interaction with effect on NOAC plasma level No ≥ 2 with anticipated impact on NOAC plasma level None or only 1 49 Other NOAC with less interactions? Yes No Concerned about disproportionate and non-modifiable bleeding risk? Yes VKA: Vitamin K antagonist NOAC at studied reduced dose • Consider dabigatran 110 mg (or edoxaban 30 mg / 15 mg, but unapproved and ischemic stroke risk↑ vs. well-controlled VKA). • Other dose reductions are “off label” and not studied in unselected patients • Re-assess alternative strategy for stroke prevention Steffel J, et al. Eur Heart J 2018; 39(16): 1330– 93. Concerned about disproportionate and non-modifiable bleeding risk? Yes Re-assess alternative strategy for stroke prevention Not opted for Consider referral for plasma level assessment in expert center+/- “off label” NOAC dose reduction No

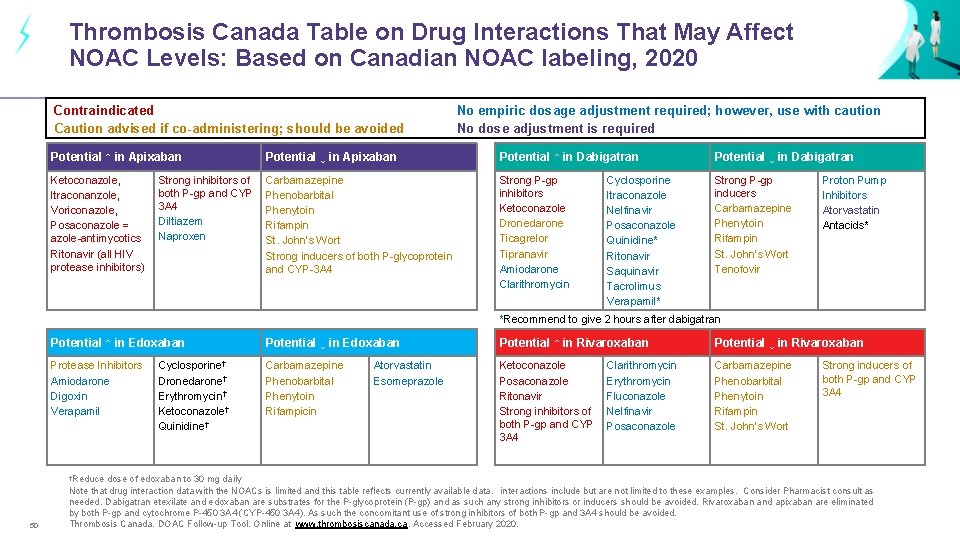
Thrombosis Canada Table on Drug Interactions That May Affect NOAC Levels: Based on Canadian NOAC labeling, 2020 Contraindicated Caution advised if co-administering; should be avoided No empiric dosage adjustment required; however, use with caution No dose adjustment is required Potential ↑ in Apixaban Potential ↓ in Apixaban Potential ↑ in Dabigatran Potential ↓ in Dabigatran Ketoconazole, Itraconanzole, Voriconazole, Posaconazole = azole-antimycotics Ritonavir (all HIV protease inhibitors) Carbamazepine Phenobarbital Phenytoin Rifampin St. John’s Wort Strong inducers of both P-glycoprotein and CYP-3 A 4 Strong P-gp inhibitors Ketoconazole Dronedarone Ticagrelor Tipranavir Amiodarone Clarithromycin Strong P-gp inducers Carbamazepine Phenytoin Rifampin St. John’s Wort Tenofovir Strong inhibitors of both P-gp and CYP 3 A 4 Diltiazem Naproxen Cyclosporine Itraconazole Nelfinavir Posaconazole Quinidine* Ritonavir Saquinavir Tacrolimus Verapamil* Proton Pump Inhibitors Atorvastatin Antacids* *Recommend to give 2 hours after dabigatran Potential ↑ in Edoxaban Potential ↓ in Edoxaban Potential ↑ in Rivaroxaban Potential ↓ in Rivaroxaban Protease Inhibitors Amiodarone Digoxin Verapamil Carbamazepine Phenobarbital Phenytoin Rifampicin Ketoconazole Posaconazole Ritonavir Strong inhibitors of both P-gp and CYP 3 A 4 Carbamazepine Phenobarbital Phenytoin Rifampin St. John’s Wort †Reduce 50 Cyclosporine† Dronedarone† Erythromycin† Ketoconazole† Quinidine† Atorvastatin Esomeprazole Clarithromycin Erythromycin Fluconazole Nelfinavir Posaconazole Strong inducers of both P-gp and CYP 3 A 4 dose of edoxaban to 30 mg daily Note that drug interaction data with the NOACs is limited and this table reflects currently available data. interactions include but are not limited to these examples. Consider Pharmacist consult as needed. Dabigatran etexilate and edoxaban are substrates for the P-glycoprotein (P-gp) and as such any strong inhibitors or inducers should be avoided. Rivaroxaban and apixaban are eliminated by both P-gp and cytochrome P-450 3 A 4 (CYP-450 3 A 4). As such the concomitant use of strong inhibitors of both P-gp and 3 A 4 should be avoided. Thrombosis Canada. DOAC Follow-up Tool. Online at www. thrombosiscanada. ca. Accessed February 2020.

Changing Case #2… What if the patient had a BMI over 30 mg/kg 2? Would a dose adjustment be required? Would you expect her to have worse outcomes?

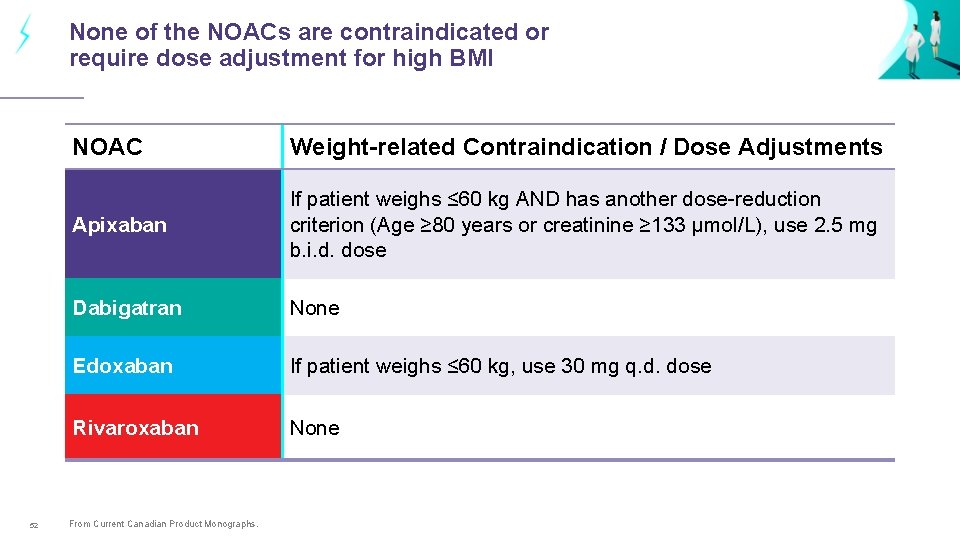
None of the NOACs are contraindicated or require dose adjustment for high BMI 52 NOAC Weight-related Contraindication / Dose Adjustments Apixaban If patient weighs ≤ 60 kg AND has another dose-reduction criterion (Age ≥ 80 years or creatinine ≥ 133 µmol/L), use 2. 5 mg b. i. d. dose Dabigatran None Edoxaban If patient weighs ≤ 60 kg, use 30 mg q. d. dose Rivaroxaban None From Current Canadian Product Monographs.

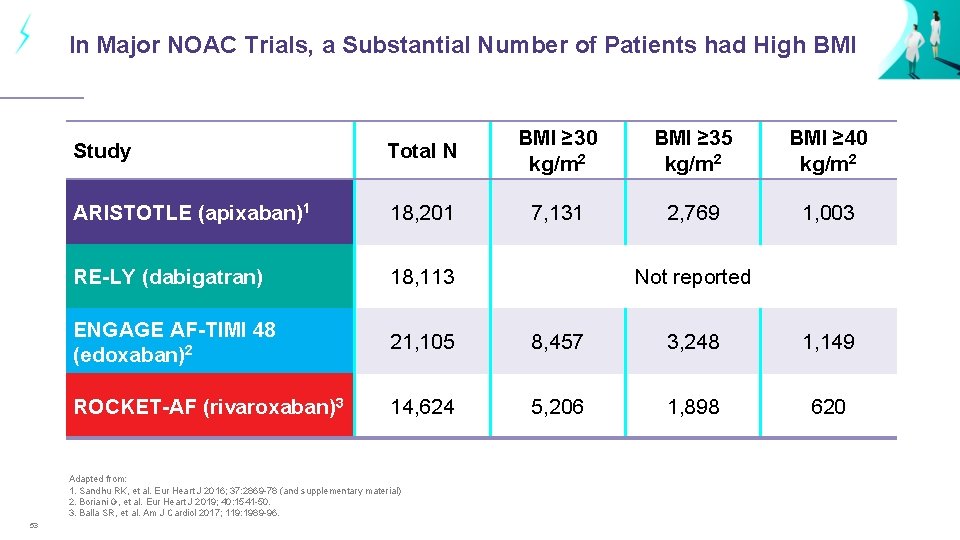
In Major NOAC Trials, a Substantial Number of Patients had High BMI Study Total N BMI ≥ 30 kg/m 2 BMI ≥ 35 kg/m 2 BMI ≥ 40 kg/m 2 ARISTOTLE (apixaban)1 18, 201 7, 131 2, 769 1, 003 RE-LY (dabigatran) 18, 113 ENGAGE AF-TIMI 48 (edoxaban)2 21, 105 8, 457 3, 248 1, 149 ROCKET-AF (rivaroxaban)3 14, 624 5, 206 1, 898 620 Adapted from: 1. Sandhu RK, et al. Eur Heart J 2016; 37: 2869 -78 (and supplementary material) 2. Boriani G, et al. Eur Heart J 2019; 40: 1541 -50. 3. Balla SR, et al. Am J Cardiol 2017; 119: 1989 -96. 53 Not reported

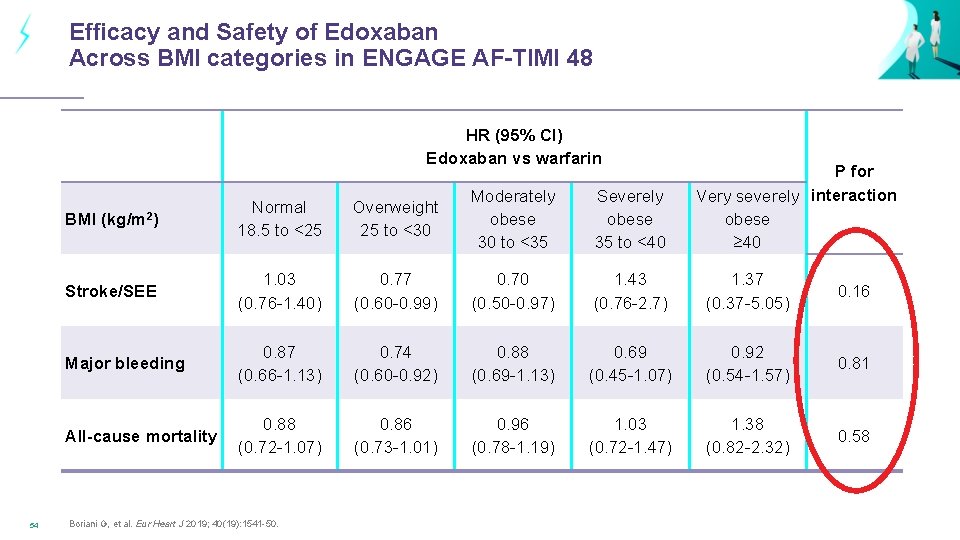
Efficacy and Safety of Edoxaban Across BMI categories in ENGAGE AF-TIMI 48 HR (95% CI) Edoxaban vs warfarin 54 P for Very severely interaction obese ≥ 40 BMI (kg/m 2) Normal 18. 5 to <25 Overweight 25 to <30 Moderately obese 30 to <35 Severely obese 35 to <40 Stroke/SEE 1. 03 (0. 76 -1. 40) 0. 77 (0. 60 -0. 99) 0. 70 (0. 50 -0. 97) 1. 43 (0. 76 -2. 7) 1. 37 (0. 37 -5. 05) 0. 16 Major bleeding 0. 87 (0. 66 -1. 13) 0. 74 (0. 60 -0. 92) 0. 88 (0. 69 -1. 13) 0. 69 (0. 45 -1. 07) 0. 92 (0. 54 -1. 57) 0. 81 All-cause mortality 0. 88 (0. 72 -1. 07) 0. 86 (0. 73 -1. 01) 0. 96 (0. 78 -1. 19) 1. 03 (0. 72 -1. 47) 1. 38 (0. 82 -2. 32) 0. 58 Boriani G, et al. Eur Heart J 2019; 40(19): 1541 -50.

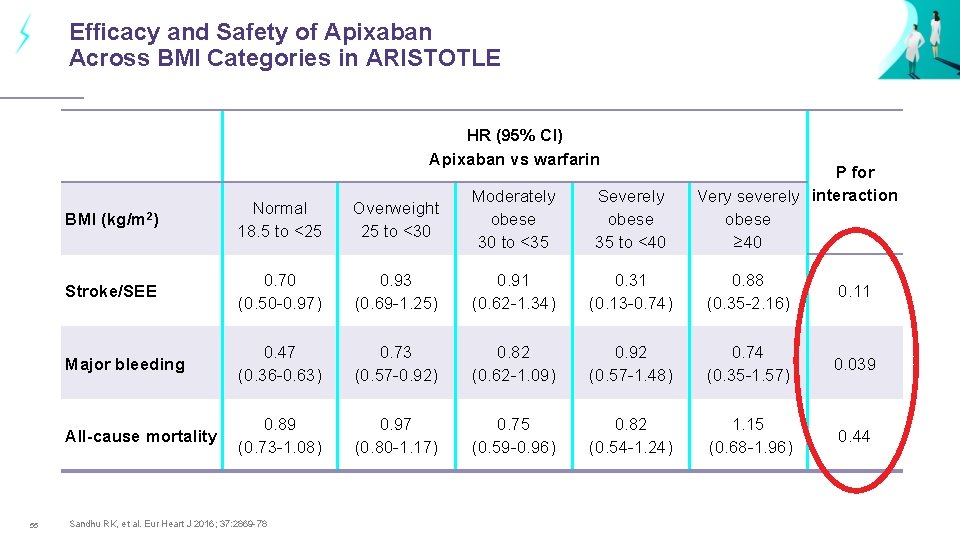
Efficacy and Safety of Apixaban Across BMI Categories in ARISTOTLE HR (95% CI) Apixaban vs warfarin 55 P for Very severely interaction obese ≥ 40 BMI (kg/m 2) Normal 18. 5 to <25 Overweight 25 to <30 Moderately obese 30 to <35 Severely obese 35 to <40 Stroke/SEE 0. 70 (0. 50 -0. 97) 0. 93 (0. 69 -1. 25) 0. 91 (0. 62 -1. 34) 0. 31 (0. 13 -0. 74) 0. 88 (0. 35 -2. 16) 0. 11 Major bleeding 0. 47 (0. 36 -0. 63) 0. 73 (0. 57 -0. 92) 0. 82 (0. 62 -1. 09) 0. 92 (0. 57 -1. 48) 0. 74 (0. 35 -1. 57) 0. 039 All-cause mortality 0. 89 (0. 73 -1. 08) 0. 97 (0. 80 -1. 17) 0. 75 (0. 59 -0. 96) 0. 82 (0. 54 -1. 24) 1. 15 (0. 68 -1. 96) 0. 44 Sandhu RK, et al. Eur Heart J 2016; 37: 2869 -78

Efficacy and Safety of Rivaroxaban Across BMI Categories in ROCKET AF Body Mass Index (kg/m 2) Outcome 56 25. 00 -29. 99 ≥ 30. 00 HR (95% CI) P-value Stroke and/or non-CNS embolism 0. 81 (0. 66 -0. 99) 0. 04 0. 69 (0. 55 -0. 86) <0. 001 Any stroke 0. 78 (0. 63 -0. 96) 0. 02 0. 64 (0. 51 -0. 81) <0. 001 Ischemic stroke 0. 78 (0. 62 -0. 99) 0. 05 0. 66 (0. 51 -0. 86) 0. 002 Major or NMCR bleeding 0. 97 (0. 88 -1. 06) 0. 49 1. 05 (0. 95 -1. 16) 0. 34 Major bleeding 1. 09 (0. 91 -1. 31) 0. 36 1. 06 (0. 87 -1. 29) 0. 55 Intracranial hemorrhage 1. 05 (0. 61 -1. 80) 0. 85 0. 75 (0. 41 -1. 38) 0. 35 Balla SR, et al. Am J Cardiol 2017; 119: 1989 -96.

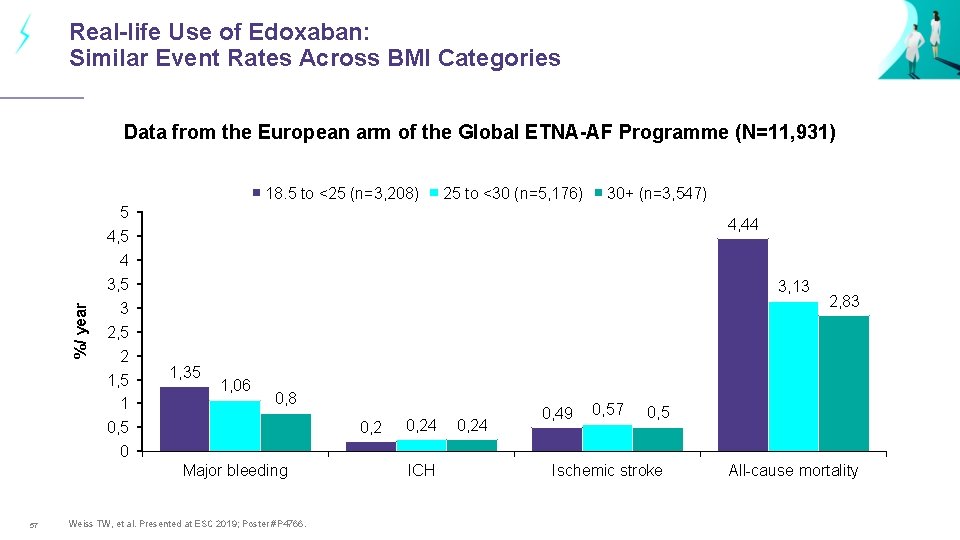
Real-life Use of Edoxaban: Similar Event Rates Across BMI Categories Data from the European arm of the Global ETNA-AF Programme (N=11, 931) %/ year 18. 5 to <25 (n=3, 208) 5 4, 5 4 3, 5 3 2, 5 2 1, 5 1 0, 5 0 30+ (n=3, 547) 4, 44 3, 13 1, 35 1, 06 0, 8 0, 2 Major bleeding 57 25 to <30 (n=5, 176) Weiss TW, et al. Presented at ESC 2019; Poster #P 4766. 0, 24 ICH 0, 24 0, 49 0, 57 2, 83 0, 5 Ischemic stroke All-cause mortality

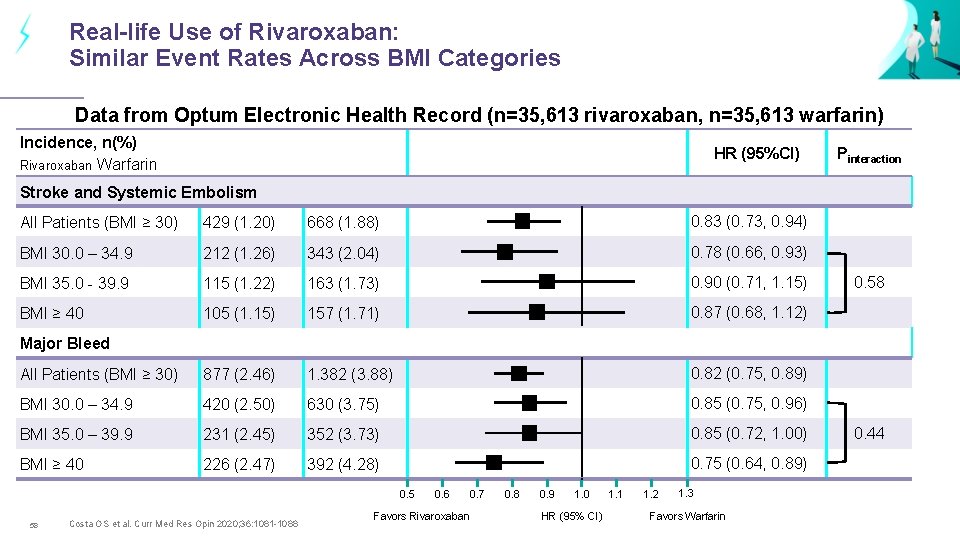
Real-life Use of Rivaroxaban: Similar Event Rates Across BMI Categories Data from Optum Electronic Health Record (n=35, 613 rivaroxaban, n=35, 613 warfarin) Incidence, n(%) Rivaroxaban Warfarin HR (95%CI) Pinteraction Stroke and Systemic Embolism All Patients (BMI ≥ 30) 429 (1. 20) 668 (1. 88) 0. 83 (0. 73, 0. 94) BMI 30. 0 – 34. 9 212 (1. 26) 343 (2. 04) 0. 78 (0. 66, 0. 93) BMI 35. 0 - 39. 9 115 (1. 22) 163 (1. 73) 0. 90 (0. 71, 1. 15) BMI ≥ 40 105 (1. 15) 157 (1. 71) 0. 87 (0. 68, 1. 12) All Patients (BMI ≥ 30) 877 (2. 46) 1. 382 (3. 88) 0. 82 (0. 75, 0. 89) BMI 30. 0 – 34. 9 420 (2. 50) 630 (3. 75) 0. 85 (0. 75, 0. 96) BMI 35. 0 – 39. 9 231 (2. 45) 352 (3. 73) 0. 85 (0. 72, 1. 00) BMI ≥ 40 226 (2. 47) 392 (4. 28) 0. 75 (0. 64, 0. 89) 0. 58 Major Bleed 0. 5 58 Costa OS et al. Curr Med Res Opin 2020; 36: 1081 -1088 0. 6 Favors Rivaroxaban 0. 7 0. 8 0. 9 1. 0 HR (95% CI) 1. 1 1. 2 1. 3 Favors Warfarin 0. 44

Retrospective Chart Audit: Similar Efficacy and Safety Apixaban, Rivaroxaban and Warfarin in Morbidly Obese Patients (BMI ≥ 40) Authors concluded that this study “enables patients with BMI ≥ 40 to benefit from more convenient and possibly safer anticoagulants. ” 1% Stroke p=0. 71 2% 1% Apixaban Rivaroxaban 3% 3% Major bleeding Warfarin p=0. 063 8% 11% 10% Composite bleeding p=0. 16 16% 0% 2% 4% 6% 8% 10% Patients 59 Kushnir M et al. Lancet Haematol 2019; 6: e 359– 65 12% 14% 16% 18%

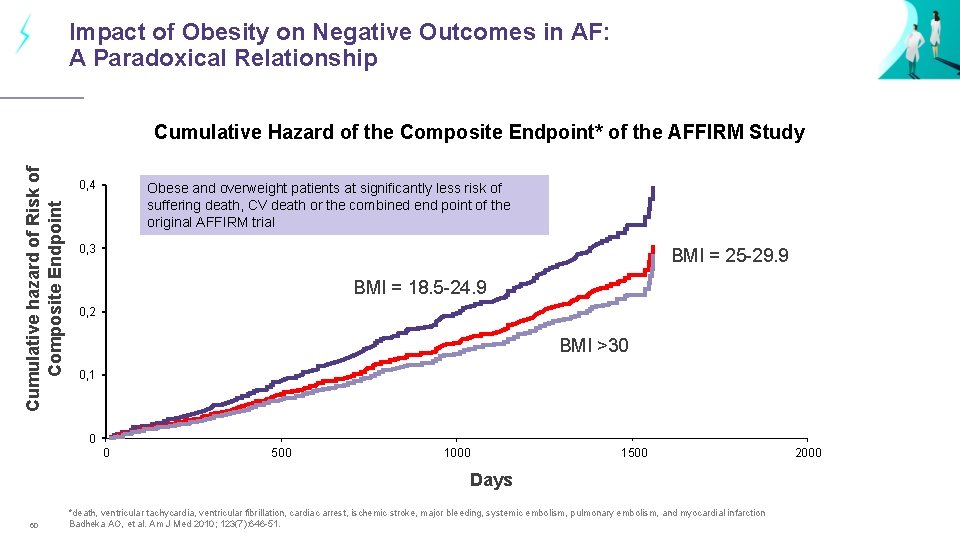
Impact of Obesity on Negative Outcomes in AF: A Paradoxical Relationship Cumulative hazard of Risk of Composite Endpoint Cumulative Hazard of the Composite Endpoint* of the AFFIRM Study 0, 4 Obese and overweight patients at significantly less risk of suffering death, CV death or the combined end point of the original AFFIRM trial 0, 3 BMI = 25 -29. 9 BMI = 18. 5 -24. 9 0, 2 BMI >30 0, 1 0 0 500 1000 1500 Days 60 *death, ventricular tachycardia, ventricular fibrillation, cardiac arrest, ischemic stroke, major bleeding, systemic embolism, pulmonary embolism, and myocardial infarction Badheka AO, et al. Am J Med 2010; 123(7): 646 -51. 2000

Key messages Dose adjustment is not indicated in patients with a BMI over 30 mg/kg 2. Obese patients do not have worse outcomes in clinical trials. 61

Changing Case #2… What if the patient had renal insufficiency? Would the treatment benefit be different? Would the risk of bleeding be different?

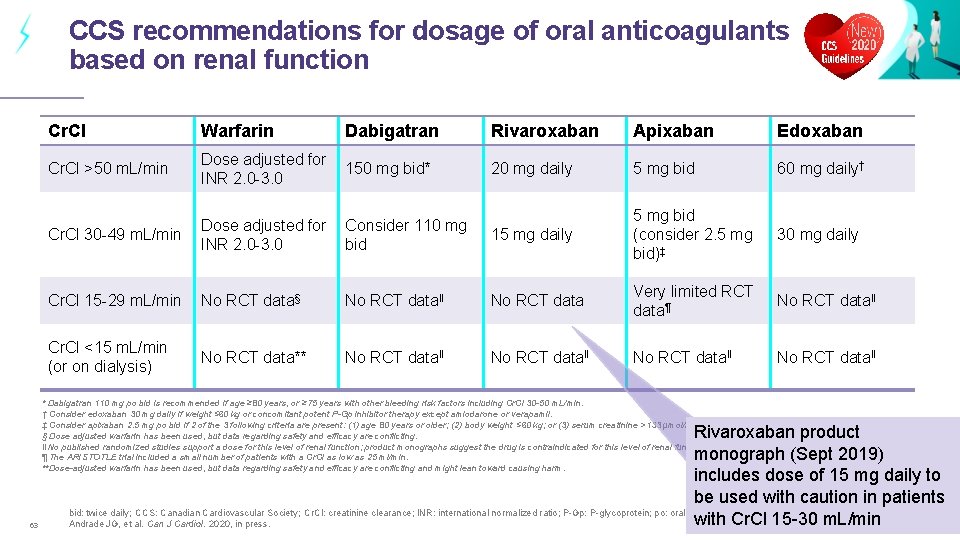
CCS recommendations for dosage of oral anticoagulants based on renal function Cr. CI Warfarin Dabigatran Rivaroxaban Apixaban Edoxaban Cr. Cl >50 m. L/min Dose adjusted for INR 2. 0 -3. 0 150 mg bid* 20 mg daily 5 mg bid 60 mg daily† Cr. Cl 30 -49 m. L/min Dose adjusted for INR 2. 0 -3. 0 Consider 110 mg bid 15 mg daily 5 mg bid (consider 2. 5 mg bid)‡ 30 mg daily Cr. Cl 15 -29 m. L/min No RCT data§ No RCT dataǁ No RCT data Very limited RCT data¶ No RCT dataǁ Cr. Cl <15 m. L/min (or on dialysis) No RCT data** No RCT dataǁ * Dabigatran 110 mg po bid is recommended if age ≥ 80 years, or ≥ 75 years with other bleeding risk factors including Cr. Cl 30 -50 m. L/min. † Consider edoxaban 30 mg daily if weight ≤ 60 kg or concomitant potent P-Gp inhibitor therapy except amiodarone or verapamil. ‡ Consider apixaban 2. 5 mg po bid if 2 of the 3 following criteria are present: (1) age 80 years or older; (2) body weight ≤ 60 kg; or (3) serum creatinine >133 µmol/L. § Dose adjusted warfarin has been used, but data regarding safety and efficacy are conflicting. ǁ No published randomized studies support a dose for this level of renal function; product monographs suggest the drug is contraindicated for this level of renal function. ¶ The ARISTOTLE trial included a small number of patients with a Cr. Cl as low as 25 ml/min. **Dose-adjusted warfarin has been used, but data regarding safety and efficacy are conflicting and might lean toward causing harm. 63 Rivaroxaban product monograph (Sept 2019) includes dose of 15 mg daily to be used with caution in patients bid: twice daily; CCS: Canadian Cardiovascular Society; Cr. Cl: creatinine clearance; INR: international normalized ratio; P-Gp: P-glycoprotein; po: orally; RCT: randomized clinical trial. with Cr. Cl 15 -30 m. L/min Andrade JG, et al. Can J Cardiol. 2020, in press.

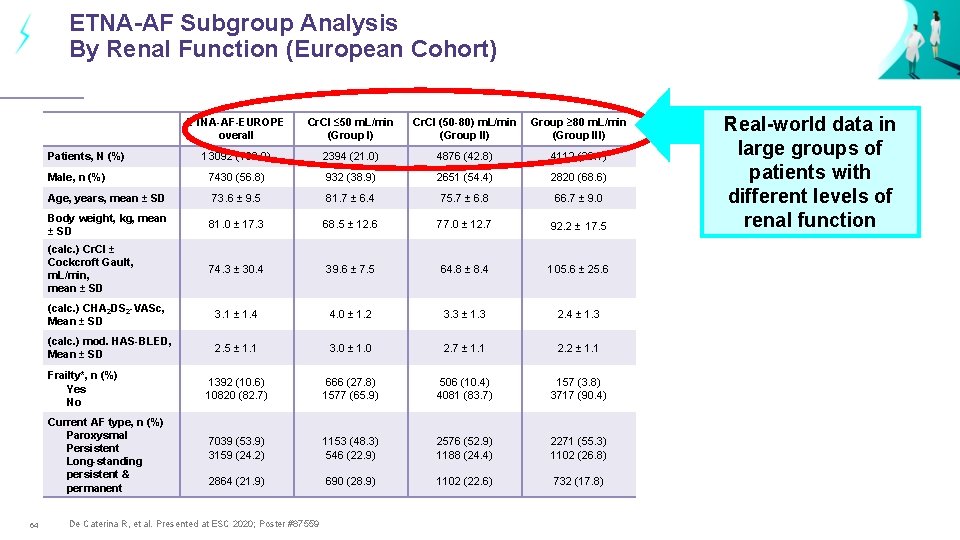
ETNA-AF Subgroup Analysis By Renal Function (European Cohort) ETNA-AF-EUROPE overall Cr. CI ≤ 50 m. L/min (Group I) Cr. CI (50 -80) m. L/min (Group II) Group ≥ 80 m. L/min (Group III) 13092 (100. 0) 2394 (21. 0) 4876 (42. 8) 4112 (36. 1) 7430 (56. 8) 932 (38. 9) 2651 (54. 4) 2820 (68. 6) Age, years, mean ± SD 73. 6 ± 9. 5 81. 7 ± 6. 4 75. 7 ± 6. 8 66. 7 ± 9. 0 Body weight, kg, mean ± SD 81. 0 ± 17. 3 68. 5 ± 12. 6 77. 0 ± 12. 7 92. 2 ± 17. 5 (calc. ) Cr. CI ± Cockcroft Gault, m. L/min, mean ± SD 74. 3 ± 30. 4 39. 6 ± 7. 5 64. 8 ± 8. 4 105. 6 ± 25. 6 (calc. ) CHA 2 DS 2 -VASc, Mean ± SD 3. 1 ± 1. 4 4. 0 ± 1. 2 3. 3 ± 1. 3 2. 4 ± 1. 3 (calc. ) mod. HAS-BLED, Mean ± SD 2. 5 ± 1. 1 3. 0 ± 1. 0 2. 7 ± 1. 1 2. 2 ± 1. 1 1392 (10. 6) 10820 (82. 7) 666 (27. 8) 1577 (65. 9) 506 (10. 4) 4081 (83. 7) 157 (3. 8) 3717 (90. 4) 7039 (53. 9) 3159 (24. 2) 1153 (48. 3) 546 (22. 9) 2576 (52. 9) 1188 (24. 4) 2271 (55. 3) 1102 (26. 8) 2864 (21. 9) 690 (28. 9) 1102 (22. 6) 732 (17. 8) Patients, N (%) Male, n (%) Frailty*, n (%) Yes No Current AF type, n (%) Paroxysmal Persistent Long-standing persistent & permanent 64 De Caterina R, et al. Presented at ESC 2020; Poster #87559 Real-world data in large groups of patients with different levels of renal function

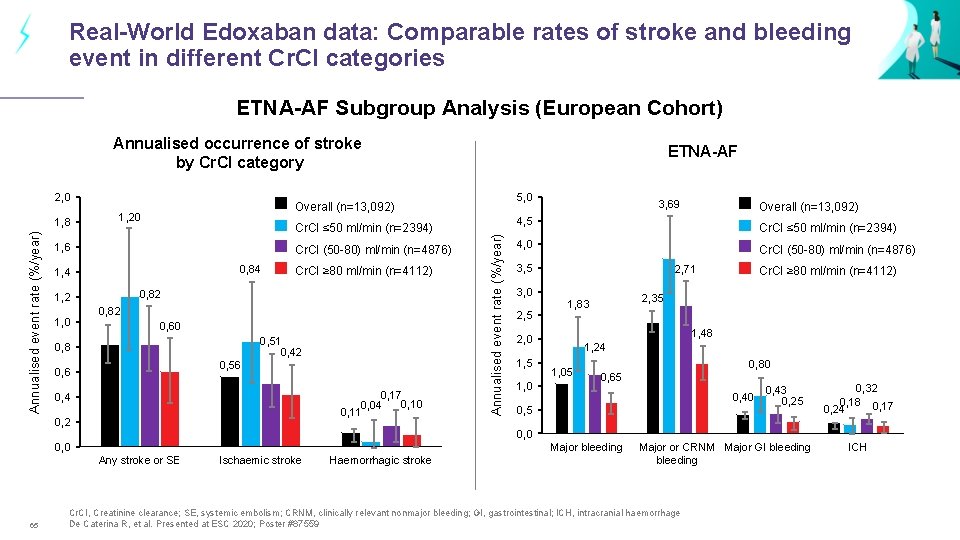
Real-World Edoxaban data: Comparable rates of stroke and bleeding event in different Cr. Cl categories ETNA-AF Subgroup Analysis (European Cohort) Annualised occurrence of stroke by Cr. CI category 2, 0 1, 20 Cr. CI ≤ 50 ml/min (n=2394) 1, 6 Cr. CI (50 -80) ml/min (n=4876) 0, 84 1, 0 Cr. CI ≥ 80 ml/min (n=4112) 0, 82 1, 2 0, 82 0, 60 0, 51 0, 42 0, 8 0, 56 0, 17 0, 10 0, 04 0, 11 0, 4 0, 2 3, 69 Overall (n=13, 092) 4, 5 Cr. CI ≤ 50 ml/min (n=2394) 4, 0 Cr. CI (50 -80) ml/min (n=4876) 3, 5 3, 0 2, 5 2, 71 1, 0 2, 35 1, 83 1, 48 2, 0 1, 5 Cr. CI ≥ 80 ml/min (n=4112) 1, 24 1, 05 0, 80 0, 65 0, 40 0, 5 0, 43 0, 25 0, 32 0, 18 0, 17 0, 24 0, 0 Major bleeding 0, 0 Any stroke or SE 65 5, 0 Overall (n=13, 092) Annualised event rate (%/year) 1, 8 ETNA-AF Ischaemic stroke Haemorrhagic stroke Major or CRNM Major GI bleeding Cr. CI, Creatinine clearance; SE, systemic embolism; CRNM, clinically relevant nonmajor bleeding; GI, gastrointestinal; ICH, intracranial haemorrhage De Caterina R, et al. Presented at ESC 2020; Poster #87559 ICH

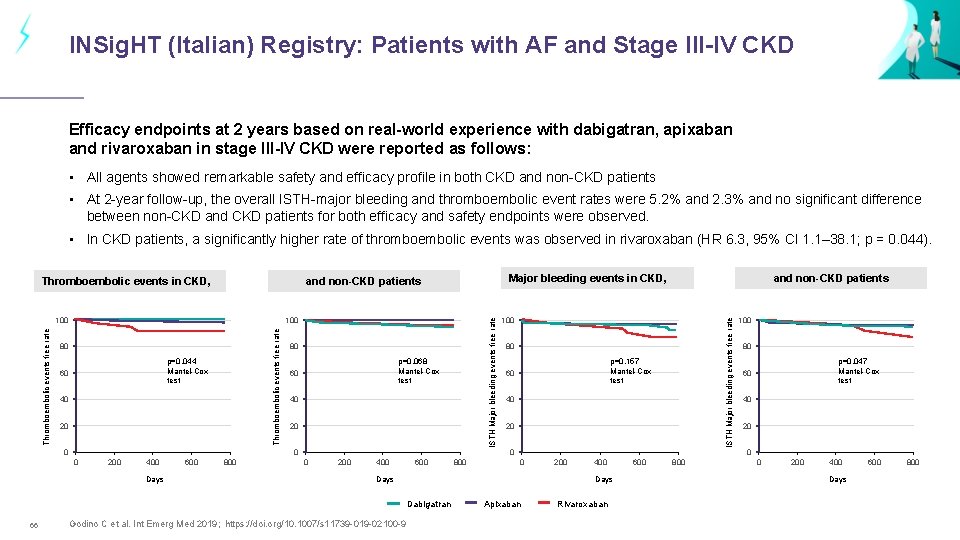
INSig. HT (Italian) Registry: Patients with AF and Stage III-IV CKD Efficacy endpoints at 2 years based on real-world experience with dabigatran, apixaban and rivaroxaban in stage III-IV CKD were reported as follows: • All agents showed remarkable safety and efficacy profile in both CKD and non-CKD patients • At 2 -year follow-up, the overall ISTH-major bleeding and thromboembolic event rates were 5. 2% and 2. 3% and no significant difference between non-CKD and CKD patients for both efficacy and safety endpoints were observed. • In CKD patients, a significantly higher rate of thromboembolic events was observed in rivaroxaban (HR 6. 3, 95% CI 1. 1– 38. 1; p = 0. 044). 80 p=0. 044 Mantel-Cox test 60 40 20 0 ISTH Major bleeding events free rate 100 Thromboembolic events free rate 100 80 p=0. 068 Mantel-Cox test 60 40 20 0 0 200 400 Days 600 800 and non-CKD patients 100 80 p=0. 157 Mantel-Cox test 60 40 20 200 400 600 800 0 Days Godino C et al. Int Emerg Med 2019; https: //doi. org/10. 1007/s 11739 -019 -02100 -9 100 80 p=0. 047 Mantel-Cox test 60 40 200 400 Days Dabigatran 66 Major bleeding events in CKD, and non-CKD patients ISTH Major bleeding events free rate Thromboembolic events in CKD, Apixaban Rivaroxaban 600 800 0 200 400 Days 600 800

Changing Case #2… What if the patient required surgery? What dose adjustment would be required?

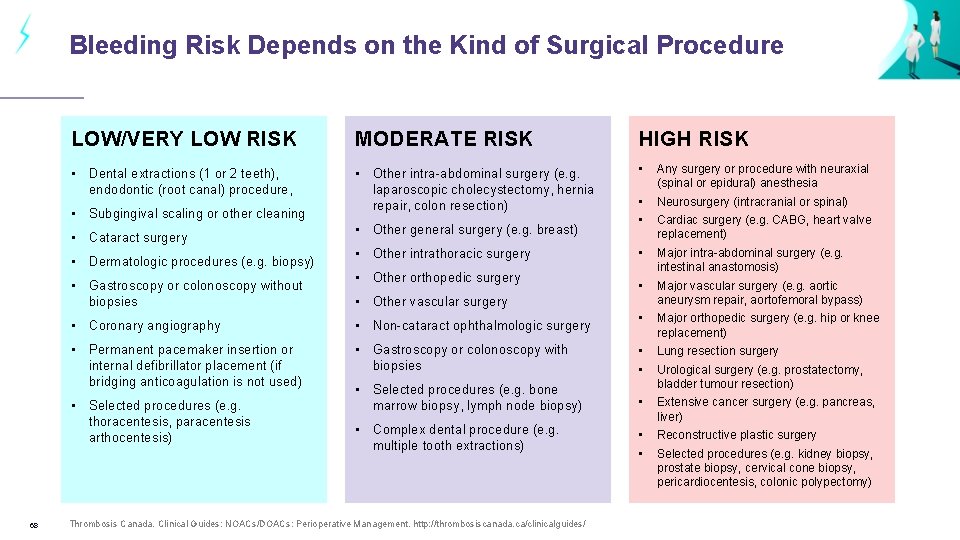
Bleeding Risk Depends on the Kind of Surgical Procedure LOW/VERY LOW RISK MODERATE RISK HIGH RISK • Dental extractions (1 or 2 teeth), endodontic (root canal) procedure, • Other intra-abdominal surgery (e. g. laparoscopic cholecystectomy, hernia repair, colon resection) • Any surgery or procedure with neuraxial (spinal or epidural) anesthesia • • Neurosurgery (intracranial or spinal) • Major intra-abdominal surgery (e. g. intestinal anastomosis) • Major vascular surgery (e. g. aortic aneurysm repair, aortofemoral bypass) • Major orthopedic surgery (e. g. hip or knee replacement) • Subgingival scaling or other cleaning • Cataract surgery • Dermatologic procedures (e. g. biopsy) • Gastroscopy or colonoscopy without biopsies • Other intrathoracic surgery • Other orthopedic surgery • Other vascular surgery Cardiac surgery (e. g. CABG, heart valve replacement) • Coronary angiography • Non-cataract ophthalmologic surgery • Permanent pacemaker insertion or internal defibrillator placement (if bridging anticoagulation is not used) • Gastroscopy or colonoscopy with biopsies • • Lung resection surgery • Selected procedures (e. g. bone marrow biopsy, lymph node biopsy) • Extensive cancer surgery (e. g. pancreas, liver) • • Reconstructive plastic surgery • Selected procedures (e. g. thoracentesis, paracentesis arthocentesis) 68 • Other general surgery (e. g. breast) • Complex dental procedure (e. g. multiple tooth extractions) Thrombosis Canada. Clinical Guides: NOACs/DOACs: Perioperative Management. http: //thrombosiscanada. ca/clinicalguides/ Urological surgery (e. g. prostatectomy, bladder tumour resection) Selected procedures (e. g. kidney biopsy, prostate biopsy, cervical cone biopsy, pericardiocentesis, colonic polypectomy)

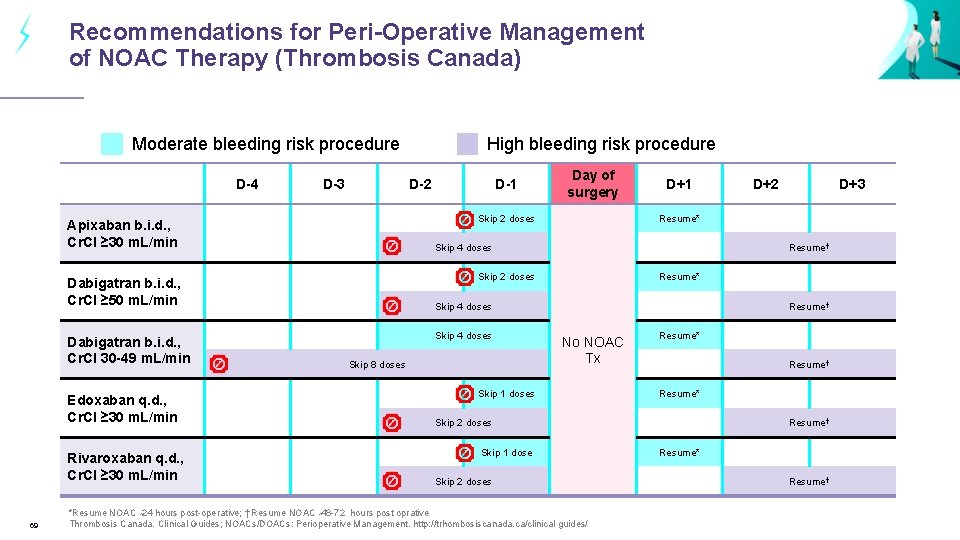
Recommendations for Peri-Operative Management of NOAC Therapy (Thrombosis Canada) Moderate bleeding risk procedure D-4 D-3 D-2 Rivaroxaban q. d. , Cr. Cl ≥ 30 m. L/min 69 Day of surgery D+1 Resume* Skip 4 doses Skip 8 doses D+3 Resume† Skip 2 doses Skip 4 doses D+2 Resume* Skip 4 doses Dabigatran b. i. d. , Cr. Cl ≥ 50 m. L/min Edoxaban q. d. , Cr. Cl ≥ 30 m. L/min D-1 Skip 2 doses Apixaban b. i. d. , Cr. Cl ≥ 30 m. L/min Dabigatran b. i. d. , Cr. Cl 30 -49 m. L/min High bleeding risk procedure Resume† No NOAC Tx Skip 1 doses Resume* Resume† Resume* Skip 2 doses Skip 1 dose Skip 2 doses *Resume NOAC 24 hours post-operative; † Resume NOAC 48 -72 hours post oprative Thrombosis Canada. Clinical Guides; NOACs/DOACs: Perioperative Management. http: //trhombosiscanada. ca/clinical guides/ Resume† Resume* Resume†

Peri-Procedural Anticoagulation Interruption: Electronic Algorithm from Thrombosis Canada • Thrombosis Canada has an online tool to guide perioperative anticoagulant management • The tool is also available as an app for Apple and Android devices 70 Thrombosis Canada. Clinical Guides: NOACs/DOACs: Perioperative Management. http: //thrombosiscanada. ca/clinicalguides/

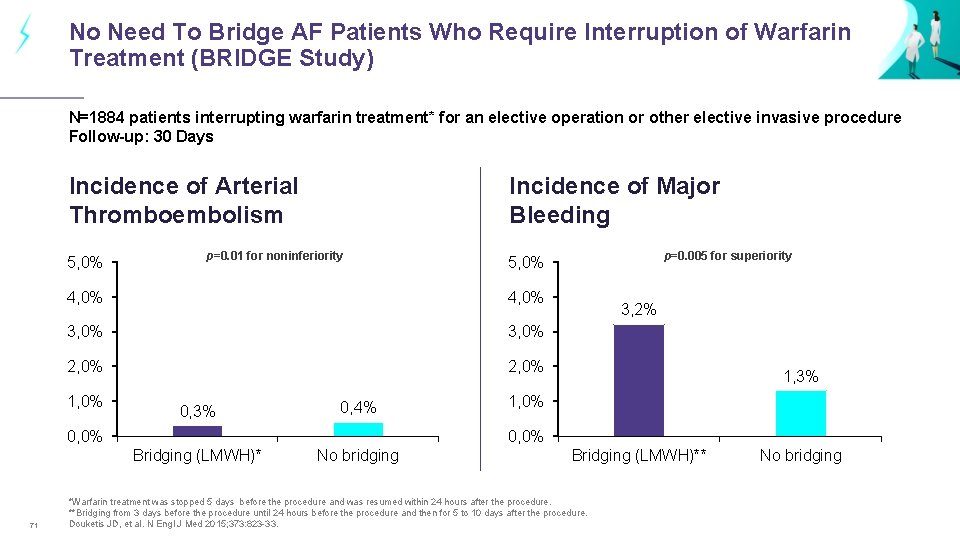
No Need To Bridge AF Patients Who Require Interruption of Warfarin Treatment (BRIDGE Study) N=1884 patients interrupting warfarin treatment* for an elective operation or other elective invasive procedure Follow-up: 30 Days Incidence of Arterial Thromboembolism 5, 0% Incidence of Major Bleeding p=0. 01 for noninferiority 4, 0% 3, 0% 2, 0% 1, 0% 0, 3% 0, 4% 0, 0% 3, 2% 1, 3% 1, 0% 0, 0% Bridging (LMWH)* 71 p=0. 005 for superiority 5, 0% No bridging Bridging (LMWH)** *Warfarin treatment was stopped 5 days before the procedure and was resumed within 24 hours after the procedure. **Bridging from 3 days before the procedure until 24 hours before the procedure and then for 5 to 10 days after the procedure. Douketis JD, et al. N Engl J Med 2015; 373: 823 -33. No bridging

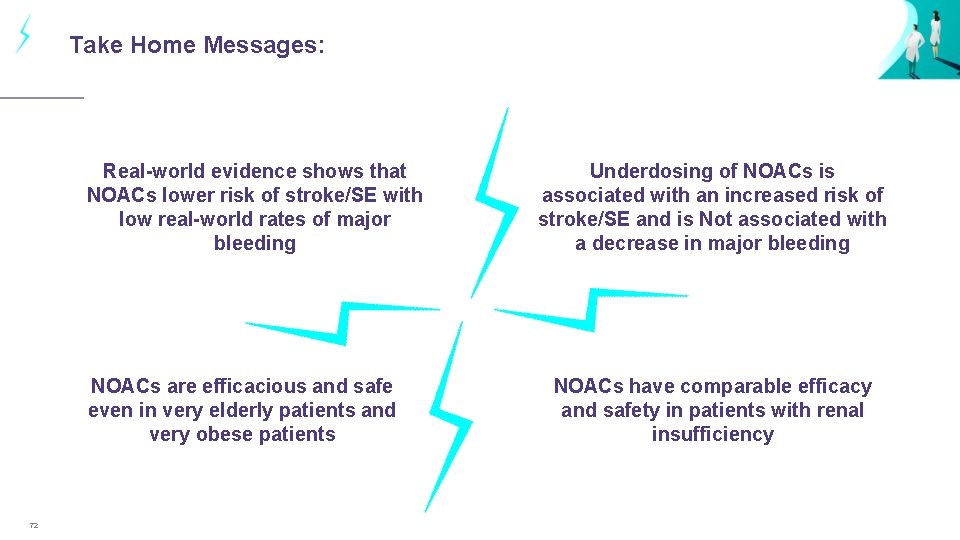
Take Home Messages: Real-world evidence shows that NOACs lower risk of stroke/SE with low real-world rates of major bleeding NOACs are efficacious and safe even in very elderly patients and very obese patients 72 Underdosing of NOACs is associated with an increased risk of stroke/SE and is Not associated with a decrease in major bleeding NOACs have comparable efficacy and safety in patients with renal insufficiency

Online Evaluation Please take 5 minutes to complete an online evaluation following the program today VISIT https: //www. accreditedevaluationonline. ca 73