MATTER AND DENSITY DENSITY Density is a measure


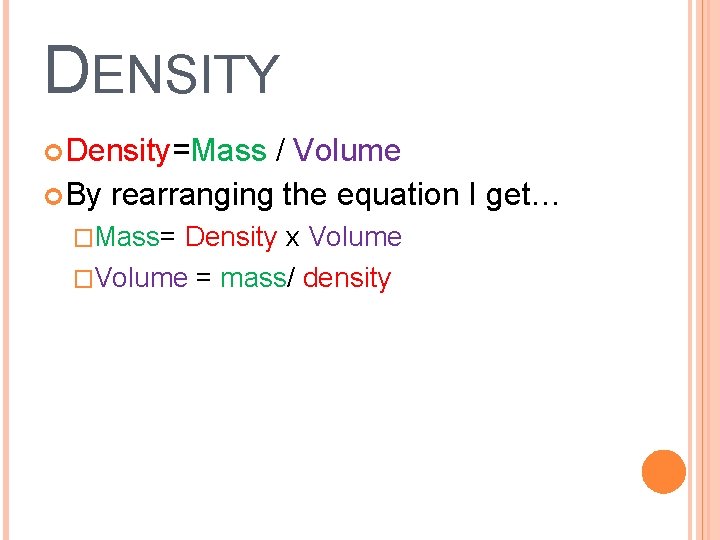




- Slides: 12

MATTER AND DENSITY

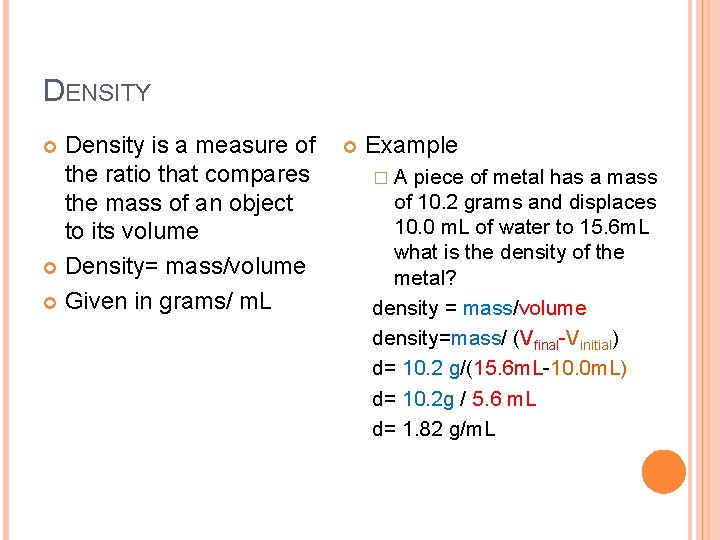
DENSITY Density is a measure of the ratio that compares the mass of an object to its volume Density= mass/volume Given in grams/ m. L Example �A piece of metal has a mass of 10. 2 grams and displaces 10. 0 m. L of water to 15. 6 m. L what is the density of the metal? density = mass/volume density=mass/ (Vfinal-Vinitial) d= 10. 2 g/(15. 6 m. L-10. 0 m. L) d= 10. 2 g / 5. 6 m. L d= 1. 82 g/m. L

All substances have a unique and individual density. � Think oil and water � Why does oil “float” on water?
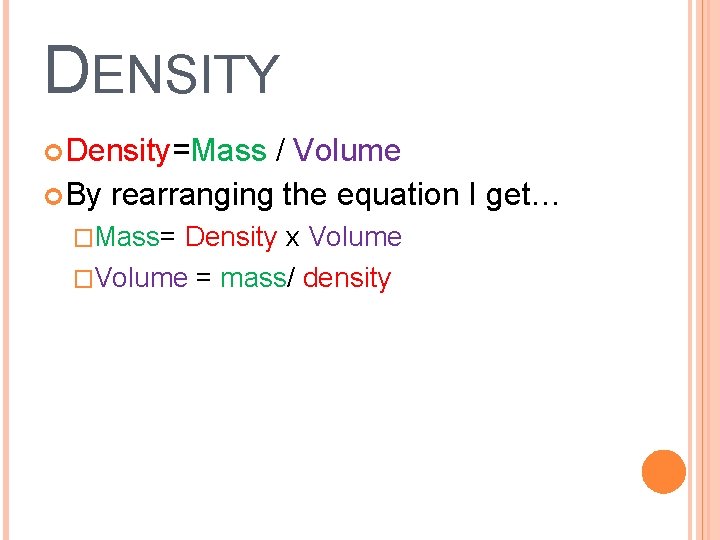
DENSITY Density=Mass / Volume By rearranging the equation I get… �Mass= Density x Volume �Volume = mass/ density

PROPERTIES OF MATTER Matter that has a uniform and unchanging composition is called a substance �Examples of substances Water (H 2 O) Salt (Na. Cl) Baking soda (Na. CO 2 H)

PHYSICAL PROPERTIES OF MATTER Physical property is one that can be observed or measured without changing the samples composition Physical properties � Color � State of matter � Melting point � Boiling point � Density � Taste � Odor � Hardness

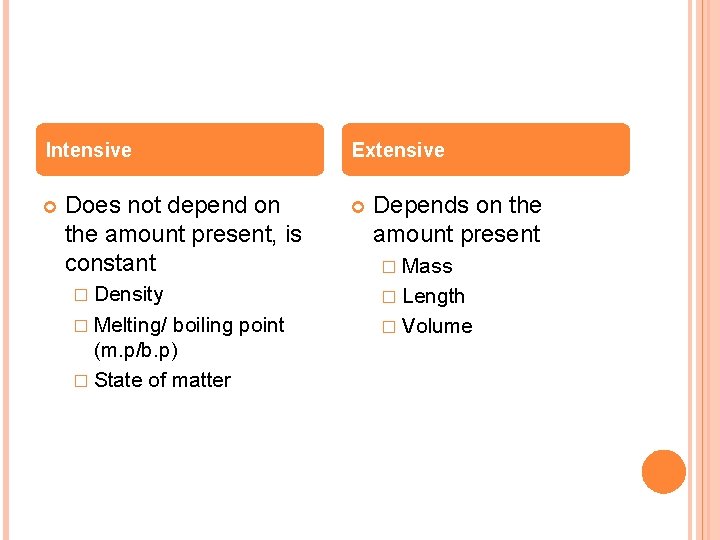
Intensive Does not depend on the amount present, is constant Extensive Depends on the amount present � Mass � Density � Length � Melting/ � Volume boiling point (m. p/b. p) � State of matter

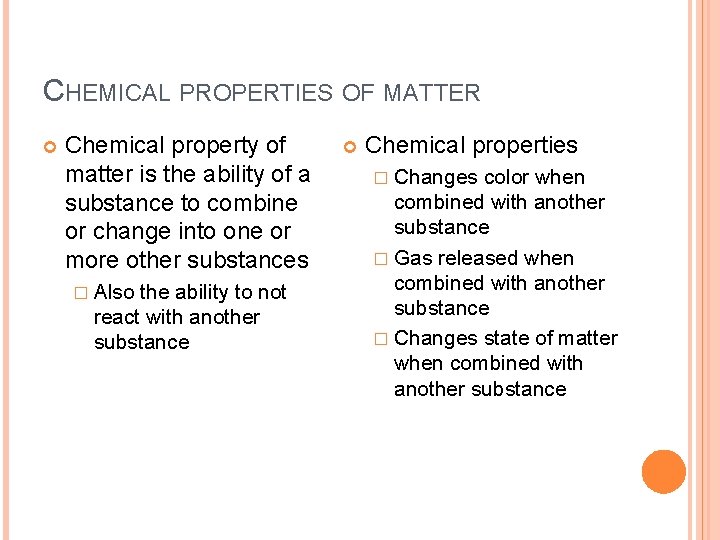
CHEMICAL PROPERTIES OF MATTER Chemical property of matter is the ability of a substance to combine or change into one or more other substances � Also the ability to not react with another substance Chemical properties � Changes color when combined with another substance � Gas released when combined with another substance � Changes state of matter when combined with another substance

CHEMICAL REACTIONS

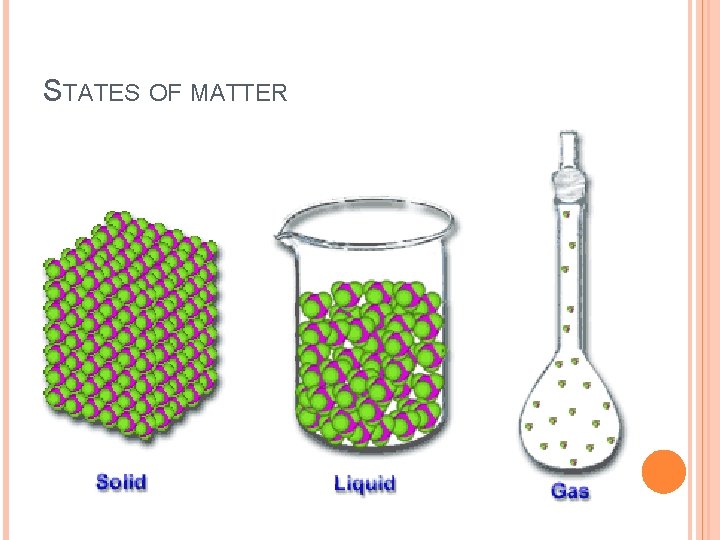
STATES OF MATTER SOLID � has a definite shape and volume, particles are tightly packed, can not be compressed into a smaller volume LIQUID � has a definite volume, but takes the shape of its container, particles are not rigidly held but able to move past each other GAS � conforms to the volume and shape of its container, takes up the entire volume of the container, particles are not tightly packed

STATES OF MATTER

Gas Vapor Gas is a compound or element that is a gas at room temperature � Ex Air is a gas A vapor is the gaseous state of a substance that is solid or liquid at room temperature � Ex. Steam is water vapor