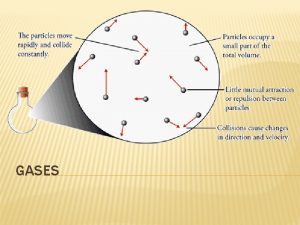
Measuring Gases College Chemistry Atmospheric Pressure The atmosphere







- Slides: 13

Measuring Gases College Chemistry

Atmospheric Pressure • The atmosphere around us exerts pressure on us – equal to the pressure we exert on it – If the atmosphere exerts more pressure we would be crushed (scuba diving) – If the atmosphere exerts less pressure we would explode (spray can in the summer)

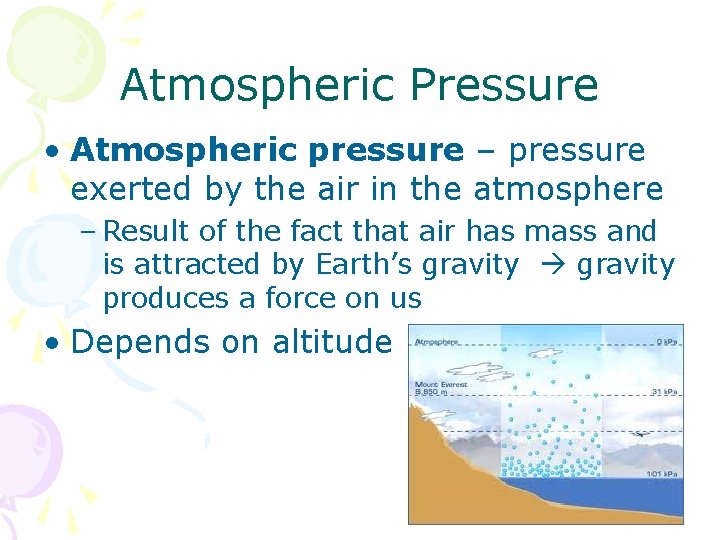
Atmospheric Pressure • Atmospheric pressure – pressure exerted by the air in the atmosphere – Result of the fact that air has mass and is attracted by Earth’s gravity produces a force on us • Depends on altitude

Atmospheric Pressure • Varies with altitude – lower the altitude, the longer and heavier the column of air above the area of earth is • Atmospheric pressure also varies with amount of water in the air – The heavier the air is the LESS water is in the air – Water vapor is NOT heavier than air!!!!

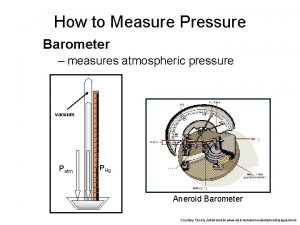
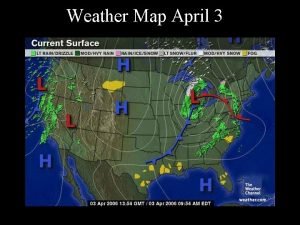
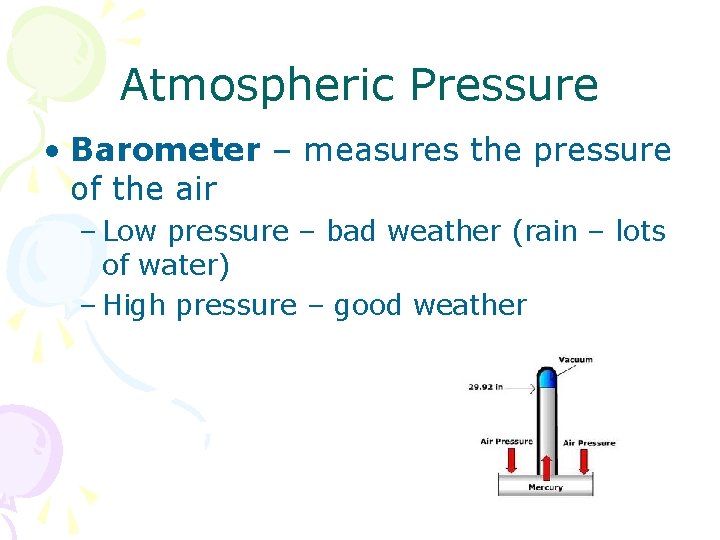
Atmospheric Pressure • Barometer – measures the pressure of the air – Low pressure – bad weather (rain – lots of water) – High pressure – good weather

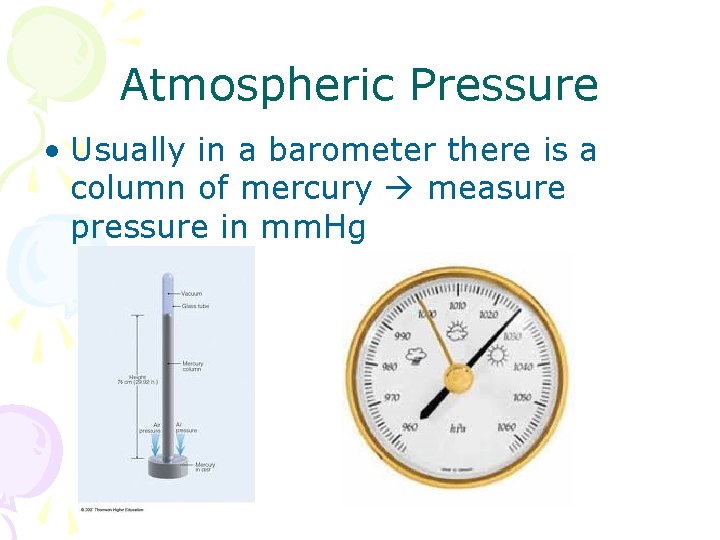
Atmospheric Pressure • Usually in a barometer there is a column of mercury measure pressure in mm. Hg

Units of Pressure • Right now you have 1 atmosphere (atm) of pressure on you • Pressure is also measured in Pascals, kilopascals, mm. Hg, torr, and bar • Learn and memorize these conversion factors!!!

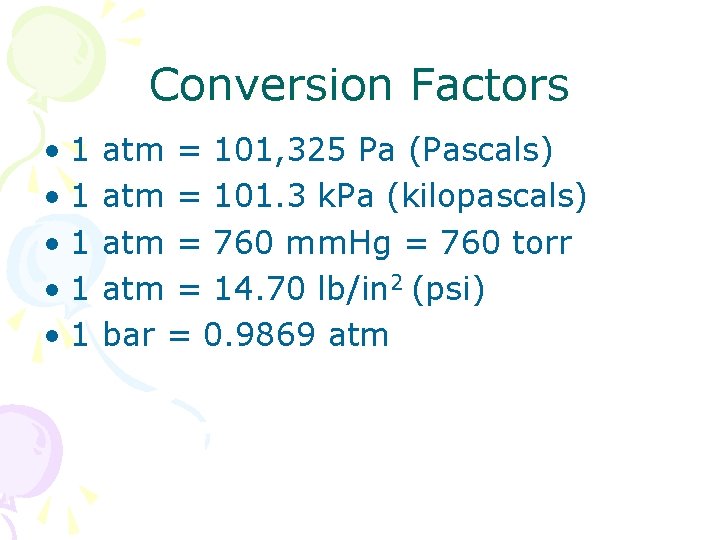
Conversion Factors • 1 • 1 • 1 atm = 101, 325 Pa (Pascals) atm = 101. 3 k. Pa (kilopascals) atm = 760 mm. Hg = 760 torr atm = 14. 70 lb/in 2 (psi) bar = 0. 9869 atm

Example • The column of mercury in a barometer is 745 mm above the bottom. What is the atmospheric pressure in Pascals? – Use dimensional analysis – 745 mm. Hg x (101, 325 Pa/760 mm. Hg) = 99, 300 Pa

Another Example • The air pressure inside the cabin of an airplane is 8. 3 lb/in 2. What is the pressure in atmospheric units? – 8. 3 lb/in 2 x (1 atm / 14. 70 lb/in 2) = 0. 56 atm

Enclosed Gases • If a gas is open to the atmosphere, some of the gas will escape • Eventually the inside gas and the atmosphere will reach equilibrium and have the same value • In an enclosed gas, the pressure inside can be different than atmospheric pressure

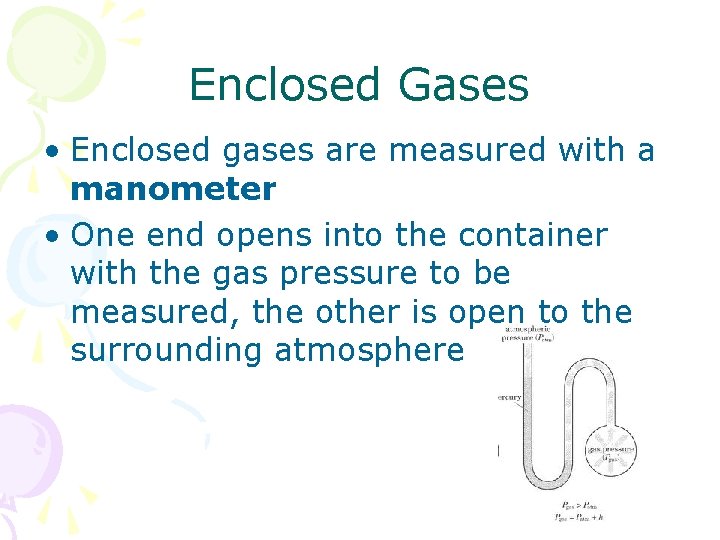
Enclosed Gases • Enclosed gases are measured with a manometer • One end opens into the container with the gas pressure to be measured, the other is open to the surrounding atmosphere

STP • Behavior of gas is STRONGLY dependent on pressure and temperature • Because of this, we usually talk about gases at STP – standard temperature and pressure – 273 K (freezing point of water, 0⁰C) and 1 atmosphere
 Atmospheric pressure at different altitudes
Atmospheric pressure at different altitudes Bernoulli's equation derivation
Bernoulli's equation derivation Sea level barometer
Sea level barometer Low atmospheric pressure
Low atmospheric pressure Atmospheric pressure in complete denture
Atmospheric pressure in complete denture Usairnet temperature map
Usairnet temperature map Atmospheric chemistry suite
Atmospheric chemistry suite Lightning elves
Lightning elves What is the mixture of several gases
What is the mixture of several gases Gases on earth's atmosphere
Gases on earth's atmosphere Do gases exert pressure on whatever surrounds them
Do gases exert pressure on whatever surrounds them Do gases exert pressure on whatever surrounds them
Do gases exert pressure on whatever surrounds them Chapter 11 review gases section 1
Chapter 11 review gases section 1 Indirect method of measuring blood pressure
Indirect method of measuring blood pressure