Speaker Disclosure SPEAKER NAME Speaker Credentials Relationships With






























- Slides: 60


Speaker Disclosure SPEAKER NAME Speaker Credentials Relationships With Financial Sponsors CONSULTING FEES/ ADVISORY BOARD MEMBER: SPEAKERS BUREAU/HONORARIA: GRANTS/RESEARCH SUPPORT: OTHER: 2

Disclosure of Financial Support This program has received • Financial support from the Boehringer Ingelheim/Eli Lilly Alliance in Diabetes in the form of an educational grant • In-kind support from the Boehringer Ingelheim/Eli Lilly Canada Alliance in Diabetes in the form of logistical support Potential for conflict of interest • The speaker has received honoraria from the CPD Network Association • Eli Lilly Canada Inc. and Boehringer Ingelheim Canada Ltd. benefit from the sale of products that may be discussed in this program

Disclosure of Financial Support Mitigation of potential bias • The CPD Network is a not-for-profit physician organization who received an educational grant to develop this program. The CPD Network engaged the scientific planning committee and participated in the content and format of this program. • The steering committee was solely and fully responsible for developing all content and was involved at all stages of CME development to achieve scientific integrity, objectivity and balance. • Boehringer Ingelheim / Eli Lilly provided funding for the content development and this CME event but were not involved in any aspect of the program development process. • Speakers have received instructions on the Conflict of Interest disclosure requirements and are required to complete all necessary documents as mandated by the CFPC. Should any conflict arise, it will be brought to the attention of the CPD Network and the subsequent course of action will be dependent on the nature of the conflict. Every effort will be made to mitigate any perceived conflicts as well. • Speakers must inform the audience if unapproved or off-label uses of a product are discussed and if any discussions represent the personal opinions of the speakers, and unsolicited questions should be directed to the speakers.

Scientific Planning Committee CHAIR ALICE Y. Y. CHENG, MD, FRCPC Associate Professor, Department of Medicine University of Toronto Endocrinologist, Trillium Health Partners in Mississauga and St. Michael’s Hospital Toronto, Ontario MEMBERS BRUNO BERNUCCI, BSc, MD, CCFP PETER J. LIN, MD, CCFP SHELLEY ZIEROTH, MD, FCCS, FRCPC Family physician Polyclinic Levasseur Montreal, Quebec Director Primary Care Initiatives Canadian Heart Research Centre Associate Editor Elsevier Web. Portal - Practice. Update Primary Care Medical Director Lin. Corp Medical Inc. Toronto, Ontario Associate Professor Director, SBH HF and Transplant Clinics University of Manitoba President, Canadian Heart Failure Society Winnipeg, Manitoba JEFFREY HABERT, MD, CCFP, FCFP JORDAN WEINSTEIN, MD, FRCPC Assistant Professor Department of Family and Community Medicine University of Toronto, Ontario Nephrologist, St. Michael’s Hospital in Toronto Associate Professor of Medicine University of Toronto, Ontario

Accreditation College of Family Physicians of Canada (CFPC) This 1 -credit-per-hour Group Learning activity has been certified by the College of Family Physicians of Canada and the Chapter for up to 1. 5 Mainpro+ credits.

Learning Objectives At the end of the program, participants will be able to: 1 Describe individualized treatment options that optimize glycemic control and offer CV and renal protection in patients with type 2 diabetes 2 Identify and manage CV risk factors in patients with type 2 diabetes 3 Screen appropriately for complications related to type 2 diabetes

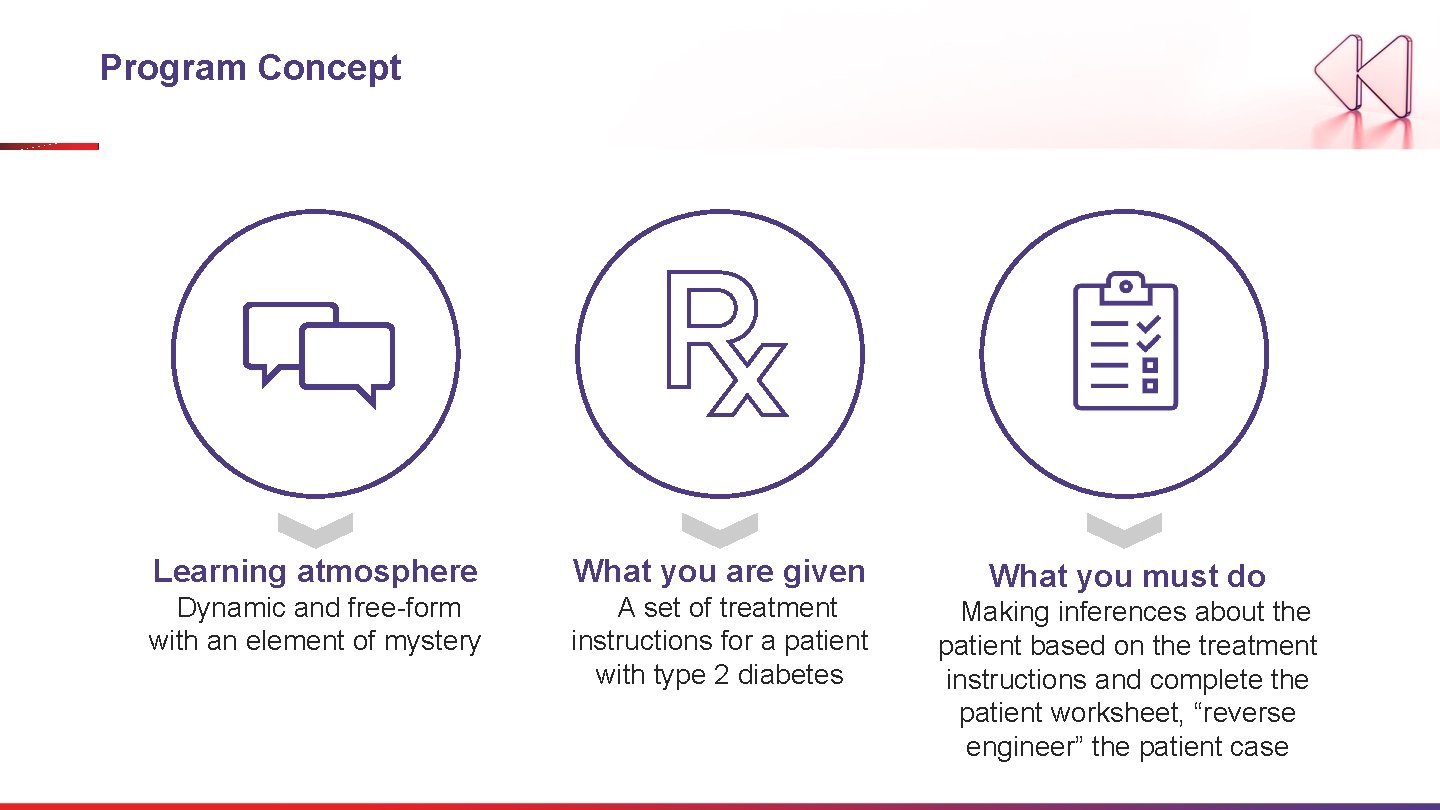
Program Concept Learning atmosphere What you are given Dynamic and free-form with an element of mystery A set of treatment instructions for a patient with type 2 diabetes What you must do Making inferences about the patient based on the treatment instructions and complete the patient worksheet, “reverse engineer” the patient case

Resource page www. cpdnetwork. org/reverseengineering Password: reverse 9

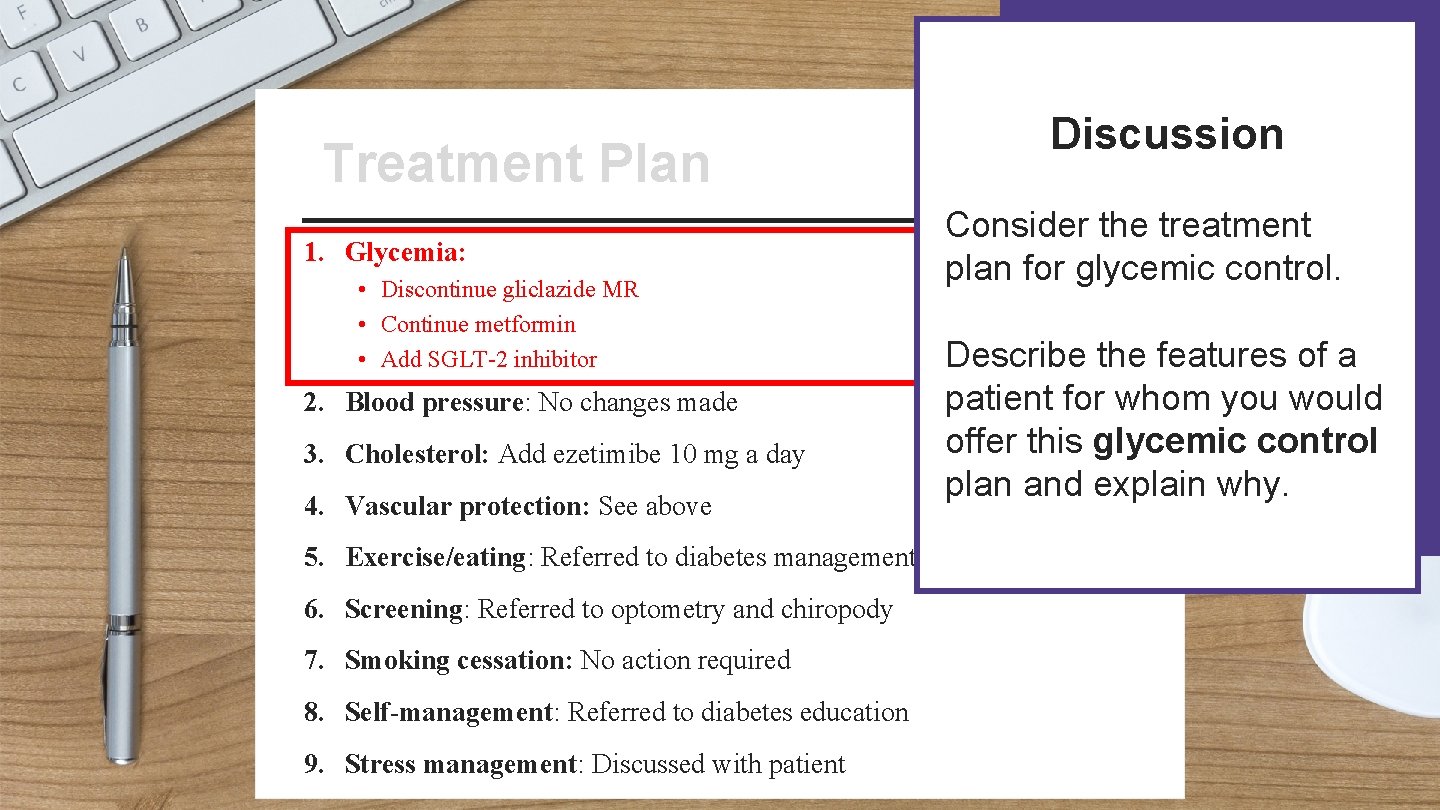
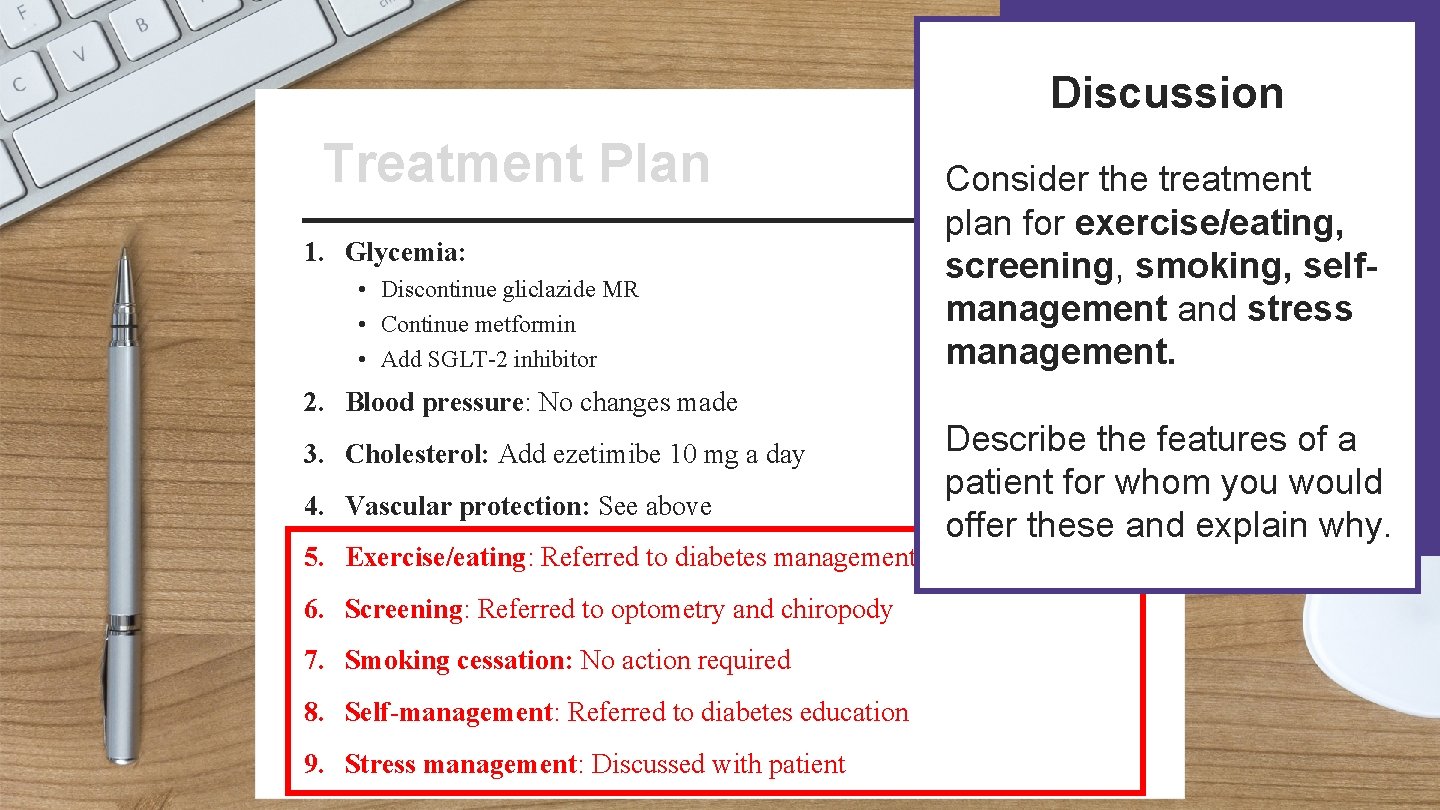
Patient Name: Treatment Plan Treatment Address: Date: 1. Glycemia: • Discontinue gliclazide MR • Continue metformin • Add SGLT-2 inhibitor 2. Blood pressure: No changes made 3. Cholesterol: Add ezetimibe 10 mg a day 4. Vascular protection: See above 5. Exercise/eating: Referred to diabetes management centre 6. Screening: Referred to optometry and chiropody 7. Smoking cessation: No action required 8. Self-management: Referred to diabetes education Signature: 9. Stress management: Discussed with patient

Discussion Patient Name: Treatment Plan Treatment Address: 1. Glycemia: • Discontinue gliclazide MR • Continue metformin • Add SGLT-2 inhibitor 2. Blood pressure: No changes made 3. Cholesterol: Add ezetimibe 10 mg a day 4. Vascular protection: See above Date: Consider the treatment plan for glycemic control. Describe the features of a patient for whom you would offer this glycemic control plan and explain why. 5. Exercise/eating: Referred to diabetes management centre 6. Screening: Referred to optometry and chiropody 7. Smoking cessation: No action required 8. Self-management: Referred to diabetes education Signature: 9. Stress management: Discussed with patient

Click here for supporting slides To continue discussing patient profiles for the treatment plan, advance to the next slide 12

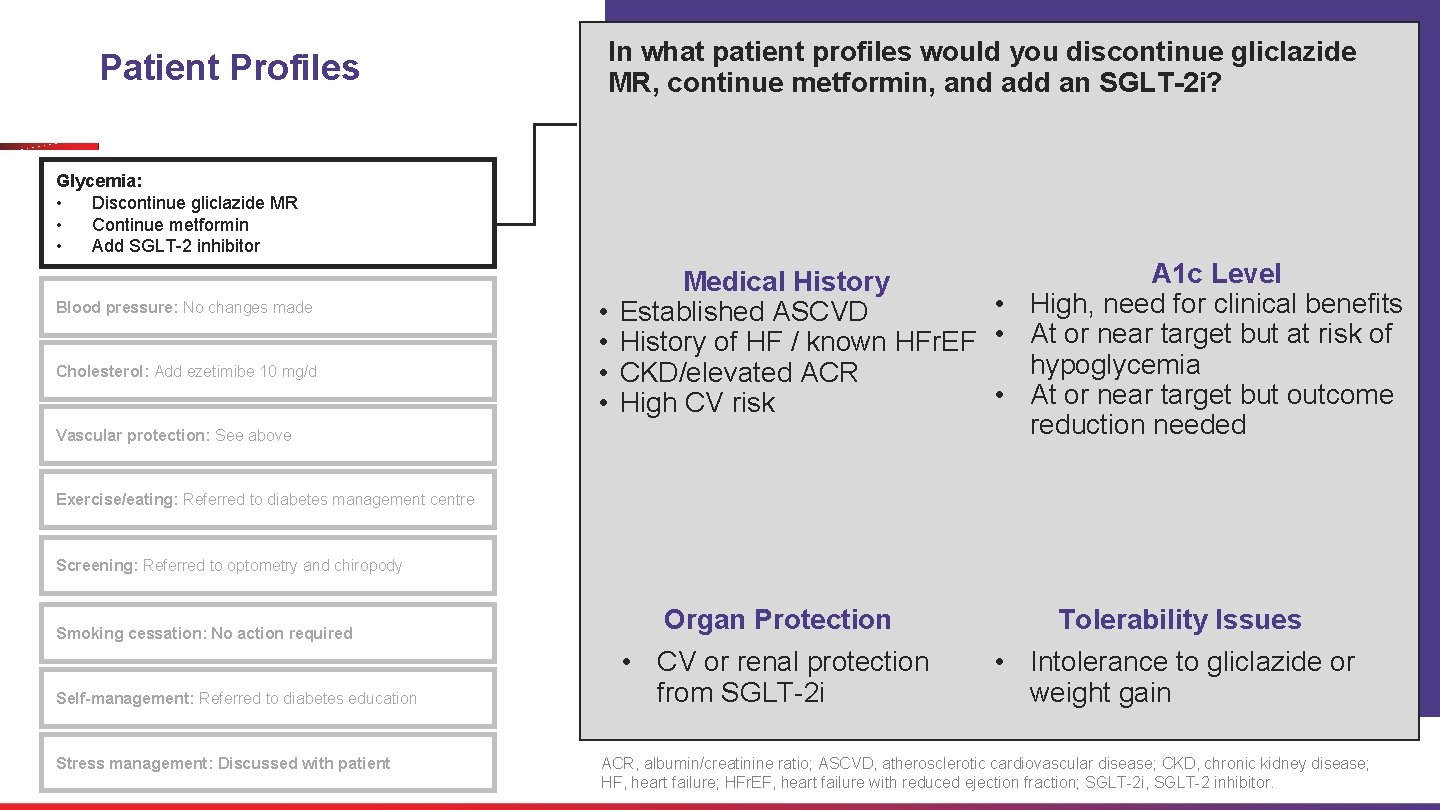
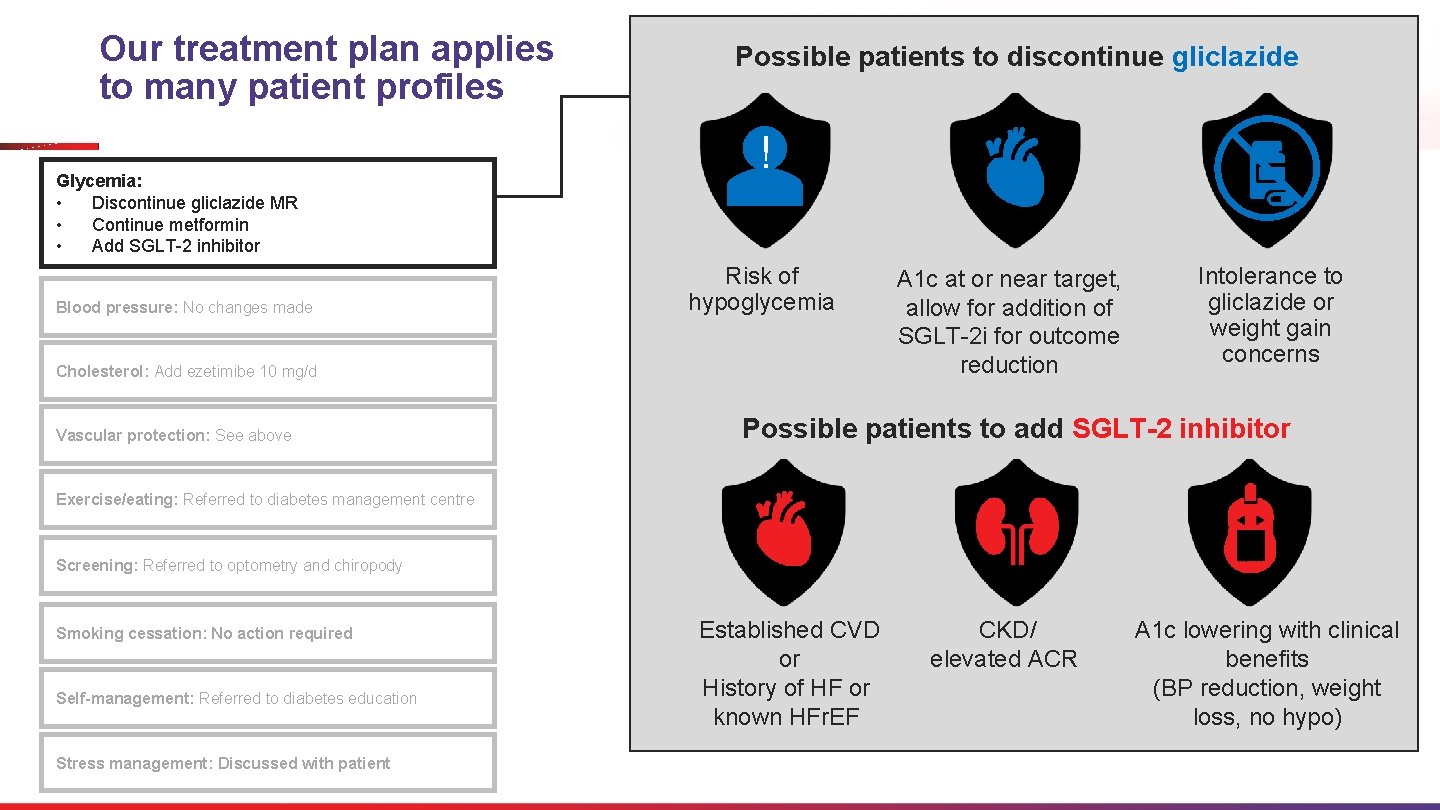
In what patient profiles would you discontinue gliclazide MR, continue metformin, and add an SGLT-2 i? Patient Profiles Glycemia: • Discontinue gliclazide MR • Continue metformin • Add SGLT-2 inhibitor Blood pressure: No changes made Cholesterol: Add ezetimibe 10 mg/d Vascular protection: See above • • A 1 c Level Medical History • High, need for clinical benefits Established ASCVD History of HF / known HFr. EF • At or near target but at risk of hypoglycemia CKD/elevated ACR • At or near target but outcome High CV risk reduction needed Exercise/eating: Referred to diabetes management centre Screening: Referred to optometry and chiropody Smoking cessation: No action required Self-management: Referred to diabetes education Stress management: Discussed with patient Organ Protection Tolerability Issues • CV or renal protection from SGLT-2 i • Intolerance to gliclazide or weight gain ACR, albumin/creatinine ratio; ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; HF, heart failure; HFr. EF, heart failure with reduced ejection fraction; SGLT-2 i, SGLT-2 inhibitor.

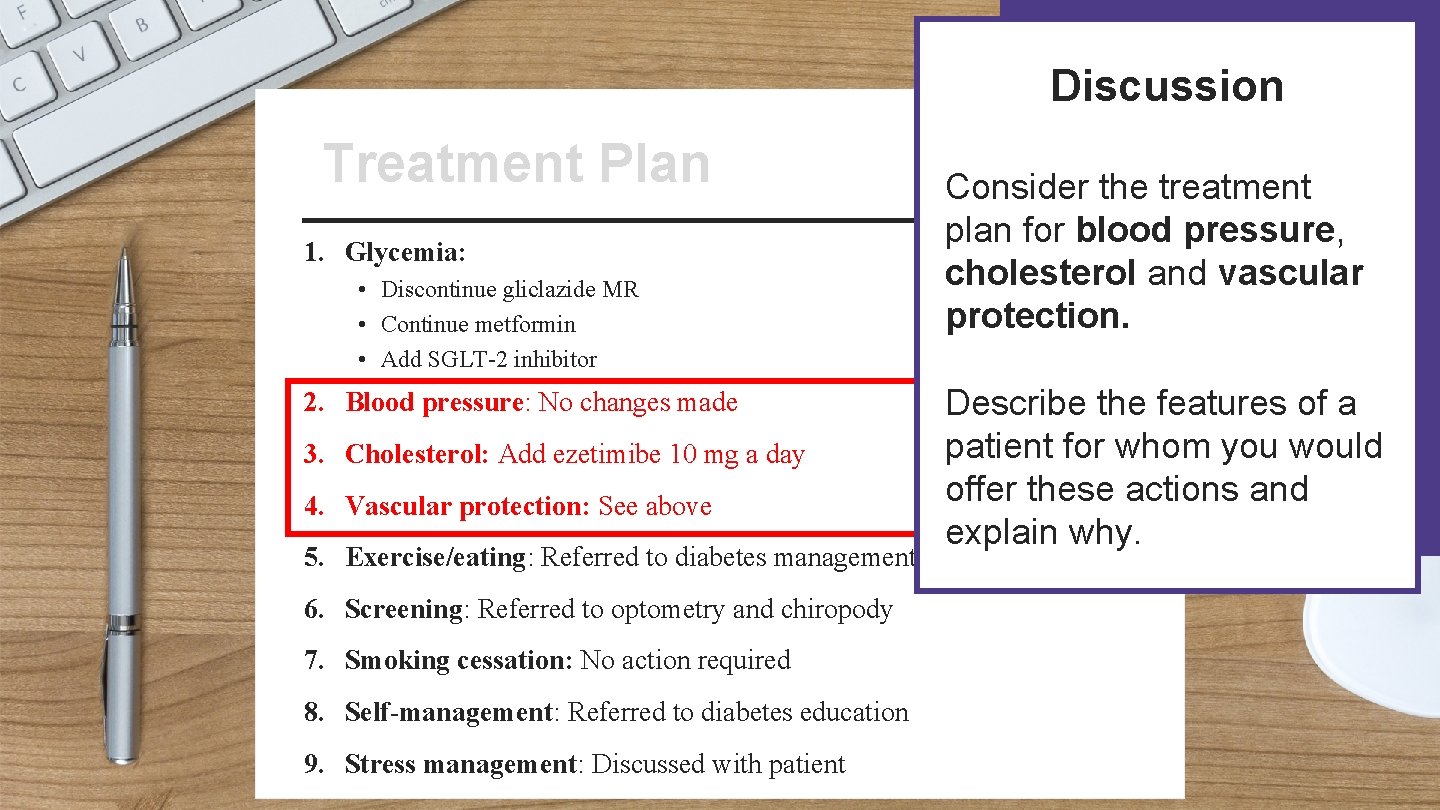
Discussion Patient Name: Treatment Plan Treatment Address: 1. Glycemia: • Discontinue gliclazide MR • Continue metformin • Add SGLT-2 inhibitor 2. Blood pressure: No changes made 3. Cholesterol: Add ezetimibe 10 mg a day 4. Vascular protection: See above Consider the treatment plan for blood pressure, cholesterol and vascular protection. Date: Describe the features of a patient for whom you would offer these actions and explain why. 5. Exercise/eating: Referred to diabetes management centre 6. Screening: Referred to optometry and chiropody 7. Smoking cessation: No action required 8. Self-management: Referred to diabetes education Signature: 9. Stress management: Discussed with patient

Click here for supporting slides To continue discussing patient profiles for the treatment plan, advance to the next slide 15

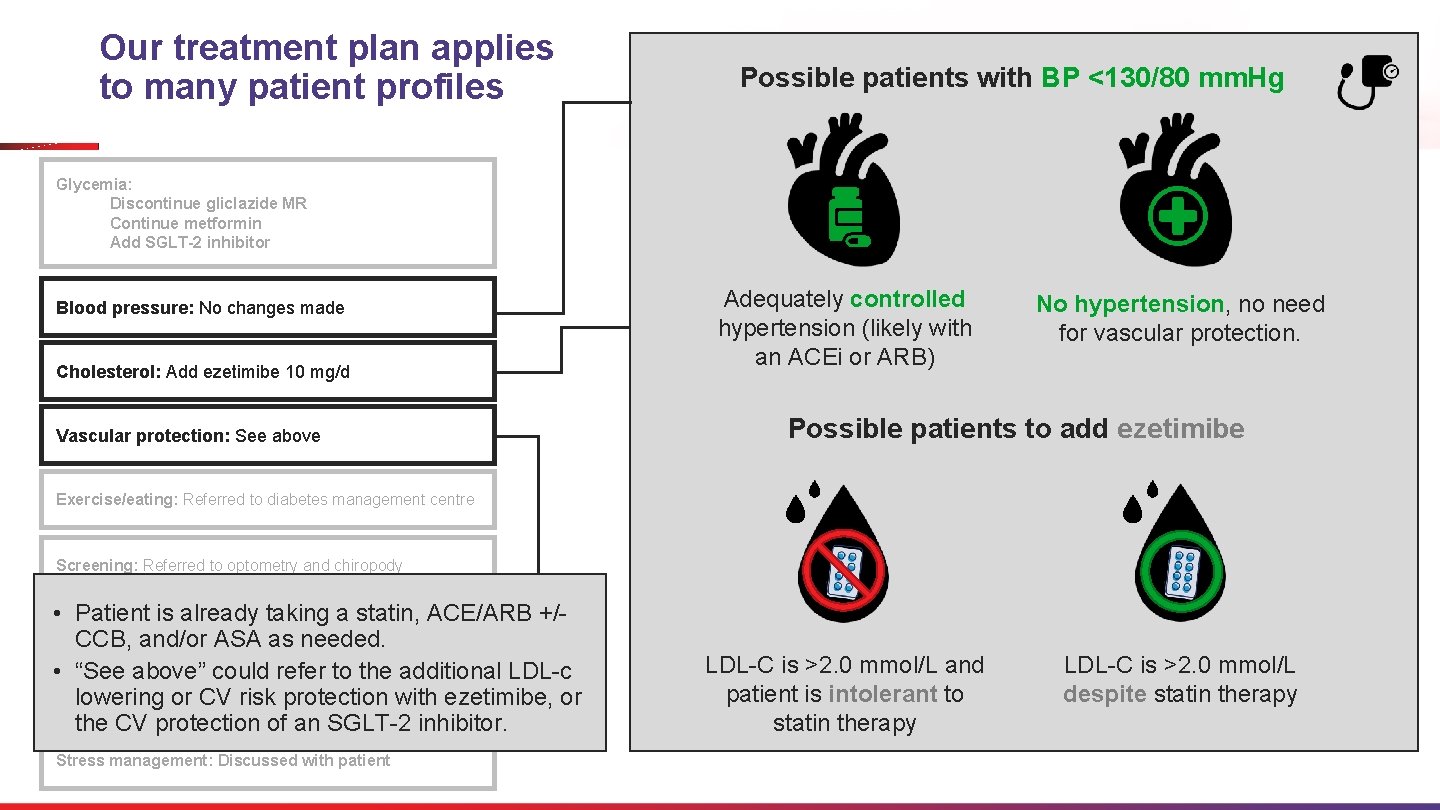
Blood pressure: No changes made Cholesterol: Add ezetimibe 10 mg/d Blood pressure Glycemia: Discontinue gliclazide MR Continue metformin Add SGLT-2 inhibitor Adequately controlled hypertension No hypertension, Cholesterol In Summary In what patient profiles would no changes be made to blood pressure medications and ezetimibe 10 mg/d be added? LDL-C is >2. 0 mmol/L and patient is intolerant to statin therapy despite statin therapy no need for vascular protection Vascular protection: See above Exercise/eating: Referred to diabetes management centre Screening: Referred to optometry and chiropody Smoking cessation: No action required Self-management: Referred to diabetes education Stress management: Discussed with patient

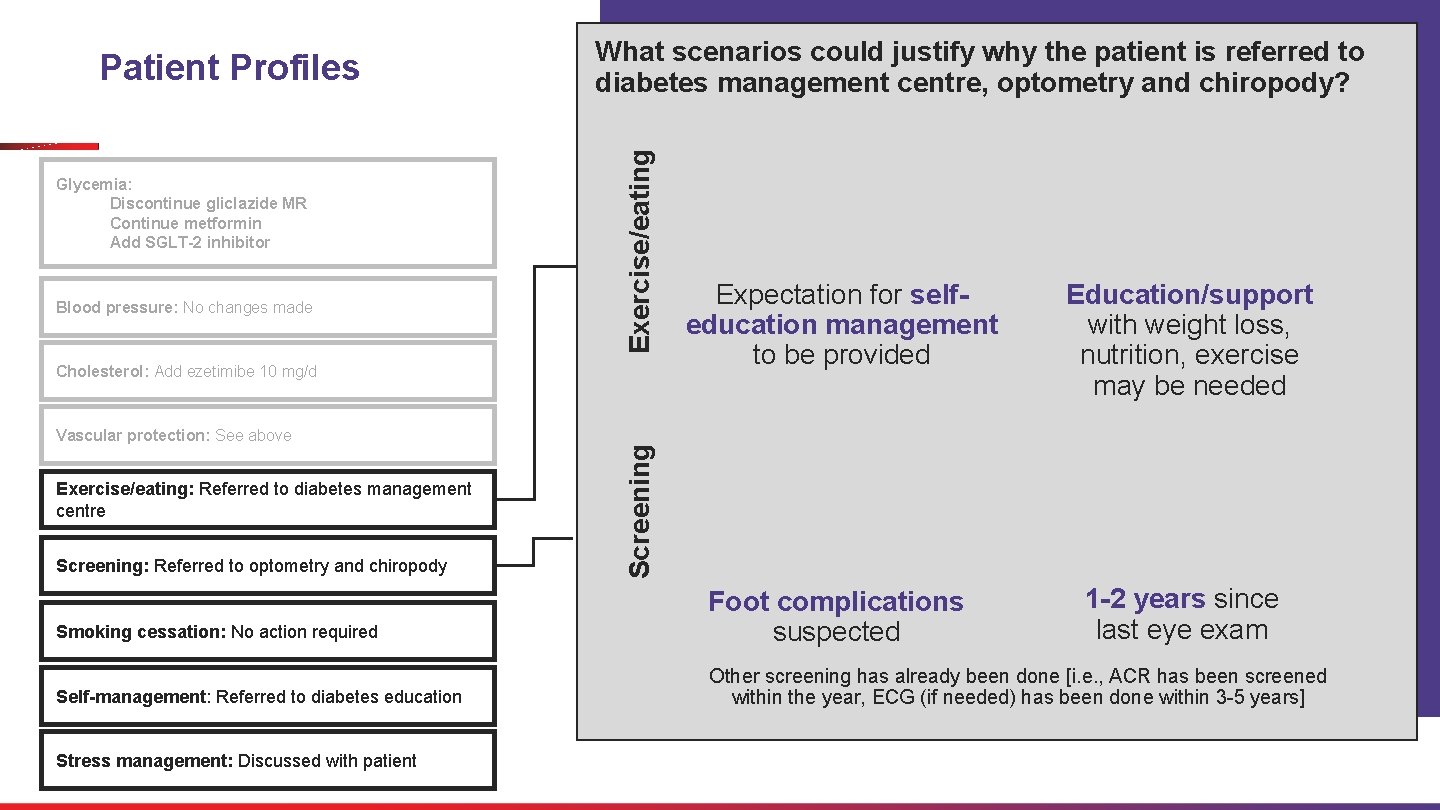
Discussion Patient Name: Treatment Plan Treatment Address: 1. Glycemia: • Discontinue gliclazide MR • Continue metformin • Add SGLT-2 inhibitor Consider the treatment plan for exercise/eating, screening, smoking, selfmanagement and stress management. Date: 2. Blood pressure: No changes made 3. Cholesterol: Add ezetimibe 10 mg a day 4. Vascular protection: See above Describe the features of a patient for whom you would offer these and explain why. 5. Exercise/eating: Referred to diabetes management centre 6. Screening: Referred to optometry and chiropody 7. Smoking cessation: No action required 8. Self-management: Referred to diabetes education Signature: 9. Stress management: Discussed with patient

Click here for supporting slides To continue discussing patient profiles for the treatment plan, advance to the next slide 18

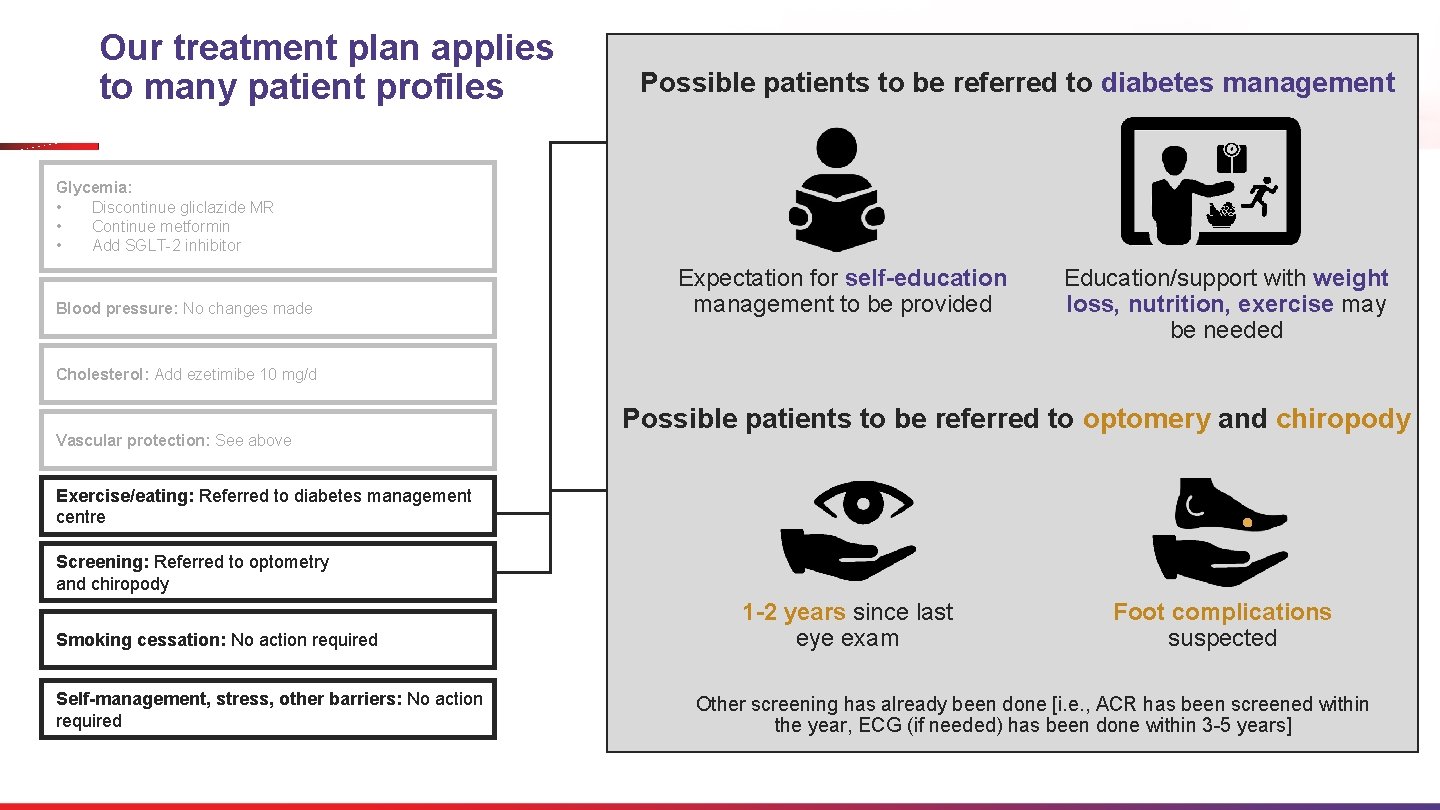
Glycemia: Discontinue gliclazide MR Continue metformin Add SGLT-2 inhibitor Exercise/eating Patient Profiles What scenarios could justify why the patient is referred to diabetes management centre, optometry and chiropody? Expectation for selfeducation management to be provided Education/support with weight loss, nutrition, exercise may be needed Smoking cessation: No action required Foot complications suspected 1 -2 years since last eye exam Self-management: Referred to diabetes education Other screening has already been done [i. e. , ACR has been screened within the year, ECG (if needed) has been done within 3 -5 years] Blood pressure: No changes made Vascular protection: See above Exercise/eating: Referred to diabetes management centre Screening: Referred to optometry and chiropody Stress management: Discussed with patient Screening Cholesterol: Add ezetimibe 10 mg/d

Recap Our treatment plan applies to many patient profiles

Our treatment plan applies to many patient profiles Glycemia: • Discontinue gliclazide MR • Continue metformin • Add SGLT-2 inhibitor Blood pressure: No changes made Possible patients to discontinue gliclazide ! Risk of hypoglycemia Cholesterol: Add ezetimibe 10 mg/d Vascular protection: See above A 1 c at or near target, allow for addition of SGLT-2 i for outcome reduction Intolerance to gliclazide or weight gain concerns Possible patients to add SGLT-2 inhibitor Exercise/eating: Referred to diabetes management centre Screening: Referred to optometry and chiropody Smoking cessation: No action required Self-management: Referred to diabetes education Stress management: Discussed with patient Established CVD or History of HF or known HFr. EF CKD/ elevated ACR A 1 c lowering with clinical benefits (BP reduction, weight loss, no hypo)

Our treatment plan applies to many patient profiles Possible patients with BP <130/80 mm. Hg Glycemia: Discontinue gliclazide MR Continue metformin Add SGLT-2 inhibitor Blood pressure: No changes made Cholesterol: Add ezetimibe 10 mg/d Vascular protection: See above Adequately controlled hypertension (likely with an ACEi or ARB) No hypertension, no need for vascular protection. Possible patients to add ezetimibe Exercise/eating: Referred to diabetes management centre Screening: Referred to optometry and chiropody • Patient is already taking a statin, ACE/ARB +/Smoking cessation: No action required CCB, and/or ASA as needed. • “See above” could refer to the additional LDL-c Self-management: diabetes education lowering or Referred CV riskto protection with ezetimibe, or the CV protection of an SGLT-2 inhibitor. Stress management: Discussed with patient LDL-C is >2. 0 mmol/L and patient is intolerant to statin therapy LDL-C is >2. 0 mmol/L despite statin therapy

Our treatment plan applies to many patient profiles Possible patients to be referred to diabetes management Glycemia: • Discontinue gliclazide MR • Continue metformin • Add SGLT-2 inhibitor Blood pressure: No changes made Expectation for self-education management to be provided Education/support with weight loss, nutrition, exercise may be needed Cholesterol: Add ezetimibe 10 mg/d Vascular protection: See above Possible patients to be referred to optomery and chiropody Exercise/eating: Referred to diabetes management centre Screening: Referred to optometry and chiropody Smoking cessation: No action required Self-management, stress, other barriers: No action required 1 -2 years since last eye exam Foot complications suspected Other screening has already been done [i. e. , ACR has been screened within the year, ECG (if needed) has been done within 3 -5 years]

Key Pearls To Retain • Several international guidelines recommend that SGLT 2 inhibitors and GLP-1 receptor agonists be considered in patients at high CV risk independent of the need to lower A 1 c. • For patients with ASCD, HF (especially HFr. EF), and/or CKD, certain SGLT 2 inhibitors and GLP-1 receptor agonists may be used to reduce the risk of CV and renal complications. • Provide or refer patients for education and support for weight loss, nutrition, and exercise. • Patients with T 2 DM should receive regular screening for retinopathy, foot complications, nephropathy, and cardiovascular complications according to the guideline recommendations.

What other key pearls will you retain from this session?

Q&A Thank you Please complete the evaluation form www. cpdnetwork. org/reverseengineering Password: reverse

Supporting slides

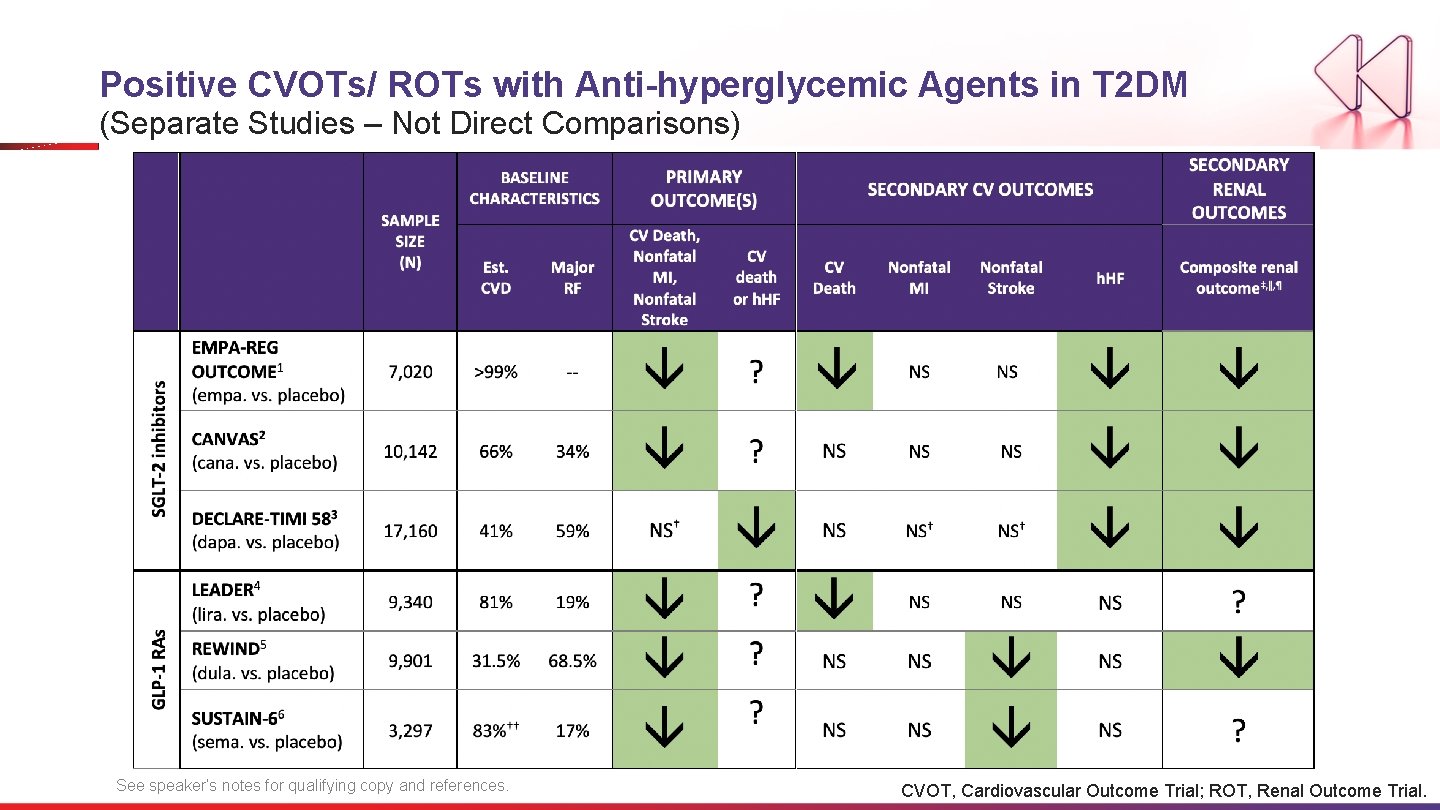
Positive CVOTs/ ROTs with Anti-hyperglycemic Agents in T 2 DM (Separate Studies – Not Direct Comparisons) See speaker’s notes for qualifying copy and references. CVOT, Cardiovascular Outcome Trial; ROT, Renal Outcome Trial.

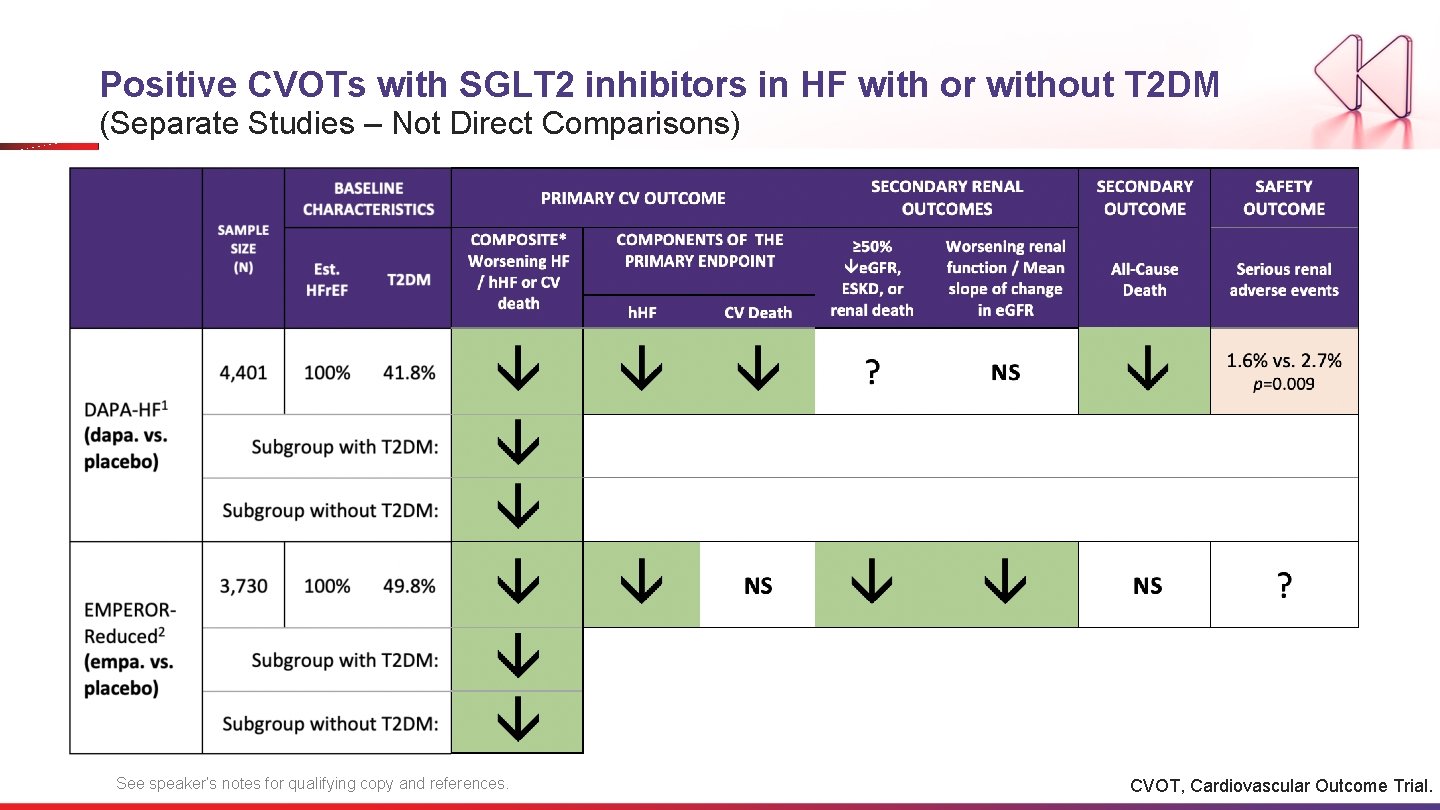
Positive CVOTs with SGLT 2 inhibitors in HF with or without T 2 DM (Separate Studies – Not Direct Comparisons) See speaker’s notes for qualifying copy and references. CVOT, Cardiovascular Outcome Trial.

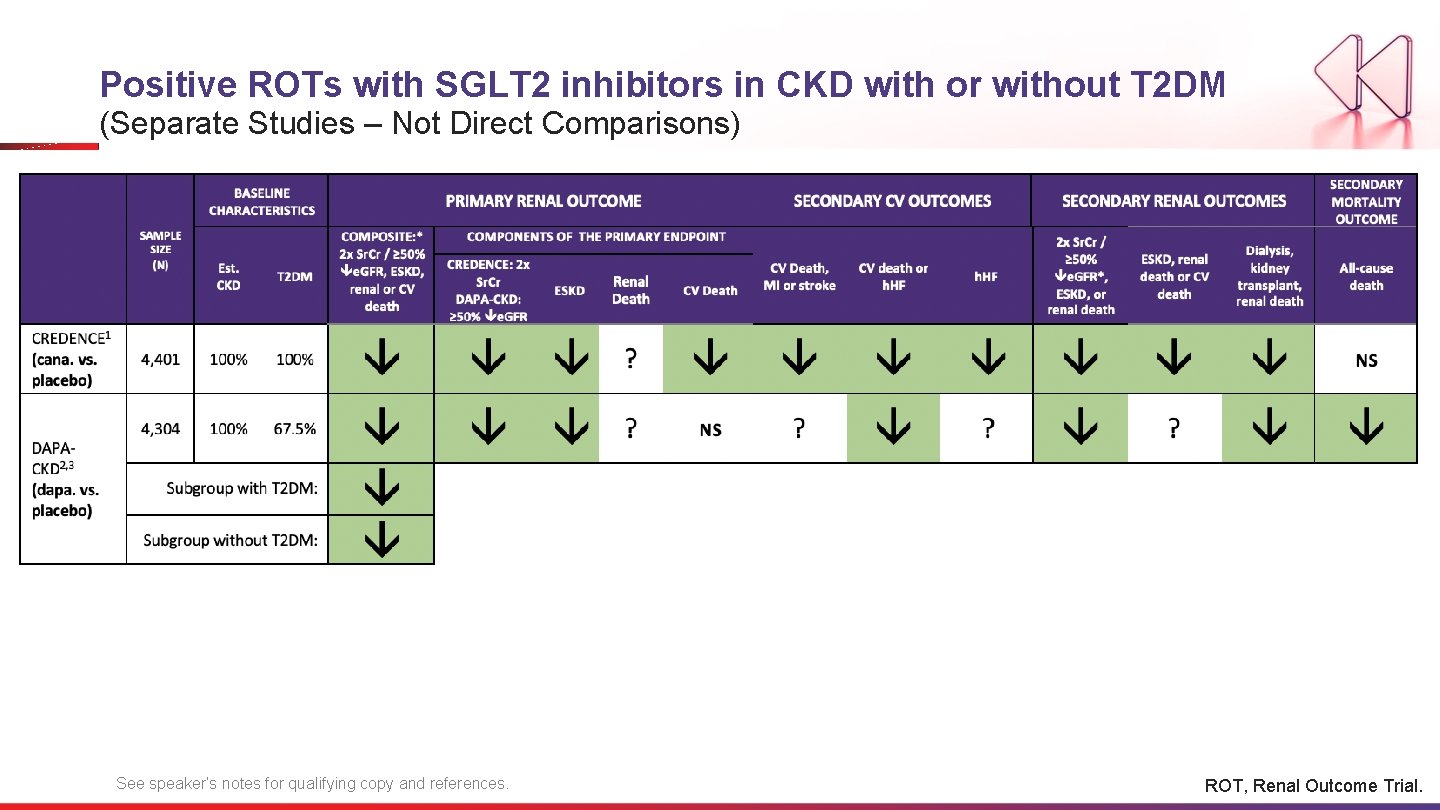
Positive ROTs with SGLT 2 inhibitors in CKD with or without T 2 DM (Separate Studies – Not Direct Comparisons) See speaker’s notes for qualifying copy and references. ROT, Renal Outcome Trial.

Discussion Question In a patient with T 2 DM at very high risk for CV event, what (if any) is your A 1 c threshold for adding an SGLT-2 inhibitor? 31

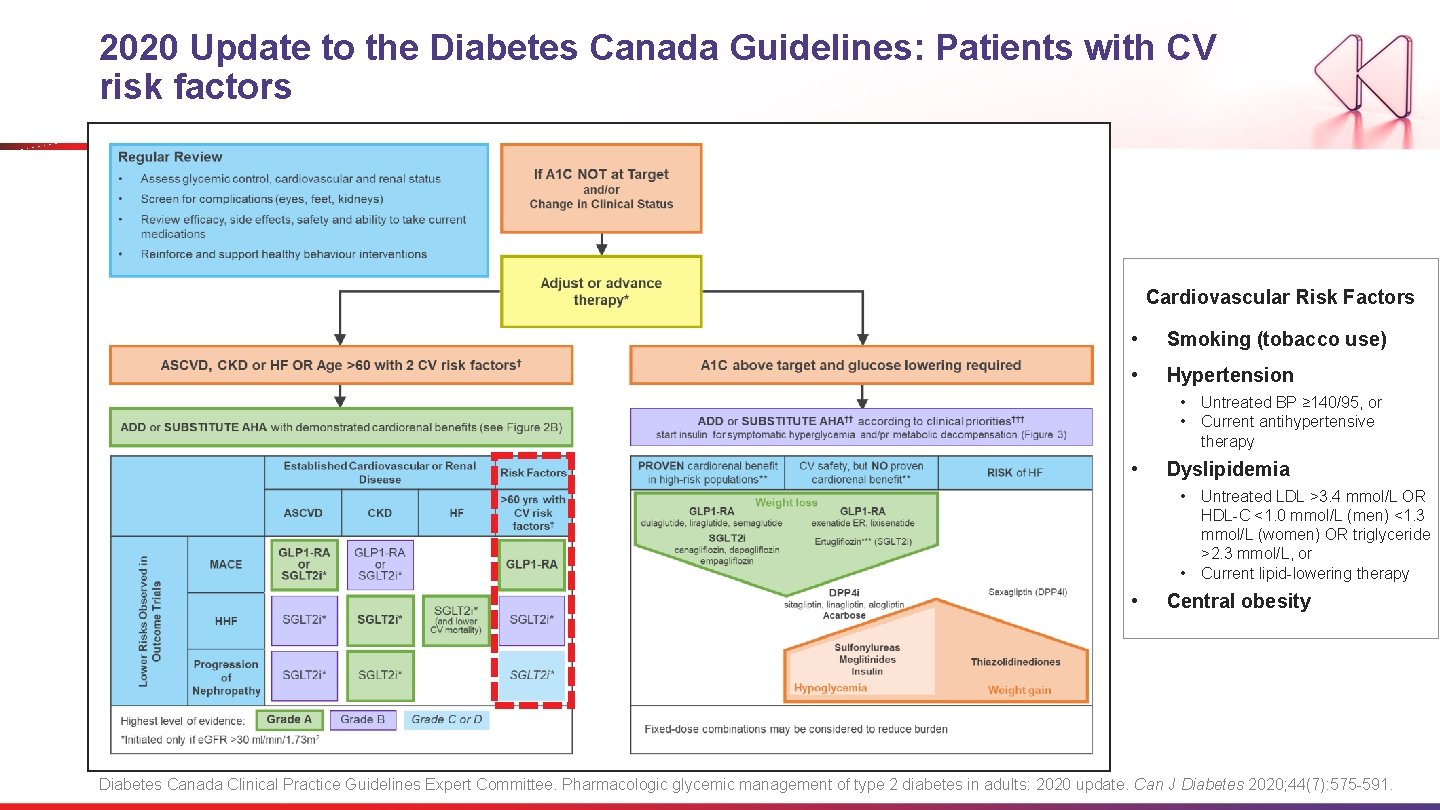
2020 Update to the Diabetes Canada Guidelines: Patients with CV risk factors Cardiovascular Risk Factors • Smoking (tobacco use) • Hypertension • Untreated BP ≥ 140/95, or • Current antihypertensive therapy • Dyslipidemia • Untreated LDL >3. 4 mmol/L OR HDL-C <1. 0 mmol/L (men) <1. 3 mmol/L (women) OR triglyceride >2. 3 mmol/L, or • Current lipid-lowering therapy • Central obesity Diabetes Canada Clinical Practice Guidelines Expert Committee. Pharmacologic glycemic management of type 2 diabetes in adults: 2020 update. Can J Diabetes 2020; 44(7): 575 -591.

2020 Update to the Diabetes Canada Guidelines: Patients with CV risk factors In adults with T 2 DM requiring treatment advancement or adjustment to improve glycemic control, the choice of antihyperglycemic medication should be individualized according to clinical priorities. In adults with type 2 diabetes aged 60 years or older with at least 2 CV risk factors, consider: • A GLP-1 RA with proven CV outcome benefit to reduce the risk of MACE • Grade A, Level 1 A for dulaglutide, Grade B, Level 2 for liraglutide, Grade C, Level 2 subcutaneous semaglutide • An SGLT-2 i with proven cardiorenal outcome benefit if estimated GFR is >30 m. L/min/1. 73 m 2 to reduce the risk of • HHF [Grade B Level 2 for dapagliflozin and canagliflozin] • Progression of nephropathy [Grade C, Level 3 for canagliflozin and dapagliflozin] Diabetes Canada Clinical Practice Guidelines Expert Committee. Pharmacologic glycemic management of type 2 diabetes in adults: 2020 update. Can J Diabetes 2020; 44(7): 575 -591.

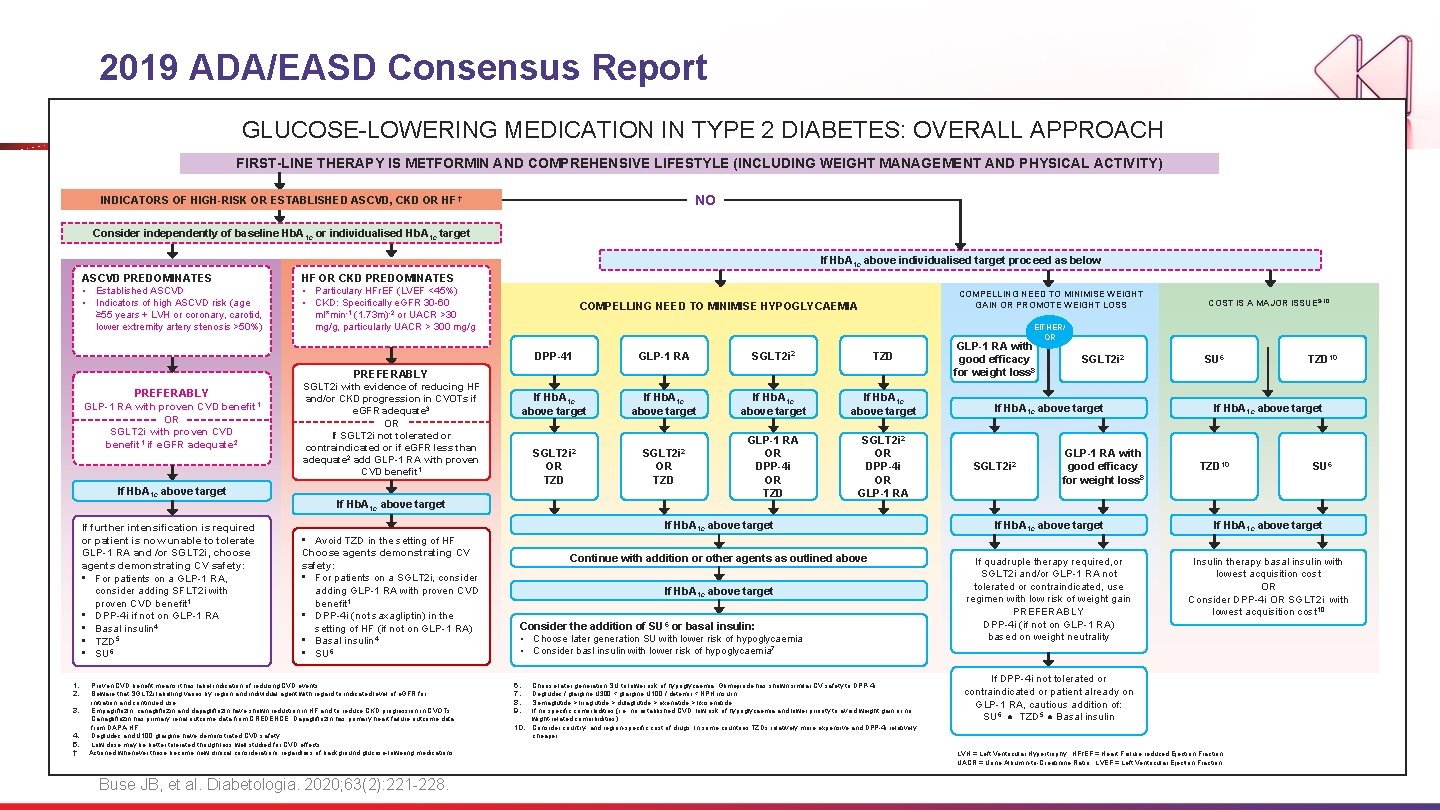
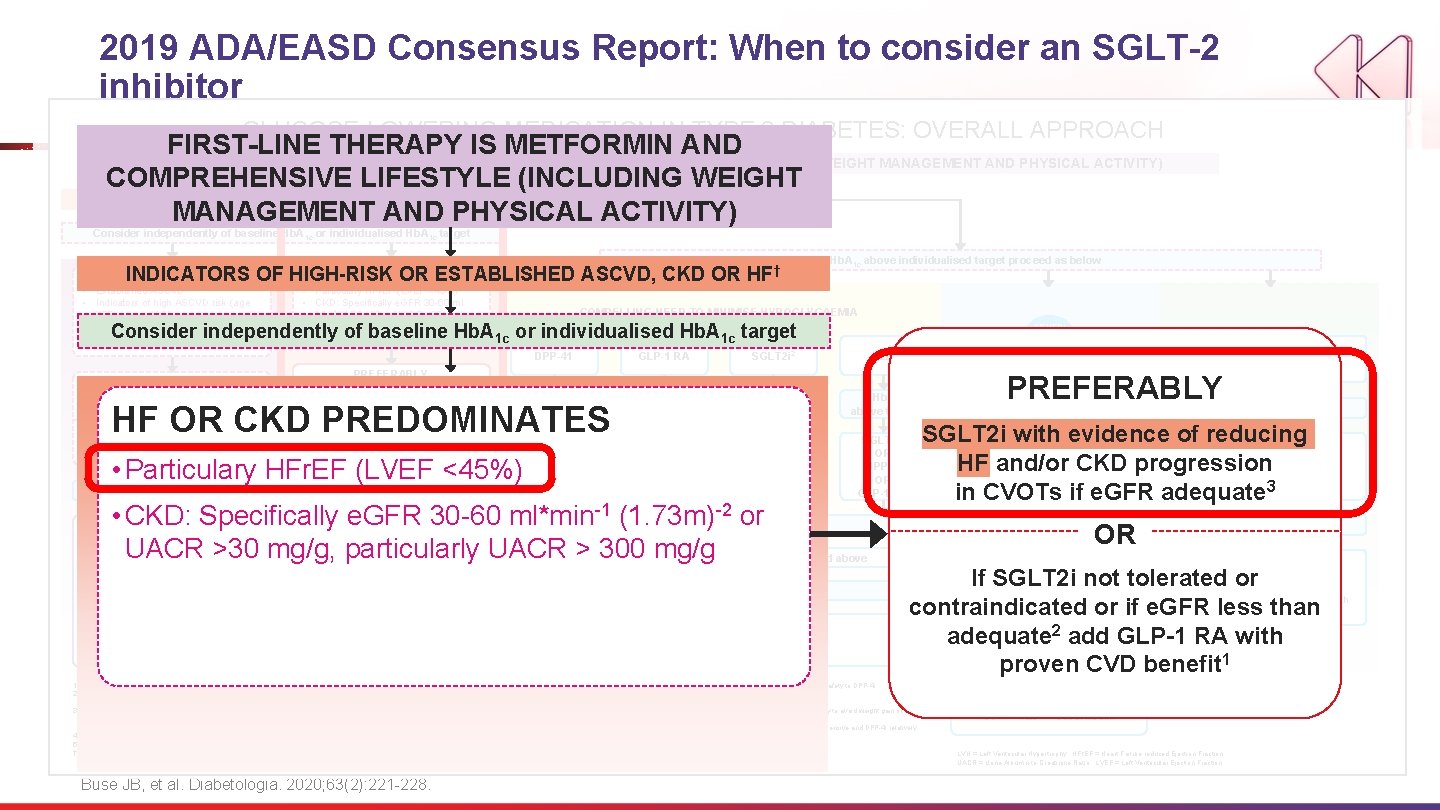
2019 ADA/EASD Consensus Report GLUCOSE-LOWERING MEDICATION IN TYPE 2 DIABETES: OVERALL APPROACH FIRST-LINE THERAPY IS METFORMIN AND COMPREHENSIVE LIFESTYLE (INCLUDING WEIGHT MANAGEMENT AND PHYSICAL ACTIVITY) NO INDICATORS OF HIGH-RISK OR ESTABLISHED ASCVD, CKD OR HF † Consider independently of baseline Hb. A 1 c or individualised Hb. A 1 c target If Hb. A 1 c above individualised target proceed as below ASCVD PREDOMINATES • Established ASCVD • Indicators of high ASCVD risk (age ≥ 55 years + LVH or coronary, carotid, lower extremity artery stenosis >50%) HF OR CKD PREDOMINATES • Particulary HFr. EF (LVEF <45%) • CKD: Specifically e. GFR 30 -60 ml*min-1 COMPELLING NEED TO MINIMISE WEIGHT GAIN OR PROMOTE WEIGHT LOSS COMPELLING NEED TO MINIMISE HYPOGLYCAEMIA (1. 73 m)-2 or UACR >30 mg/g, particularly UACR > 300 mg/g EITHER/ OR DPP-41 GLP-1 RA SGLT 2 i 2 TZD If Hb. A 1 c above target SGLT 2 i 2 OR TZD GLP-1 RA OR DPP-4 i OR TZD SGLT 2 i 2 OR DPP-4 i OR GLP-1 RA PREFERABLY GLP-1 RA with proven CVD benefit 1 OR SGLT 2 i with proven CVD benefit 1 if e. GFR adequate 2 If Hb. A 1 c above target If further intensification is required or patient is now unable to tolerate GLP-1 RA and /or SGLT 2 i, choose agents demonstrating CV safety: • For patients on a GLP-1 RA, consider adding SFLT 2 i with proven CVD benefit 1 • DPP-4 i if not on GLP-1 RA • Basal insulin 4 • TZD 5 • SU 6 1. 2. 3. 4. 5. † COST IS A MAJOR ISSUE 9 -10 SGLT 2 i with evidence of reducing HF and/or CKD progression in CVOTs if e. GFR adequate 3 GLP-1 RA with good efficacy for weight loss 8 SGLT 2 i 2 If Hb. A 1 c above target SU 6 TZD 10 If Hb. A 1 c above target OR If SGLT 2 i not tolerated or contraindicated or if e. GFR less than adequate 2 add GLP-1 RA with proven CVD benefit 1 If Hb. A 1 c above target • Avoid TZD in the setting of HF Choose agents demonstrating CV safety: • For patients on a SGLT 2 i, consider adding GLP-1 RA with proven CVD benefit 1 • DPP-4 i (not saxagliptin) in the setting of HF (if not on GLP-1 RA) • Basal insulin 4 • SU 6 Proven CVD benefit means it has label indication of reducing CVD events. Beware that SGLT 2 i labelling varies by region and individual agent with regard to indicated level of e. GFR for initiation and continued use. Empagliflozin, canagliflozin and dapagliflozin have shown reduction in HF and to reduce CKD progression in CVOTs. Canagliflozin has primary renal outcome data from CREDENCE, Dapagliflozin has primary heart failure outcome data from DAPA-HF. Degludec and U 100 glargine have demonstrated CVD safety. Low dose may be better tolerated though less well studied for CVD effects Actioned whenever these become new clinical considerations regardless of background glucose-lowering medications Buse JB, et al. Diabetologia. 2020; 63(2): 221 -228. TZD 10 SU 6 If Hb. A 1 c above target Continue with addition or other agents as outlined above If quadruple therapy required, or SGLT 2 i and/or GLP-1 RA not tolerated or contraindicated, use regimen with low risk of weight gain PREFERABLY DPP-4 i (if not on GLP-1 RA) based on weight neutrality Insulin therapy basal insulin with lowest acquisition cost OR Consider DPP-4 i OR SGLT 2 i with lowest acquisition cost 10 Consider the addition of SU 6 or basal insulin: • Choose later generation SU with lower risk of hypoglycaemia • Consider basl insulin with lower risk of hypoglycaemia 7 10. GLP-1 RA with good efficacy for weight loss 8 If Hb. A 1 c above target 6. 7. 8. 9. SGLT 2 i 2 Choose later generation SU to lower risk of hypoglycaemia, Glimepiride has shown similar CV safety to DPP-4 i. Degludec / glargine U 300 < glargine U 100 / detemir < NPH insulin. Semaglutide > liraglutide > dulaglutide > exenatide > lixisenatide If no specific comorbidities (i. e. no established CVD, low risk of hypoglycaemia and lower priority to avoid weight gain or no wight-related comorbidities) Consider country- and region-specific cost of drugs. In some countries TZDs relatively more expensive and DPP-4 i relatively cheaper If DPP-4 i not tolerated or contraindicated or patient already on GLP-1 RA, cautious addition of: SU 6 ● TZD 5 ● Basal insulin LVH = Left Ventricular Hypertrophy; HFr. EF = Heart Failure reduced Ejection Fraction UACR = Urine Albumin-to-Creatinine Ratio; LVEF = Left Ventricular Ejection Fraction

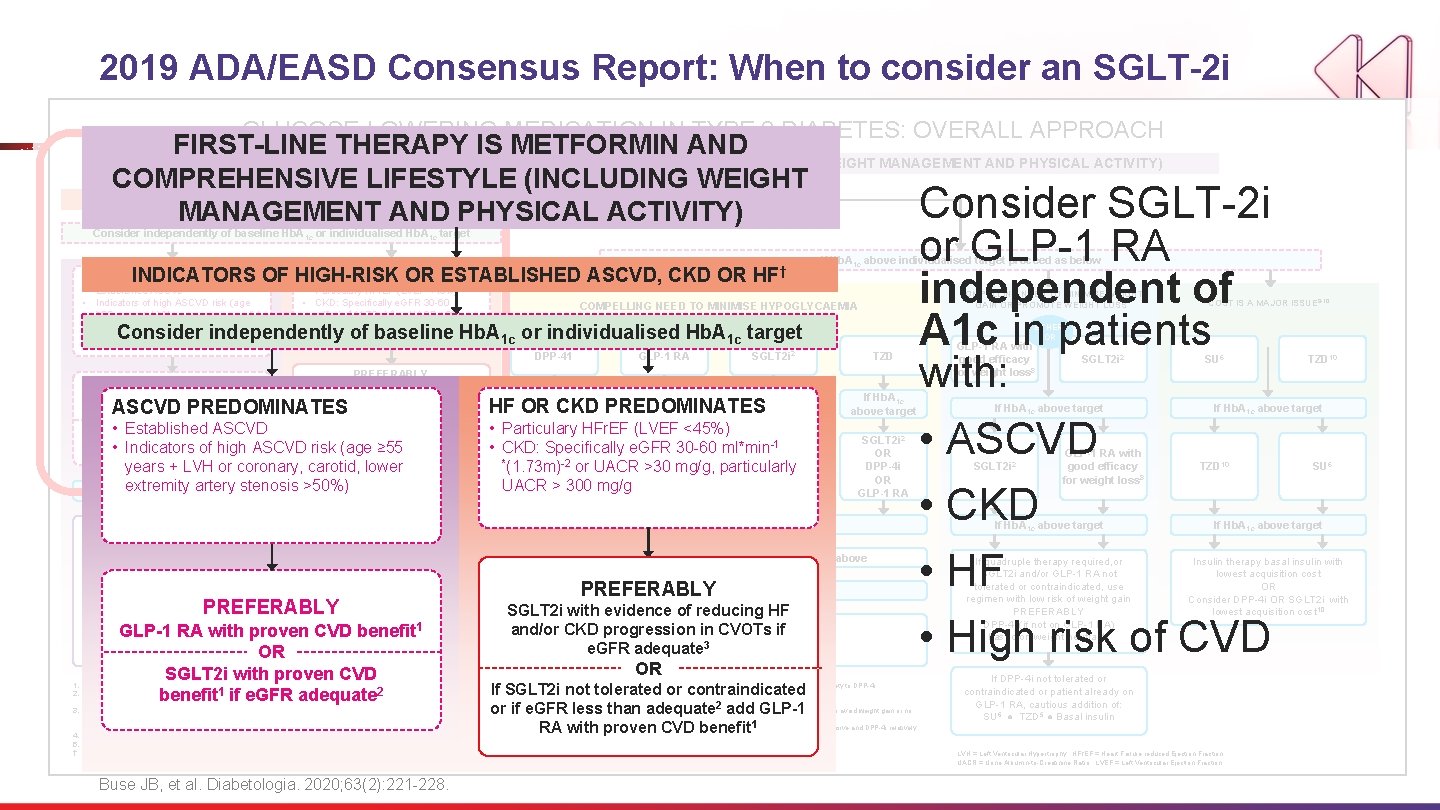
2019 ADA/EASD Consensus Report: When to consider an SGLT-2 i GLUCOSE-LOWERING MEDICATION IN TYPE 2 DIABETES: OVERALL APPROACH FIRST-LINE THERAPY IS METFORMIN AND COMPREHENSIVE LIFESTYLE (INCLUDING WEIGHT MANAGEMENT AND PHYSICAL ACTIVITY) COMPREHENSIVE LIFESTYLE (INCLUDING WEIGHT NO Consider SGLT-2 i MANAGEMENT AND PHYSICAL ACTIVITY) INDICATORS OF HIGH-RISK OR ESTABLISHED ASCVD, CKD OR HF † Consider independently of baseline Hb. A 1 c or individualised Hb. A 1 c target HF OR CKD PREDOMINATES INDICATORS OF HIGH-RISK OR ESTABLISHED ASCVD, CKD OR ASCVD PREDOMINATES • Established ASCVD • Indicators of high ASCVD risk (age ≥ 55 years + LVH or coronary, carotid, lower extremity artery stenosis >50%) • Particulary HFr. EF (LVEF <45%) • CKD: Specifically e. GFR 30 -60 ml*min-1 (1. 73 m)-2 or UACR >30 mg/g, particularly UACR > 300 mg/g HF † COMPELLING NEED TO MINIMISE WEIGHT GAIN OR PROMOTE WEIGHT LOSS COMPELLING NEED TO MINIMISE HYPOGLYCAEMIA Consider independently of baseline Hb. A 1 c or individualised Hb. A 1 c target PREFERABLY GLP-1 RA with proven CVD benefit 1 OR OR • Established ASCVD SGLT 2 i with proven CVD If SGLT 2 i not tolerated or 1 if e. GFR adequate 2 benefit or if e. GFR less than • Indicators of high ASCVDcontraindicated risk (age ≥ 55 adequate 2 add GLP-1 RA with proven years + LVH or coronary, carotid, CVD lower benefit 1 ASCVD PREDOMINATES DPP-41 GLP-1 RA SGLT 2 i 2 TZD If Hb. A 1 c above target If extremity Hb. A 1 c above artery target stenosis >50%) If further intensification is required or patient is now unable to tolerate GLP-1 RA and /or SGLT 2 i, choose agents demonstrating CV safety: • For patients on a GLP-1 RA, consider adding SFLT 2 i with proven CVD benefit 1 • DPP-4 i if not on GLP-1 RA • Basal insulin 4 • TZD 5 • SU 6 If Hb. A 1 c above target 3. 4. 5. † • Particulary HFr. EF (LVEF <45%) GLP-1 -1 RA • CKD: SGLT 2 i Specifically e. GFR 30 -60 ml*min 2 2 SGLT 2 i OR *(1. 73 m) OR-2 or UACR >30 OR mg/g, particularly DPP-4 i OR UACRTZD > 300 mg/g TZD SGLT 2 i 2 OR DPP-4 i OR GLP-1 RA If Hb. A 1 c above target • Avoid TZD in the setting of HF Choose agents demonstrating CV safety: • For patients on a SGLT 2 i, consider adding GLP-1 RA with proven CVD benefit 1 • DPP-4 i (not saxagliptin) in the setting of HF (if not on 1 GLP-1 RA) • Basal insulin 4 • SU 6 PREFERABLY 1. 2. HF OR PREDOMINATES above CKD target above target GLP-1 RA with proven CVD benefit OR SGLT 2 i with proven CVD benefit 1 if e. GFR adequate 2 Proven CVD benefit means it has label indication of reducing CVD events. Beware that SGLT 2 i labelling varies by region and individual agent with regard to indicated level of e. GFR for initiation and continued use. Empagliflozin, canagliflozin and dapagliflozin have shown reduction in HF and to reduce CKD progression in CVOTs. Canagliflozin has primary renal outcome data from CREDENCE, Dapagliflozin has primary heart failure outcome data from DAPA-HF. Degludec and U 100 glargine have demonstrated CVD safety. Low dose may be better tolerated though less well studied for CVD effects Actioned whenever these become new clinical considerations regardless of background glucose-lowering medications Buse JB, et al. Diabetologia. 2020; 63(2): 221 -228. Continue with addition or other agents as outlined above If Hb. A above target PREFERABLY 1 c SGLT 2 i with evidence of reducing HF Consider. CKD the addition of SU 6 or basal and/or progression in insulin: CVOTs if • Choose later generation SU with lower risk of hypoglycaemia e. GFR adequate • Consider basl insulin with lower risk of 3 hypoglycaemia 7 OR If SGLT 2 i not tolerated or contraindicated or if e. GFR less than adequate add GLP-1 10. RA with proven CVD benefit 6. 7. 8. 9. COST IS A MAJOR ISSUE 9 -10 EITHER/ OR PREFERABLY SGLT 2 i with evidence of reducing HF and/or CKD progression in CVOTs if e. GFR adequate 3 or GLP-1 RA independent of A 1 c in patients with: If Hb. A 1 c above individualised target proceed as below Choose later generation SU to lower risk of hypoglycaemia, Glimepiride has shown similar CV safety to DPP-4 i. Degludec / glargine U 300 < glargine U 100 / detemir < NPH insulin. Semaglutide > liraglutide > dulaglutide > exenatide > lixisenatide 2 If no specific comorbidities (i. e. no established CVD, low risk of hypoglycaemia and lower priority to avoid weight gain or no wight-related comorbidities) 1 relatively more expensive and DPP-4 i relatively Consider country- and region-specific cost of drugs. In some countries TZDs cheaper GLP-1 RA with good efficacy for weight loss 8 SGLT 2 i 2 If Hb. A 1 c above target SU 6 If Hb. A 1 c above target • ASCVD • CKD • HF • High risk of CVD SGLT 2 i 2 GLP-1 RA with good efficacy for weight loss 8 TZD 10 SU 6 If Hb. A 1 c above target If quadruple therapy required, or SGLT 2 i and/or GLP-1 RA not tolerated or contraindicated, use regimen with low risk of weight gain PREFERABLY DPP-4 i (if not on GLP-1 RA) based on weight neutrality Insulin therapy basal insulin with lowest acquisition cost OR Consider DPP-4 i OR SGLT 2 i with lowest acquisition cost 10 If DPP-4 i not tolerated or contraindicated or patient already on GLP-1 RA, cautious addition of: SU 6 ● TZD 5 ● Basal insulin LVH = Left Ventricular Hypertrophy; HFr. EF = Heart Failure reduced Ejection Fraction UACR = Urine Albumin-to-Creatinine Ratio; LVEF = Left Ventricular Ejection Fraction

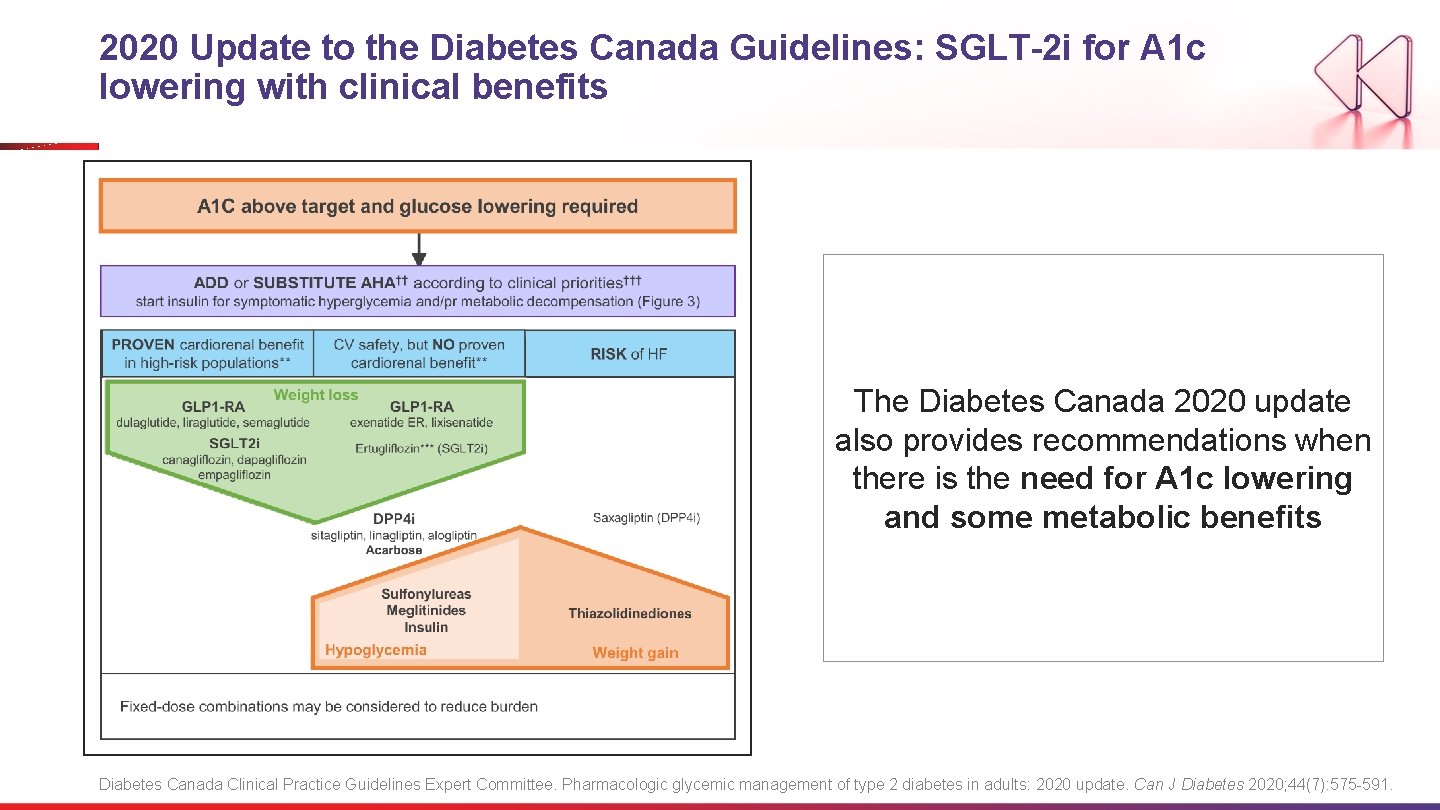
2020 Update to the Diabetes Canada Guidelines: SGLT-2 i for A 1 c lowering with clinical benefits The Diabetes Canada 2020 update also provides recommendations when there is the need for A 1 c lowering and some metabolic benefits Diabetes Canada Clinical Practice Guidelines Expert Committee. Pharmacologic glycemic management of type 2 diabetes in adults: 2020 update. Can J Diabetes 2020; 44(7): 575 -591.

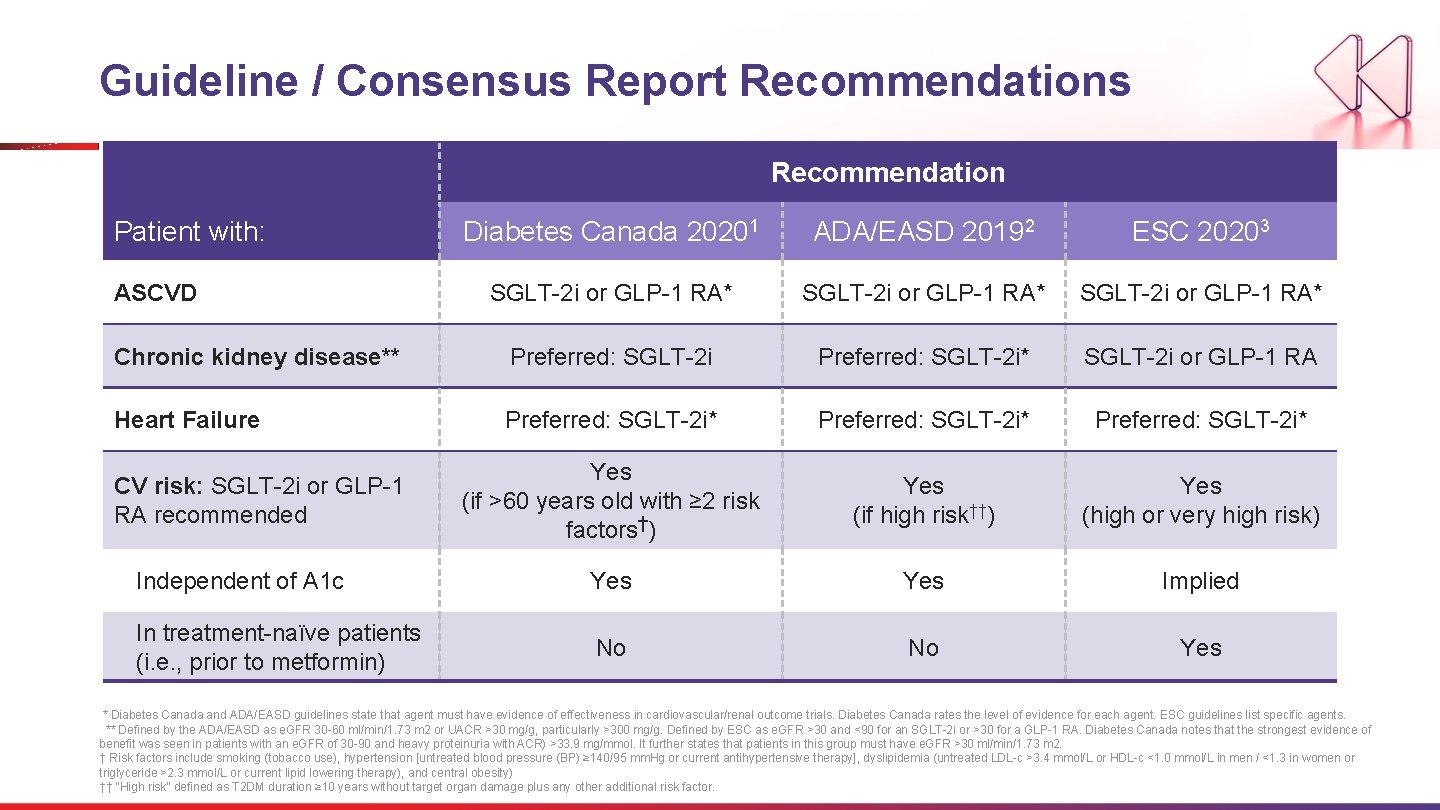
Guideline / Consensus Report Recommendations Recommendation Patient with: Diabetes Canada 20201 ADA/EASD 20192 ESC 20203 SGLT-2 i or GLP-1 RA* Chronic kidney disease** Preferred: SGLT-2 i* SGLT-2 i or GLP-1 RA Heart Failure Preferred: SGLT-2 i* Yes (if >60 years old with ≥ 2 risk factors†) Yes (if high risk††) Yes (high or very high risk) Independent of A 1 c Yes Implied In treatment-naïve patients (i. e. , prior to metformin) No No Yes ASCVD CV risk: SGLT-2 i or GLP-1 RA recommended * Diabetes Canada and ADA/EASD guidelines state that agent must have evidence of effectiveness in cardiovascular/renal outcome trials. Diabetes Canada rates the level of evidence for each agent. ESC guidelines list specific agents. ** Defined by the ADA/EASD as e. GFR 30 -60 ml/min/1. 73 m 2 or UACR >30 mg/g, particularly >300 mg/g. Defined by ESC as e. GFR >30 and <90 for an SGLT-2 i or >30 for a GLP-1 RA. Diabetes Canada notes that the strongest evidence of benefit was seen in patients with an e. GFR of 30 -90 and heavy proteinuria with ACR) >33. 9 mg/mmol. It further states that patients in this group must have e. GFR >30 ml/min/1. 73 m 2. † Risk factors include smoking (tobacco use), hypertension [untreated blood pressure (BP) ≥ 140/95 mm. Hg or current antihypertensive therapy], dyslipidemia (untreated LDL-c >3. 4 mmol/L or HDL-c <1. 0 mmol/L in men / <1. 3 in women or triglyceride >2. 3 mmol/L or current lipid lowering therapy), and central obesity) †† “High risk” defined as T 2 DM duration ≥ 10 years without target organ damage plus any other additional risk factor.

Discussion Question For a patient with T 2 DM, history of HF on metformin with an A 1 c of 6. 7%, what would you do next? 38

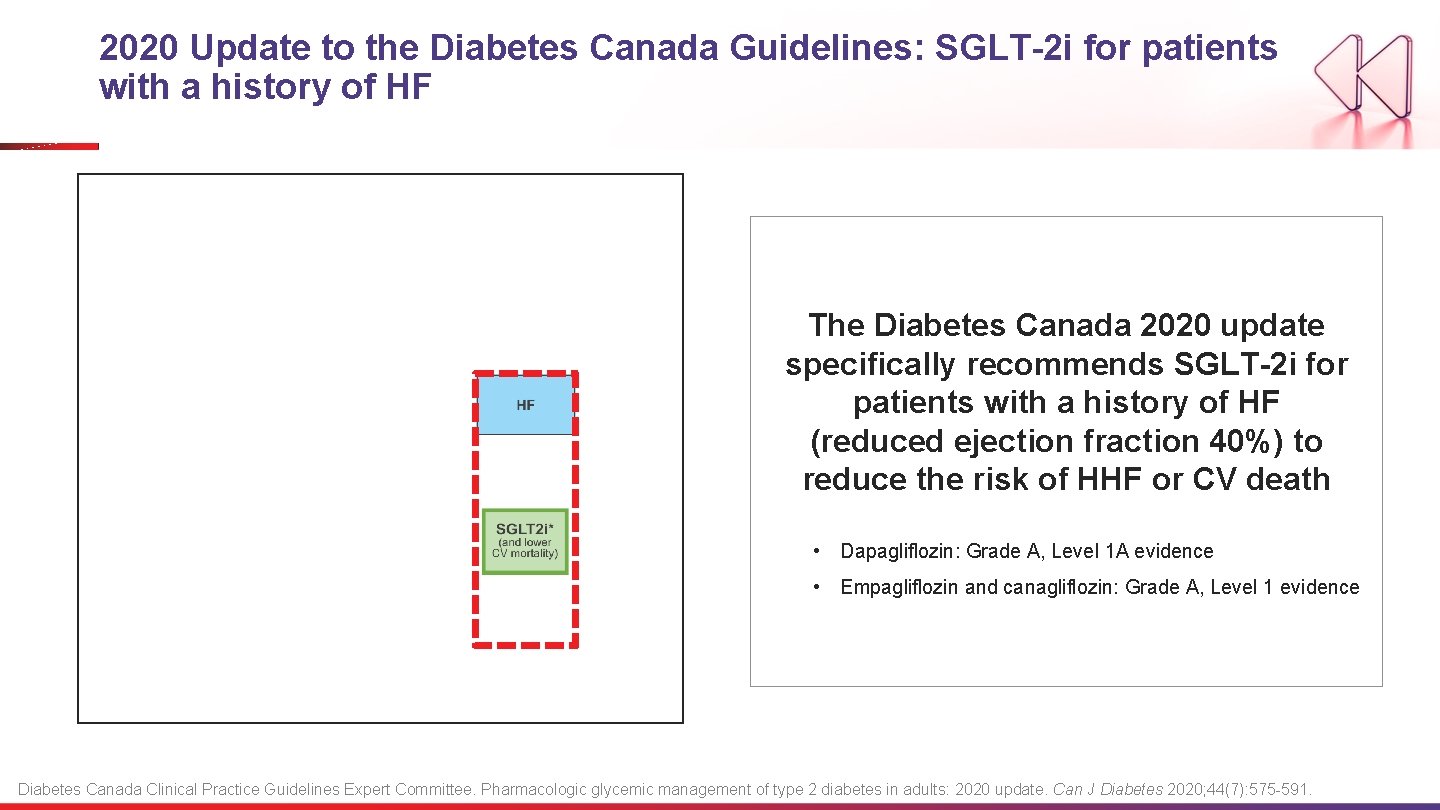
2020 Update to the Diabetes Canada Guidelines: SGLT-2 i for patients with a history of HF The Diabetes Canada 2020 update specifically recommends SGLT-2 i for patients with a history of HF (reduced ejection fraction 40%) to reduce the risk of HHF or CV death • Dapagliflozin: Grade A, Level 1 A evidence • Empagliflozin and canagliflozin: Grade A, Level 1 evidence Diabetes Canada Clinical Practice Guidelines Expert Committee. Pharmacologic glycemic management of type 2 diabetes in adults: 2020 update. Can J Diabetes 2020; 44(7): 575 -591.

2019 ADA/EASD Consensus Report: When to consider an SGLT-2 inhibitor GLUCOSE-LOWERING MEDICATION IN TYPE 2 DIABETES: OVERALL APPROACH FIRST-LINE THERAPY IS METFORMIN AND COMPREHENSIVE LIFESTYLE (INCLUDING WEIGHT MANAGEMENT AND PHYSICAL ACTIVITY) COMPREHENSIVE LIFESTYLE (INCLUDING WEIGHT NO MANAGEMENT AND PHYSICAL ACTIVITY) INDICATORS OF HIGH-RISK OR ESTABLISHED ASCVD, CKD OR HF † Consider independently of baseline Hb. A 1 c or individualised Hb. A 1 c target INDICATORS OF HIGH-RISK OR ESTABLISHED ASCVD, CKD OR HF OR CKD PREDOMINATES ASCVD PREDOMINATES • Established ASCVD • Indicators of high ASCVD risk (age ≥ 55 years + LVH or coronary, carotid, lower extremity artery stenosis >50%) • Particulary HFr. EF (LVEF <45%) • CKD: Specifically e. GFR 30 -60 ml • Min-1 (1. 73 m)-2 or UACR >30 mg/g, particularly UACR > 300 mg/g HF † If Hb. A 1 c above individualised target proceed as below COMPELLING NEED TO MINIMISE HYPOGLYCAEMIA Consider independently of baseline Hb. A 1 c or individualised Hb. A 1 c target EITHER/ OR GLP-1 RA with good efficacy for weight loss 8 DPP-41 GLP-1 RA SGLT 2 i 2 TZD If Hb. A 1 c above target SGLT 2 i 2 OR TZD GLP-1 RA OR DPP-4 i OR TZD SGLT 2 i 2 OR DPP-4 i OR GLP-1 RA PREFERABLY GLP-1 RA with proven CVD benefit 1 OR SGLT 2 i with proven CVD benefit 1 if e. GFR adequate 2 SGLT 2 i with evidence of reducing HF and/or CKD progression in CVOTs if e. GFR adequate 3 HF OR CKD PREDOMINATES OR If SGLT 2 i not tolerated or contraindicated or if e. GFR less than adequate 2 add GLP-1 RA with proven CVD benefit 1 • Particulary HFr. EF (LVEF <45%) If Hb. A 1 c above target • CKD: Specifically e. GFR 30 -60 ml*min-1 (1. 73 m)-2 or UACR >30 mg/g, particularly UACR > 300 mg/g If further intensification is required or patient is now unable to tolerate GLP-1 RA and /or SGLT 2 i, choose agents demonstrating CV safety: • For patients on a GLP-1 RA, consider adding SFLT 2 i with proven CVD benefit 1 • DPP-4 i if not on GLP-1 RA • Basal insulin 4 • TZD 5 • SU 6 1. 2. 3. 4. 5. † • Avoid TZD in the setting of HF Choose agents demonstrating CV safety: • For patients on a SGLT 2 i, consider adding GLP-1 RA with proven CVD benefit 1 • DPP-4 i (not saxagliptin) in the setting of HF (if not on GLP-1 RA) • Basal insulin 4 • SU 6 Proven CVD benefit means it has label indication of reducing CVD events. Beware that SGLT 2 i labelling varies by region and individual agent with regard to indicated level of e. GFR for initiation and continued use. Empagliflozin, canagliflozin and dapagliflozin have shown reduction in HF and to reduce CKD progression in CVOTs. Canagliflozin has primary renal outcome data from CREDENCE, Dapagliflozin has primary heart failure outcome data from DAPA-HF. Degludec and U 100 glargine have demonstrated CVD safety. Low dose may be better tolerated though less well studied for CVD effects Actioned whenever these become new clinical considerations regardless of background glucose-lowering medications Buse JB, et al. Diabetologia. 2020; 63(2): 221 -228. TZD 10 PREFERABLY If Hb. A 1 c above target SGLT 2 i with evidence of reducing GLP-1 RA with SGLT 2 i efficacyprogression TZD SU HF and/orgood CKD for weight loss in CVOTs if e. GFR adequate 3 2 10 6 8 If Hb. A 1 c above target Continue with addition or other agents as outlined above If quadruple therapy required, or SGLT 2 i and/or GLP-1 RA not tolerated or contraindicated, use regimen with low risk of weight gain PREFERABLY DPP-4 i (if not on GLP-1 RA) 2 neutrality based on weight Insulin therapy basal insulin with lowest acquisition cost OR Consider DPP-4 i OR SGLT 2 i with lowest acquisition cost 10 Consider the addition of SU 6 or basal insulin: • Choose later generation SU with lower risk of hypoglycaemia • Consider basl insulin with lower risk of hypoglycaemia 7 10. SU 6 If Hb. A 1 c above target 6. 7. 8. 9. SGLT 2 i 2 OR If SGLT 2 i not tolerated or contraindicated or if e. GFR less than adequate add GLP-1 RA with 1 proven CVD benefit If DPP-4 i not tolerated or Choose later generation SU to lower risk of hypoglycaemia, Glimepiride has shown similar CV safety to DPP-4 i. Degludec / glargine U 300 < glargine U 100 / detemir < NPH insulin. Semaglutide > liraglutide > dulaglutide > exenatide > lixisenatide If no specific comorbidities (i. e. no established CVD, low risk of hypoglycaemia and lower priority to avoid weight gain or no wight-related comorbidities) Consider country- and region-specific cost of drugs. In some countries TZDs relatively more expensive and DPP-4 i relatively cheaper contraindicated or patient already on GLP-1 RA, cautious addition of: SU 6 ● TZD 5 ● Basal insulin LVH = Left Ventricular Hypertrophy; HFr. EF = Heart Failure reduced Ejection Fraction UACR = Urine Albumin-to-Creatinine Ratio; LVEF = Left Ventricular Ejection Fraction

Discussion Question In a drug-naïve patient with CV risk factors, would you add an SGLT-2 inhibitor as firstline therapy for T 2 DM rather than standard of care metformin? 41

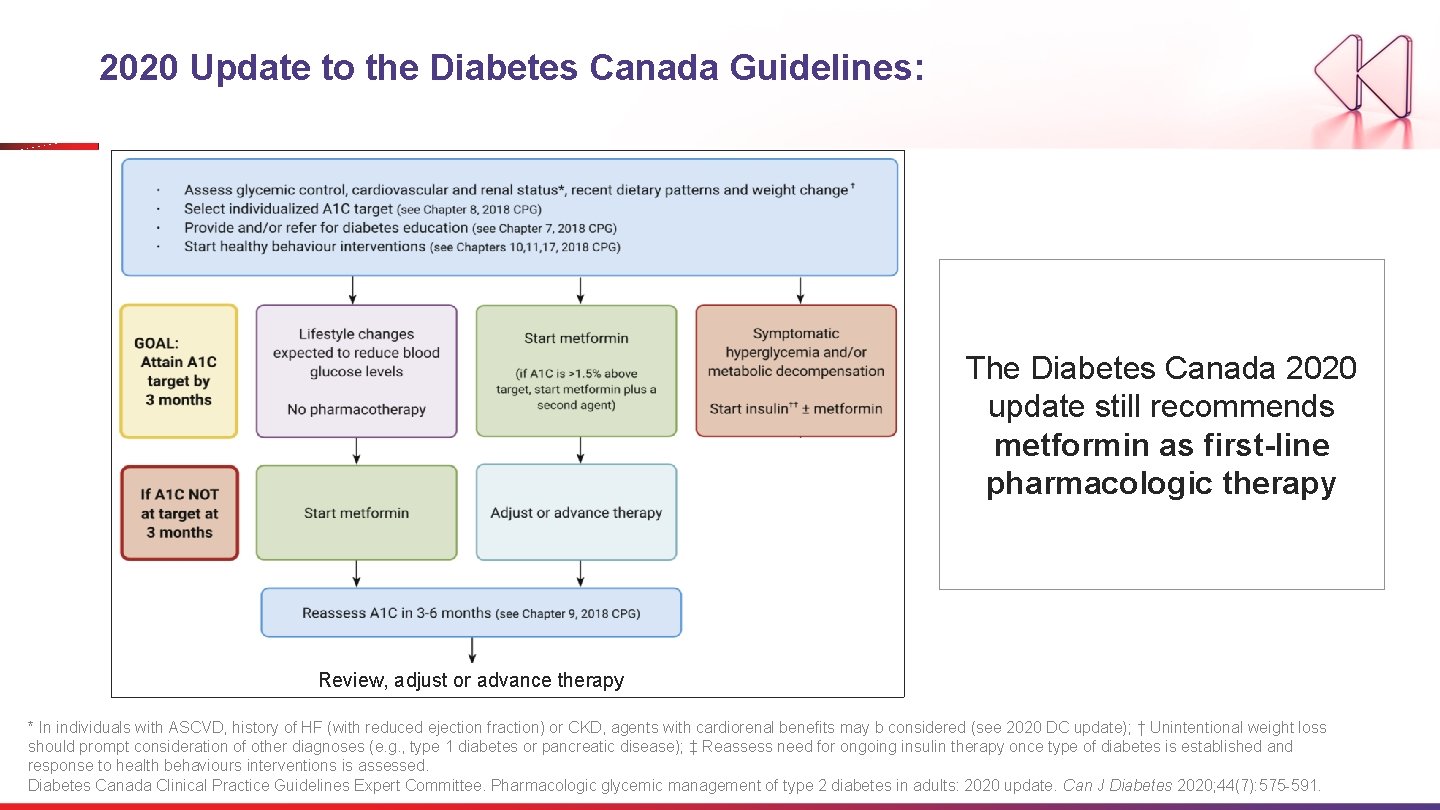
2020 Update to the Diabetes Canada Guidelines: The Diabetes Canada 2020 update still recommends metformin as first-line pharmacologic therapy Review, adjust or advance therapy * In individuals with ASCVD, history of HF (with reduced ejection fraction) or CKD, agents with cardiorenal benefits may b considered (see 2020 DC update); † Unintentional weight loss should prompt consideration of other diagnoses (e. g. , type 1 diabetes or pancreatic disease); ‡ Reassess need for ongoing insulin therapy once type of diabetes is established and response to health behaviours interventions is assessed. Diabetes Canada Clinical Practice Guidelines Expert Committee. Pharmacologic glycemic management of type 2 diabetes in adults: 2020 update. Can J Diabetes 2020; 44(7): 575 -591.

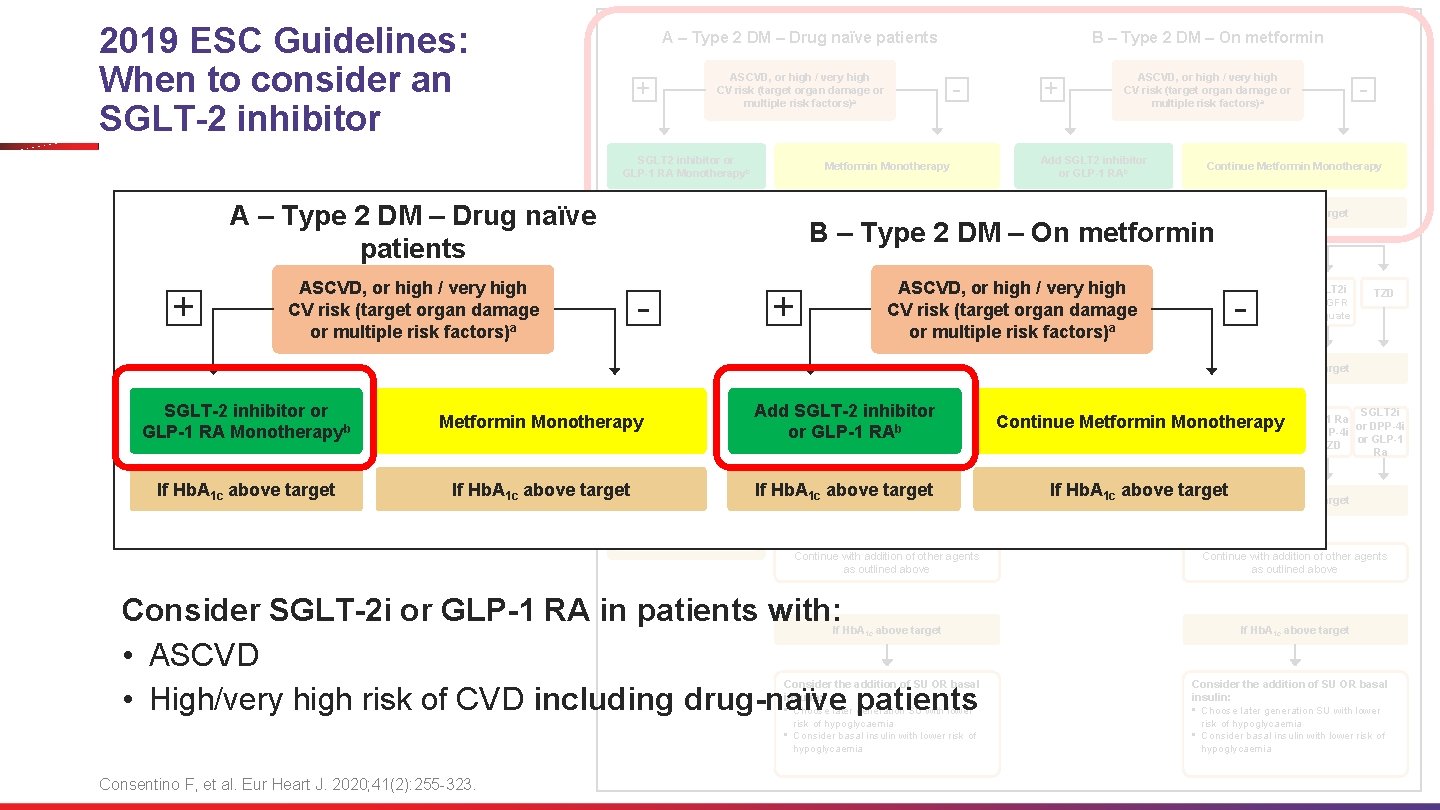
2019 ESC Guidelines: When to consider an SGLT-2 inhibitor + ASCVD, or high / very high CV risk (target organ damage or multiple risk factors)a - - Continue Metformin Monotherapy If Hb. A 1 c above target - Add Metformin B – Type 2 DM – On metformin + DPP-4 i GLP-1 RA SGLT 2 i ASCVD, or high • /Consider very adding highthe TZD IF e. GFR other class (GLP-1 RA CV risk (target organ damage adequate SGLT 2 i) with a proven or multiple risk or factors) CVD benefit If Hb. A 1 c above target • Consider adding the Metformin Monotherapy other class (GLP-1 RA Add. SGLT 2 i SGLT-2 inhibitor SGLT 2 i GLP-1 Ra b DPP-4 i or GLP-1 or RAor or If Hb. A 1 c above target • • • or SGLT 2 i) with proven CVD benefit DPP-4 i if not on GLP-1 RA Basal insulin TZD (not in HF pat) SU TZD or TZD SGLT 2 i or DPP-4 i or GLP-1 Ra If Hb. A 1 c above target If Hb. A above target 1 c Continue with addition of other agents as outlined above Consider SGLT-2 i or GLP-1 RA in patients with: • ASCVD • High/very high risk of CVD including drug-naïve patients If Hb. A 1 c above target Consider the addition of SU OR basal insulin: • Choose later generation SU with lower risk of hypoglycaemia • Consider basal insulin with lower risk of hypoglycaemia Consentino F, et al. Eur Heart J. 2020; 41(2): 255 -323. + Add SGLT 2 inhibitor or GLP-1 RAb • If Hb. A 1 c above target ASCVD, or high / very high CV risk (target organ damage or multiple risk factors)a Metformin Monotherapy ASCVD, or high / very high CV risk (target organ damage or multiple risk factors)a SGLT-2 inhibitor or GLP-1 RA Monotherapyb B – Type 2 DM – On metformin SGLT 2 inhibitor or GLP-1 RA Monotherapyb A – Type 2 DM – Drug naïve patients + A – Type 2 DM – Drug naïve patients DPP-4 i • DPP-4 i if not on GLP-1 RA • Basal insulin • TZD (not in HF pat) • SU - GLP-1 RA SGLT 2 i IF e. GFR adequate TZD If Hb. A 1 c above target SGLT 2 i Continue Metformin Monotherapy or or TZD If Hb. A 1 c above target TZD SGLT 2 i GLP-1 Ra or DPP-4 i or GLP-1 or TZD Ra If Hb. A 1 c above target Continue with addition of other agents as outlined above If Hb. A 1 c above target Consider the addition of SU OR basal insulin: • Choose later generation SU with lower risk of hypoglycaemia • Consider basal insulin with lower risk of hypoglycaemia

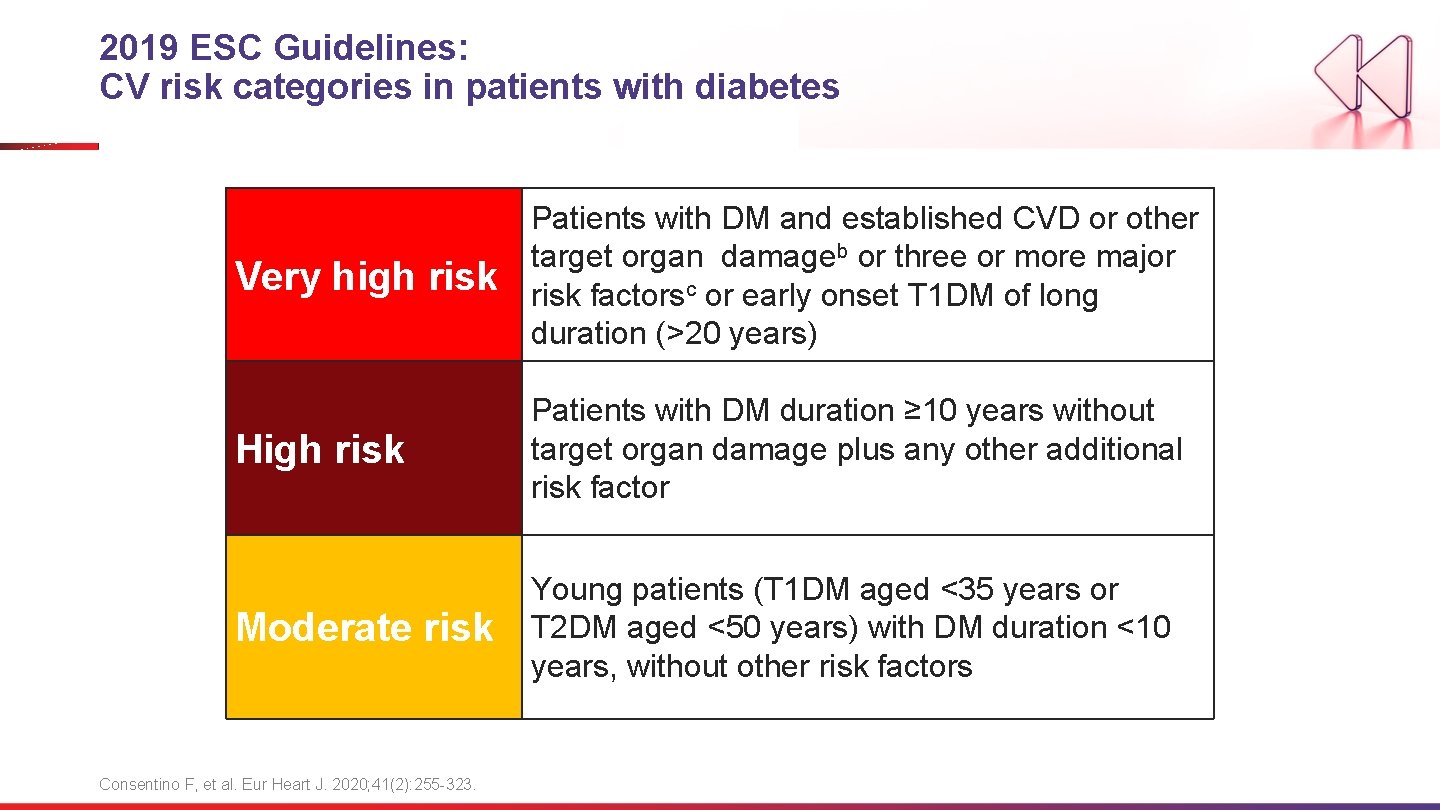
2019 ESC Guidelines: CV risk categories in patients with diabetes Very high risk Patients with DM and established CVD or other target organ damageb or three or more major risk factorsc or early onset T 1 DM of long duration (>20 years) High risk Patients with DM duration ≥ 10 years without target organ damage plus any other additional risk factor Moderate risk Young patients (T 1 DM aged <35 years or T 2 DM aged <50 years) with DM duration <10 years, without other risk factors Consentino F, et al. Eur Heart J. 2020; 41(2): 255 -323.

Discussion Question To reduce the risk of hospitalization for heart failure in a T 2 DM patient with risk factors for ASCVD, which SGLT-2 inhibitor would you consider first? 45

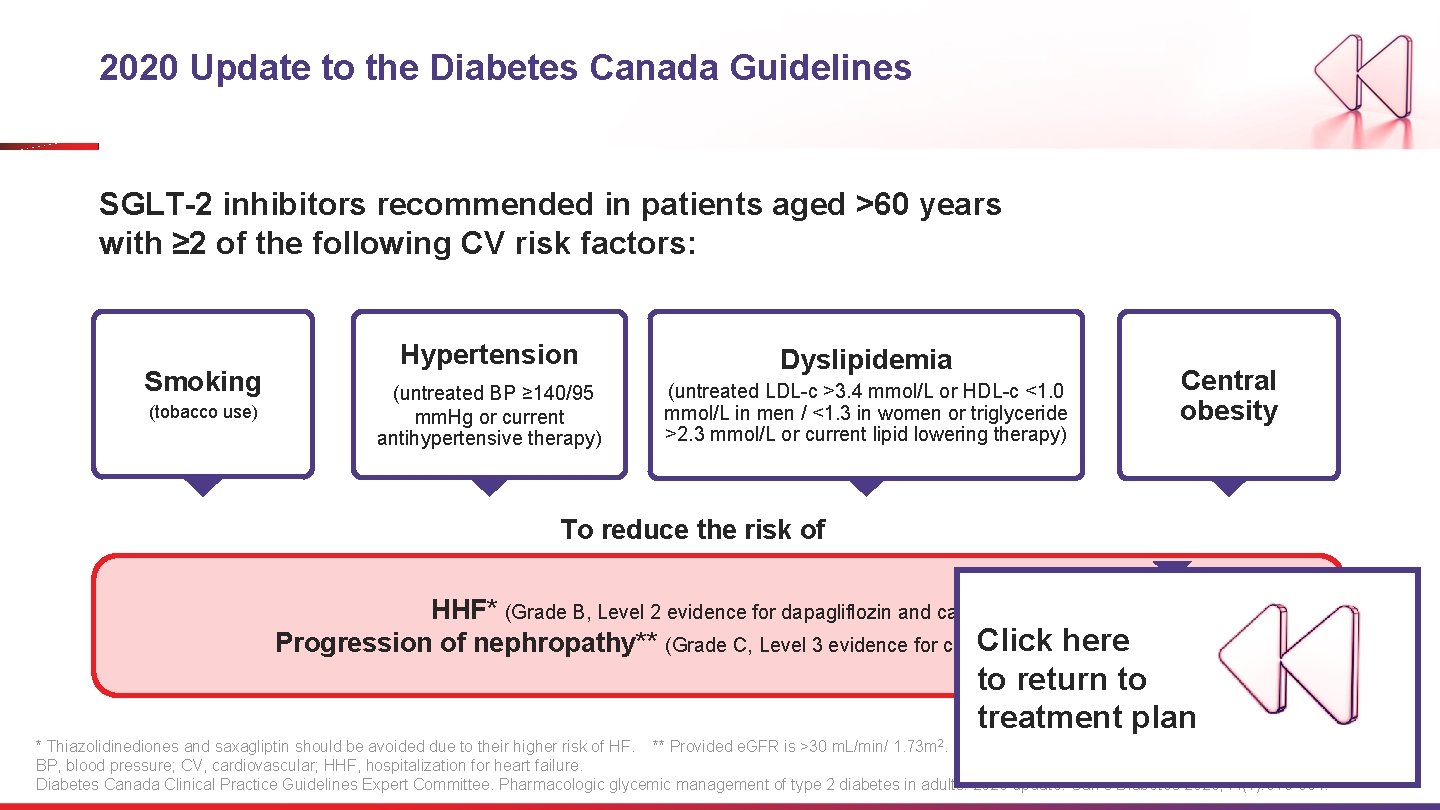
2020 Update to the Diabetes Canada Guidelines SGLT-2 inhibitors recommended in patients aged >60 years with ≥ 2 of the following CV risk factors: Smoking (tobacco use) Hypertension Dyslipidemia (untreated BP ≥ 140/95 mm. Hg or current antihypertensive therapy) (untreated LDL-c >3. 4 mmol/L or HDL-c <1. 0 mmol/L in men / <1. 3 in women or triglyceride >2. 3 mmol/L or current lipid lowering therapy) Central obesity To reduce the risk of HHF* (Grade B, Level 2 evidence for dapagliflozin and canagliflozin). Click and here Progression of nephropathy** (Grade C, Level 3 evidence for canagliflozin dapagliflozin) to return to treatment plan * Thiazolidinediones and saxagliptin should be avoided due to their higher risk of HF. ** Provided e. GFR is >30 m. L/min/ 1. 73 m 2. BP, blood pressure; CV, cardiovascular; HHF, hospitalization for heart failure. Diabetes Canada Clinical Practice Guidelines Expert Committee. Pharmacologic glycemic management of type 2 diabetes in adults: 2020 update. Can J Diabetes 2020; 44(7): 575 -591.

2018 Diabetes Canada CPG – The Essentials 3 ABCDES of Diabetes Care üA • A 1 C – optimal glycemic control (usually ≤ 7%) ü B • BP – optimal blood pressure control (<130/80) ü C • Cholesterol – LDL <2. 0 mmol/L or >50% reduction ü D • Drugs to protect the heart A – ACEi or ARB │ S – Statin │ A – ASA if indicated │SGLT 2 i/GLP-1 RA with demonstrated CV benefit if type 2 DM with CVD and A 1 C not at target üE • Exercise / Healthy Eating ü S • Screening for complications ü S • Smoking cessation ü S • Self-management, stress and other barriers

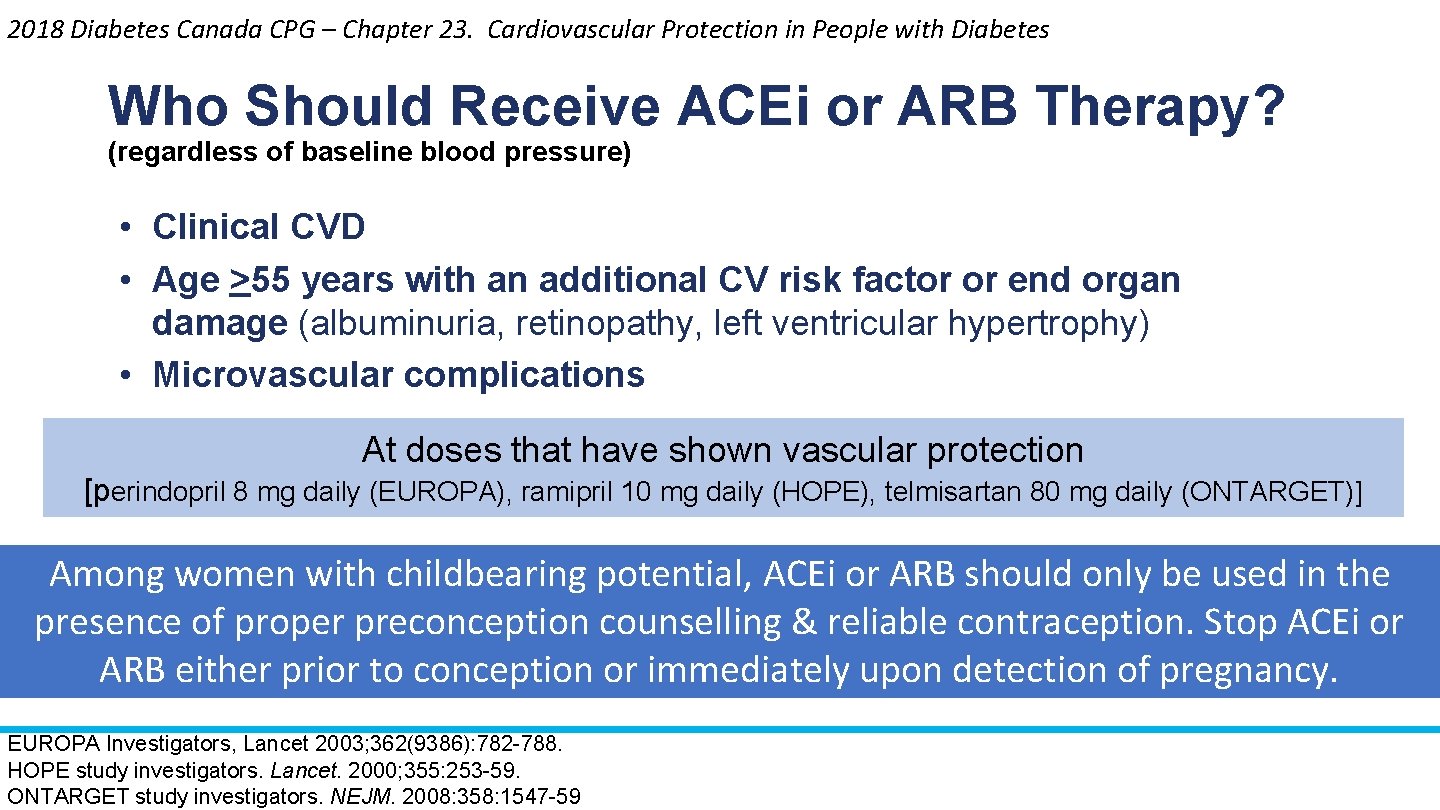
2018 Diabetes Canada CPG – Chapter 23. Cardiovascular Protection in People with Diabetes Who Should Receive ACEi or ARB Therapy? (regardless of baseline blood pressure) • Clinical CVD • Age >55 years with an additional CV risk factor or end organ damage (albuminuria, retinopathy, left ventricular hypertrophy) • Microvascular complications At doses that have shown vascular protection [perindopril 8 mg daily (EUROPA), ramipril 10 mg daily (HOPE), telmisartan 80 mg daily (ONTARGET)] Among women with childbearing potential, ACEi or ARB should only be used in the presence of proper preconception counselling & reliable contraception. Stop ACEi or ARB either prior to conception or immediately upon detection of pregnancy. EUROPA Investigators, Lancet 2003; 362(9386): 782 -788. HOPE study investigators. Lancet. 2000; 355: 253 -59. ONTARGET study investigators. NEJM. 2008: 358: 1547 -59

2018 Diabetes Canada CPG – Chapter 23. Cardiovascular Protection in People with Diabetes Who Should Receive Statins? (regardless of baseline LDL-C) • Clinical CVD or • Age ≥ 40 yrs or • Microvascular complications or • Diabetes >15 yrs duration and age >30 yr or • Warrants therapy based on the 2016 Canadian Cardiovascular Society Guidelines for the Diagnosis and Treatment of Dyslipidemia Among women with childbearing potential, statins should only be used Click here in the presence of proper preconception counselling & reliable to return to contraception. Stop statins prior to conception. treatment plan CVD, cardiovascular disease

2018 Diabetes Canada CPG – The Essentials 3 ABCDES of Diabetes Care üA • A 1 C – optimal glycemic control (usually ≤ 7%) ü B • BP – optimal blood pressure control (<130/80) ü C • Cholesterol – LDL <2. 0 mmol/L or >50% reduction ü D • Drugs to protect the heart A – ACEi or ARB │ S – Statin │ A – ASA if indicated │SGLT 2 i/GLP-1 RA with demonstrated CV benefit if type 2 DM with CVD and A 1 C not at target üE • Exercise / Healthy Eating ü S • Screening for complications ü S • Smoking cessation ü S • Self-management, stress and other barriers

2018 Diabetes Canada CPG – Chapter 11. Nutrition Checklist ü REFER for nutrition counseling by a registered dietitian ü FOLLOW Eating Well with Canada’s Food Guide ü INDIVIDUALIZE dietary advice based on preferences and treatment goals ü CHOOSE low glycemic index carbohydrate food sources

2018 Diabetes Canada CPG – Chapter 10. Physical Activity Checklist ü TRY TO DO a minimum of 150 minutes of moderate-to vigorousintensity aerobic exercise per week. ü INCLUDE resistance exercise (strength training) ≥ 2 times a week. ü SET physical activity goals and INVOLVE a multi-disciplinary team if available. ü MINIMIZE uninterrupted sedentary time.

Discussion Question What percentage of your patients with T 2 DM do you believe exercise minimum of 150 minutes of moderate-to vigorous-intensity aerobic exercise per week? 53

Discussion Question What percentage of your patients with T 2 DM receive a regular eye exam at 1 -2 -year intervals to screen for retinopathy? 54

2018 Diabetes Canada CPG – Chapter 30. Retinopathy Screening for Retinopathy

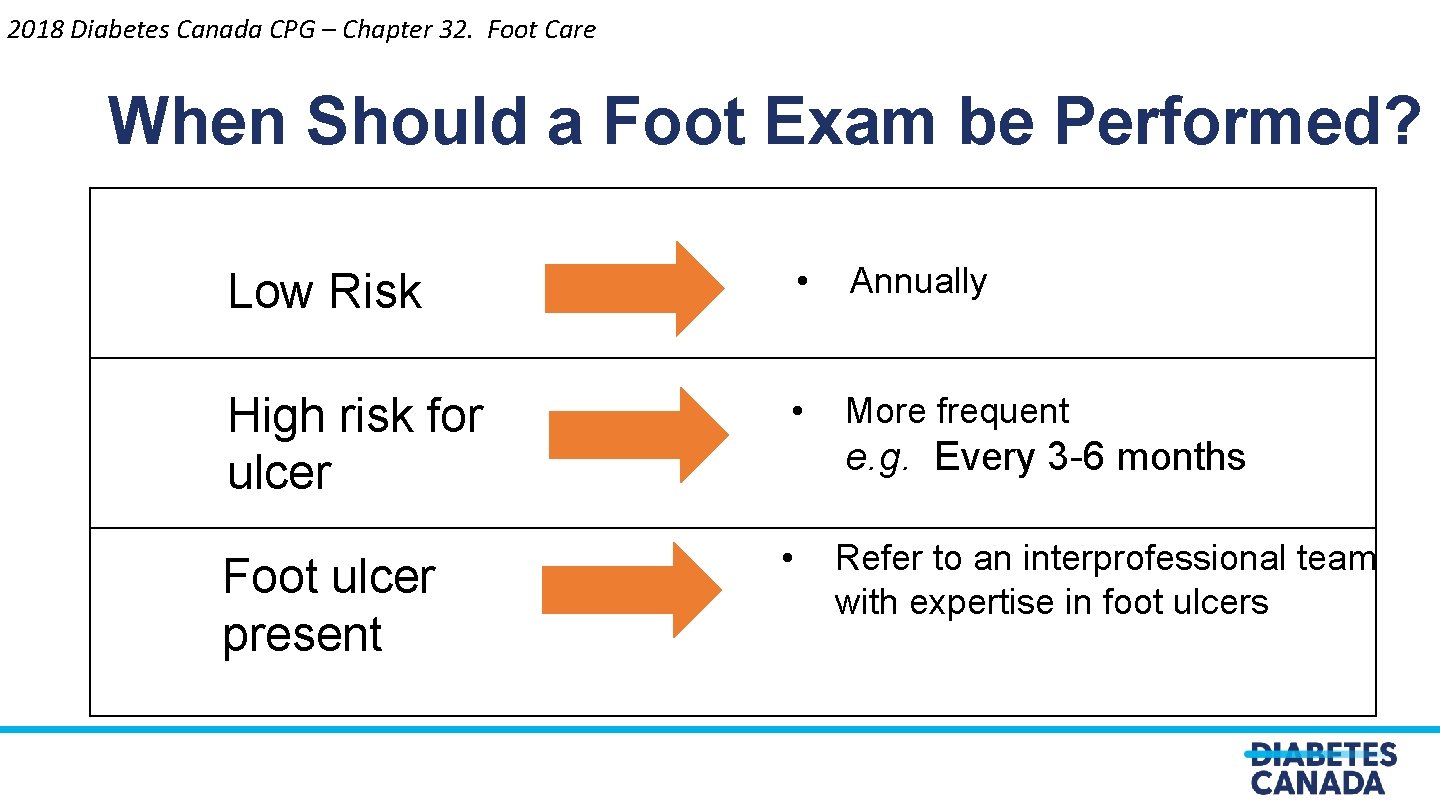
2018 Diabetes Canada CPG – Chapter 32. Foot Care When Should a Foot Exam be Performed? Low Risk • Annually High risk for ulcer • More frequent Foot ulcer present • e. g. Every 3 -6 months Refer to an interprofessional team with expertise in foot ulcers

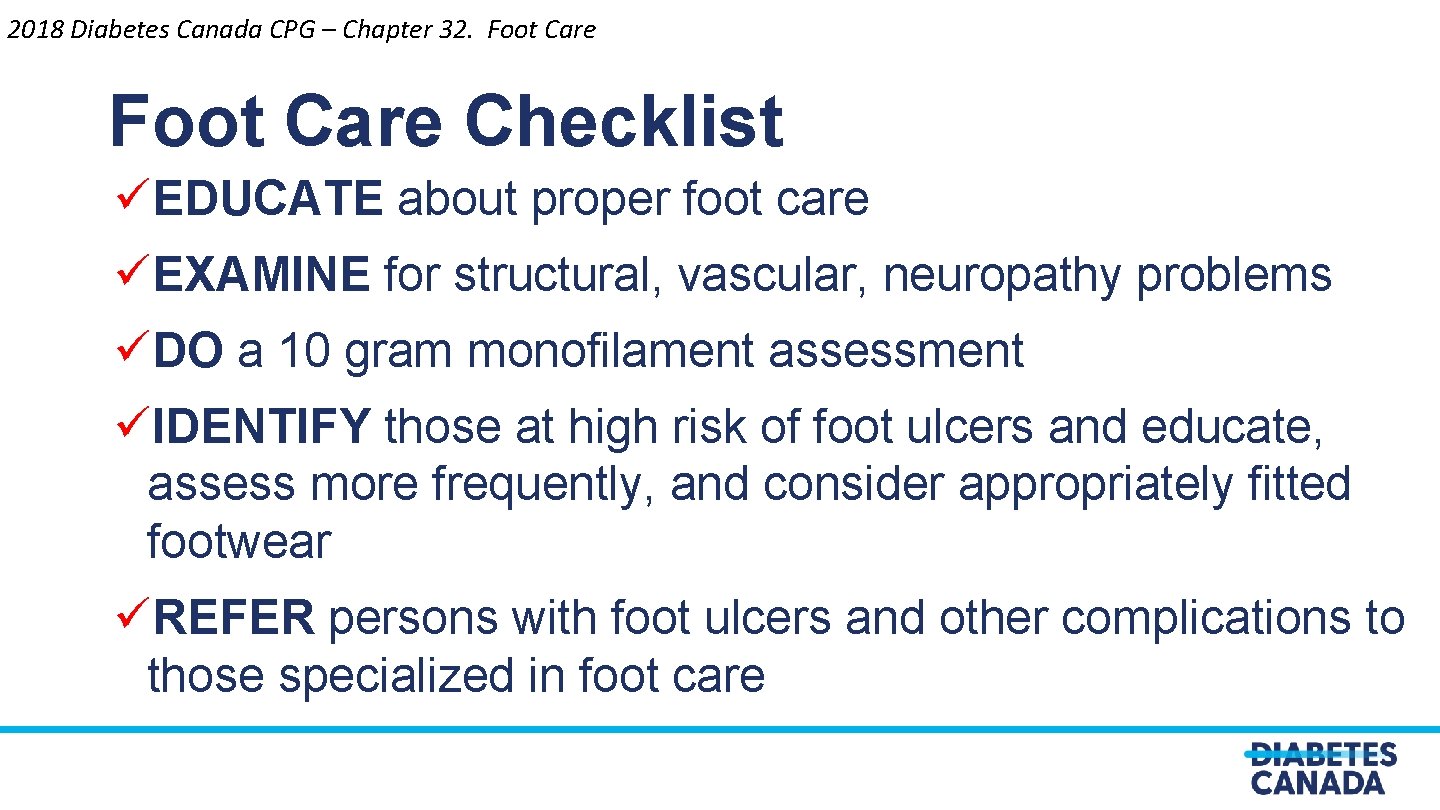
2018 Diabetes Canada CPG – Chapter 32. Foot Care Checklist üEDUCATE about proper foot care üEXAMINE for structural, vascular, neuropathy problems üDO a 10 gram monofilament assessment üIDENTIFY those at high risk of foot ulcers and educate, assess more frequently, and consider appropriately fitted footwear üREFER persons with foot ulcers and other complications to those specialized in foot care

Discussion Question In a 45 -year-old patient with T 2 DM and no CV risk factors, when do you screen with an ECG? 58

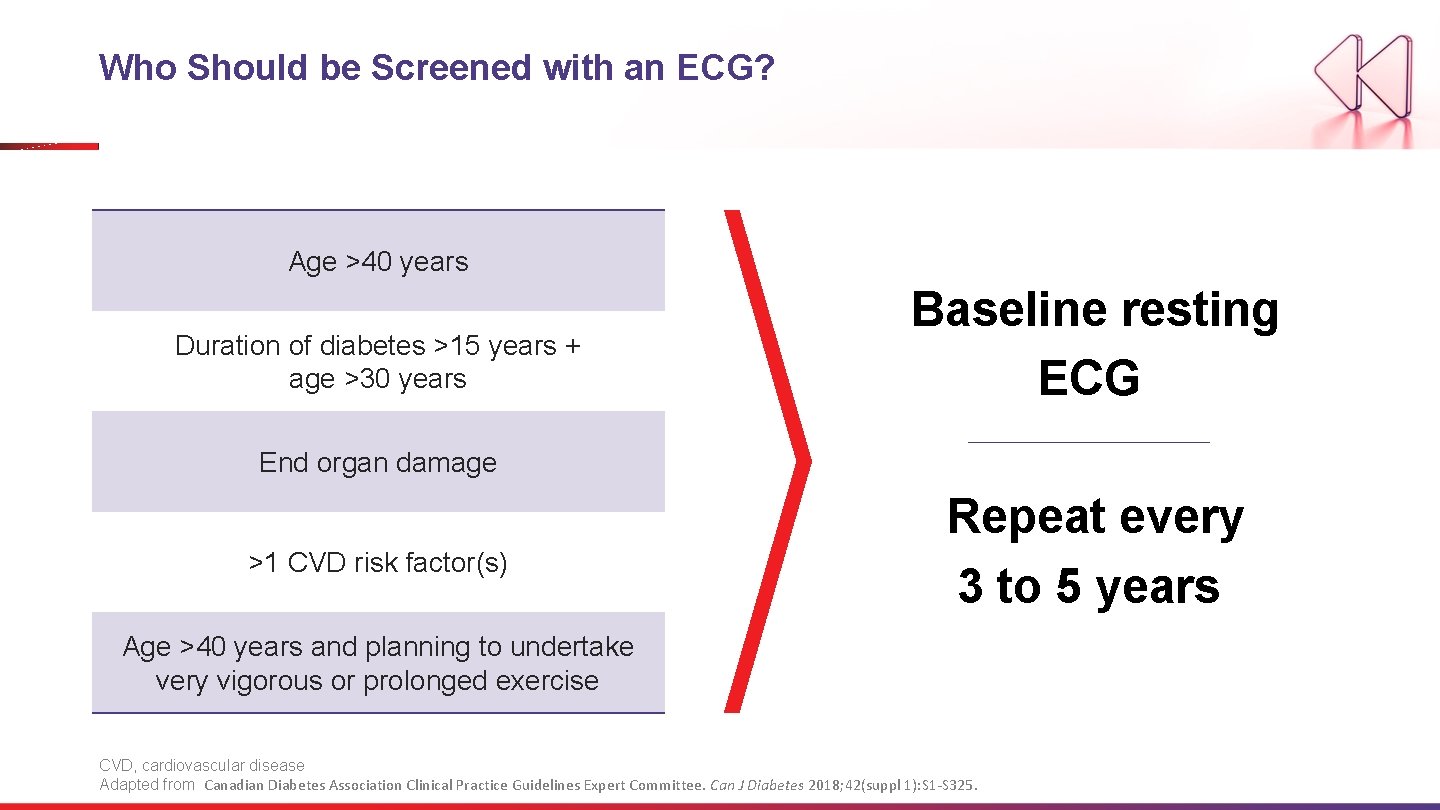
Who Should be Screened with an ECG? Age >40 years Duration of diabetes >15 years + age >30 years Baseline resting ECG End organ damage >1 CVD risk factor(s) Repeat every 3 to 5 years Age >40 years and planning to undertake very vigorous or prolonged exercise CVD, cardiovascular disease Adapted from Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Can J Diabetes 2018; 42(suppl 1): S 1 -S 325.

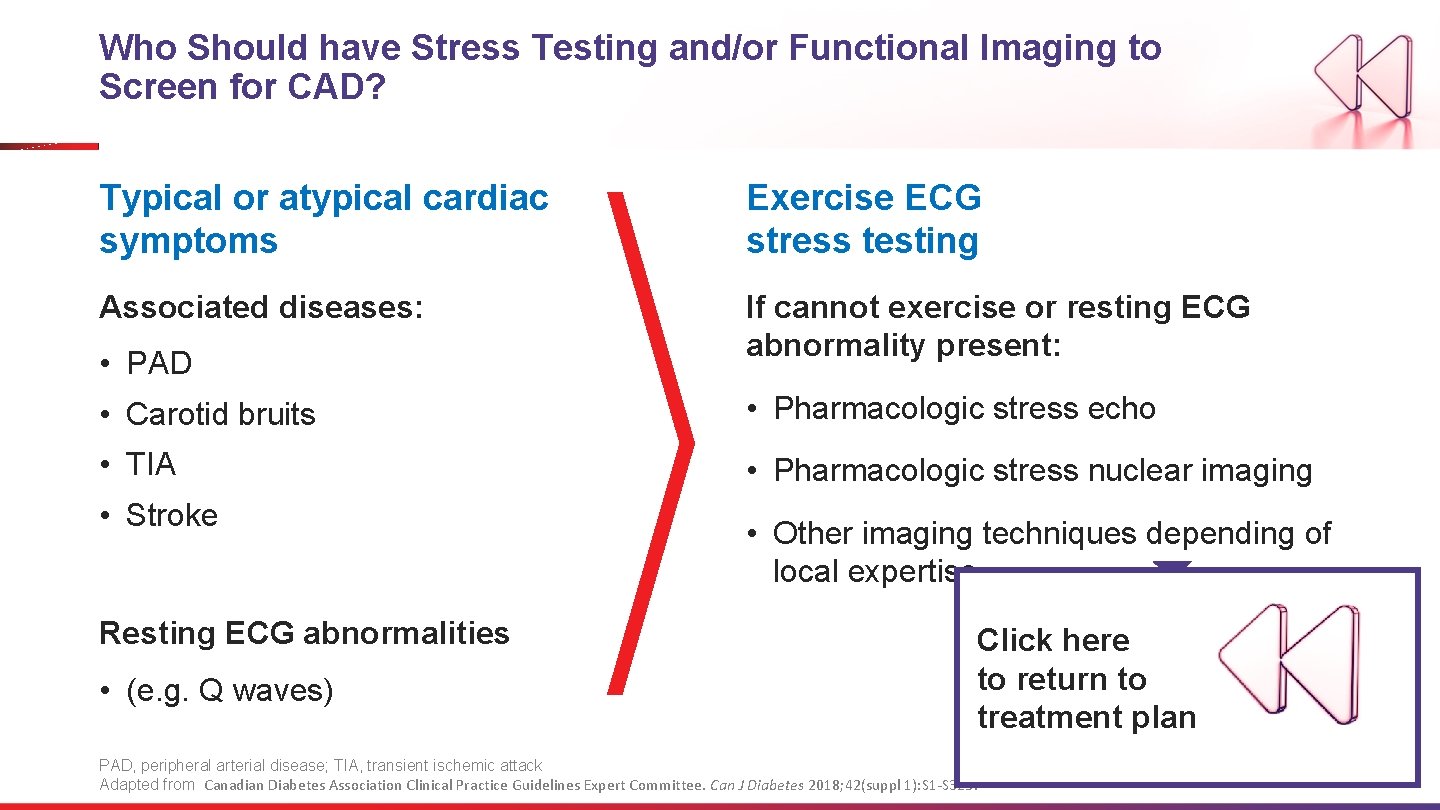
Who Should have Stress Testing and/or Functional Imaging to Screen for CAD? Typical or atypical cardiac symptoms Exercise ECG stress testing Associated diseases: If cannot exercise or resting ECG abnormality present: • PAD • Carotid bruits • Pharmacologic stress echo • TIA • Pharmacologic stress nuclear imaging • Stroke Resting ECG abnormalities • (e. g. Q waves) • Other imaging techniques depending of local expertise Click here to return to treatment plan PAD, peripheral arterial disease; TIA, transient ischemic attack Adapted from Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Can J Diabetes 2018; 42(suppl 1): S 1 -S 325.