Density and Pressure Density and Pressure Define density








- Slides: 20

Density and Pressure

Density and Pressure Define density Understand relative density Define and use the term pressure Learn the instruments used for measuring pressure • Brownian Motion and the Kinetic Model • Pressure in a Gas • Melting, Boiling and Evaporation • •

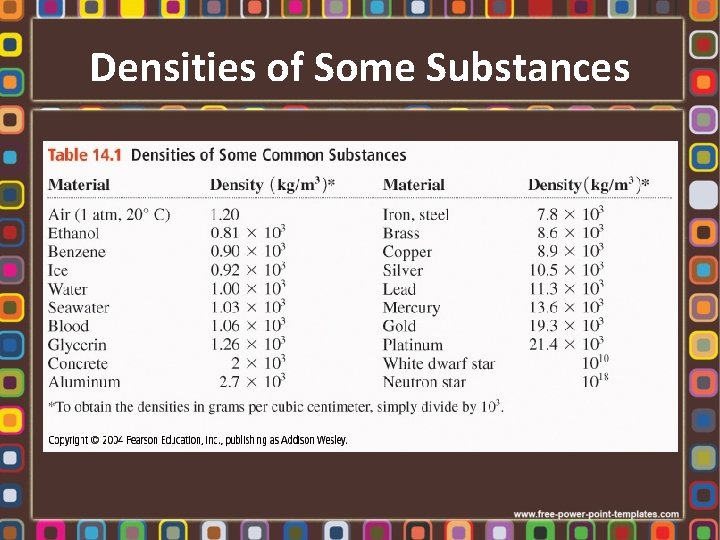
Density • • Mass per unit volume Units: kgm-3, gcm-3, gml-1 Density is changes with temperature Relative Density – Density of a substance divided density of water – Dimensionless – Density of water is 1000 kgm-3

Densities of Some Substances

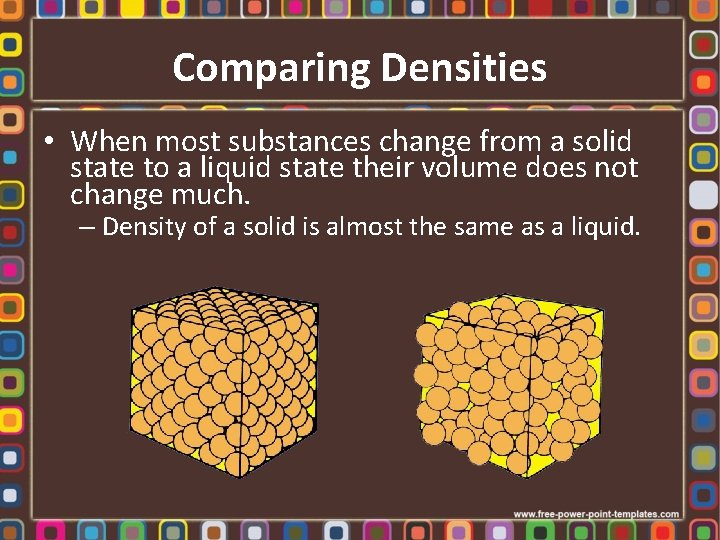
Comparing Densities • When most substances change from a solid state to a liquid state their volume does not change much. – Density of a solid is almost the same as a liquid.

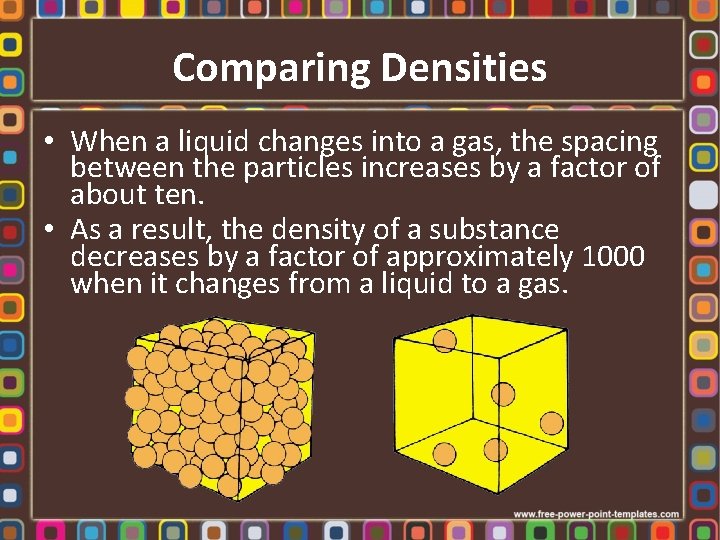
Comparing Densities • When a liquid changes into a gas, the spacing between the particles increases by a factor of about ten. • As a result, the density of a substance decreases by a factor of approximately 1000 when it changes from a liquid to a gas.

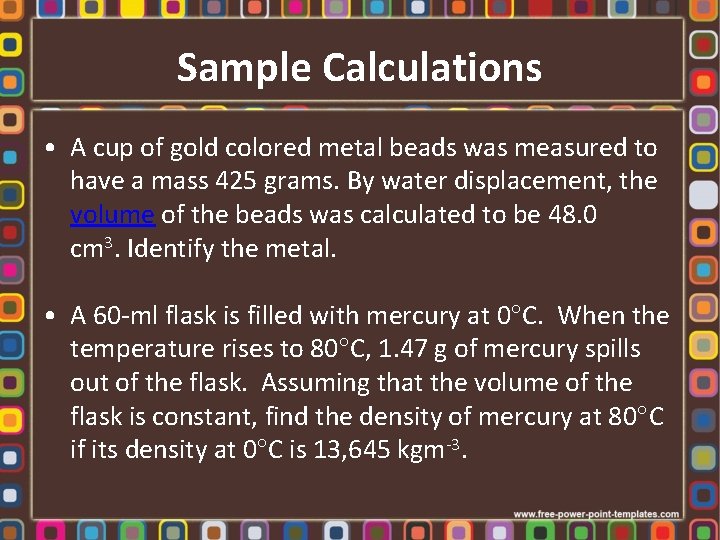
Sample Calculations • A cup of gold colored metal beads was measured to have a mass 425 grams. By water displacement, the volume of the beads was calculated to be 48. 0 cm 3. Identify the metal. • A 60 -ml flask is filled with mercury at 0 C. When the temperature rises to 80 C, 1. 47 g of mercury spills out of the flask. Assuming that the volume of the flask is constant, find the density of mercury at 80 C if its density at 0 C is 13, 645 kgm-3.

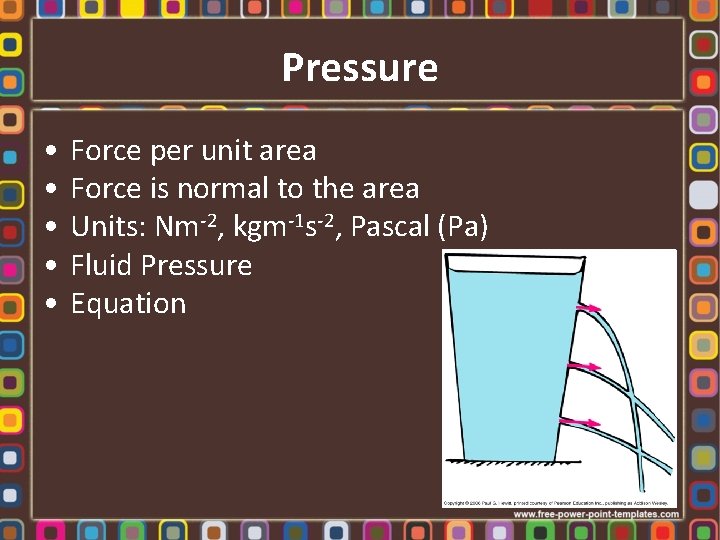
Pressure • • • Force per unit area Force is normal to the area Units: Nm-2, kgm-1 s-2, Pascal (Pa) Fluid Pressure Equation

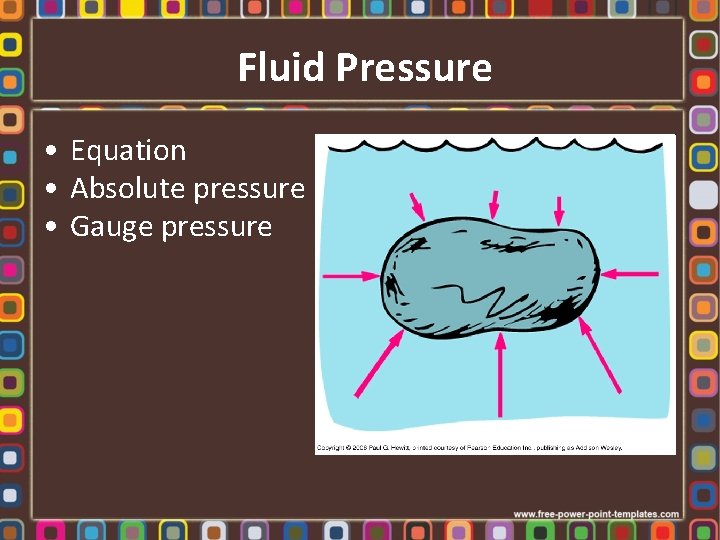
Fluid Pressure • Equation • Absolute pressure • Gauge pressure

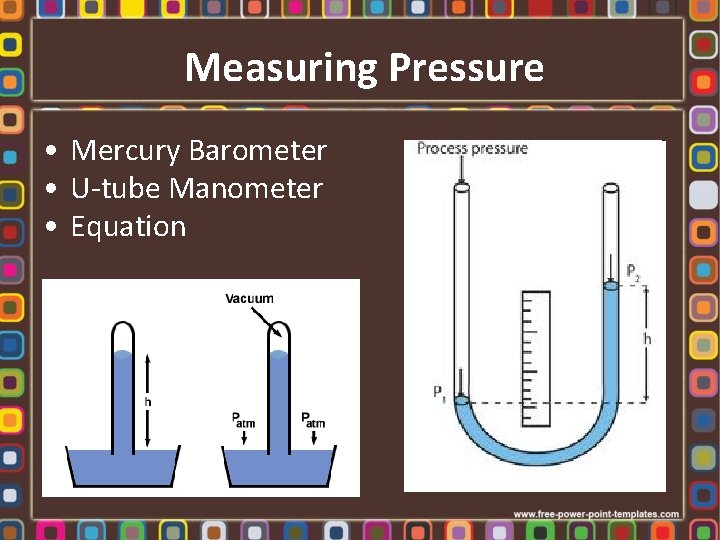
Measuring Pressure • Mercury Barometer • U-tube Manometer • Equation

Sample Calculations • A U-tube contains liquid of unknown density. An oil of density 800 kgm-3 is poured into one arm of the tube until the oil column is 12 cm high. The oil-air interface is 5. 0 cm above the liquid level in the other arm of the tube. Find the density of the liquid. • P 400, #22. 18 (a)

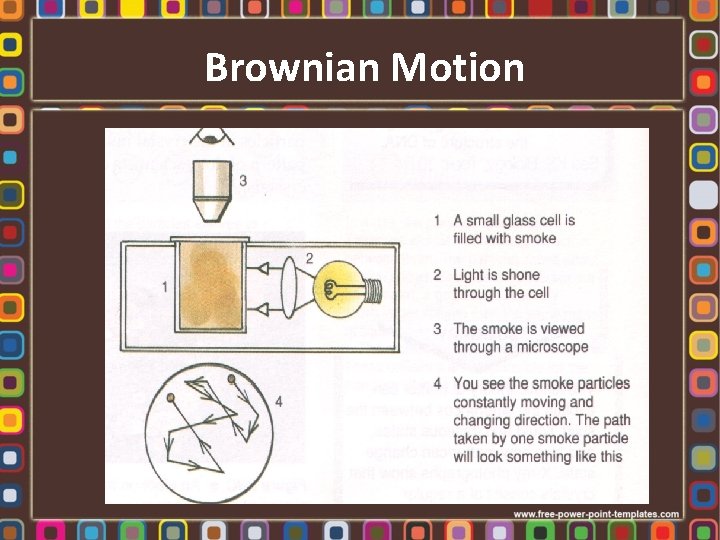
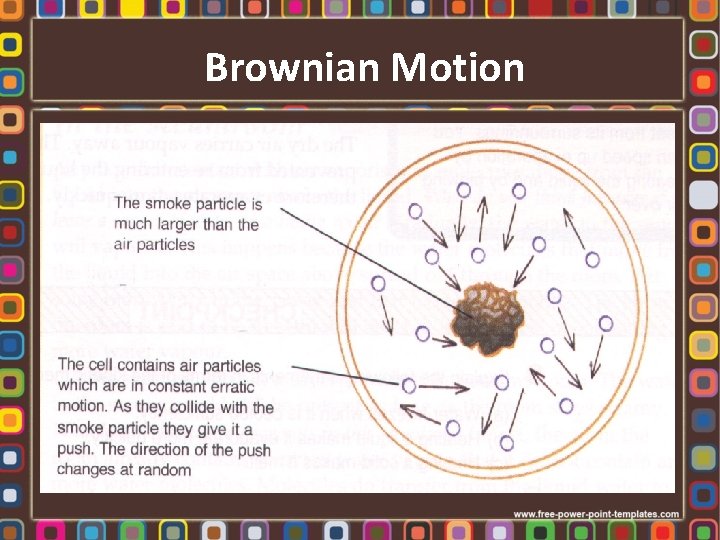
Brownian Motion

Brownian Motion

Brownian Motion Video 1

Brownian Motion Video 2

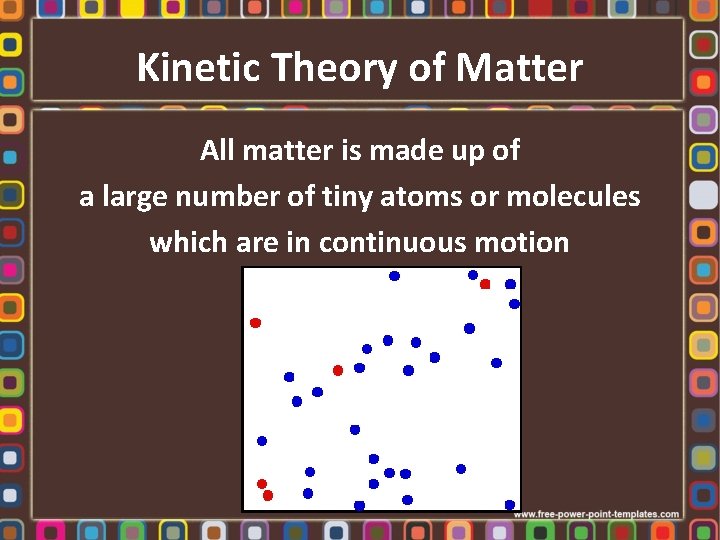
Kinetic Theory of Matter All matter is made up of a large number of tiny atoms or molecules which are in continuous motion

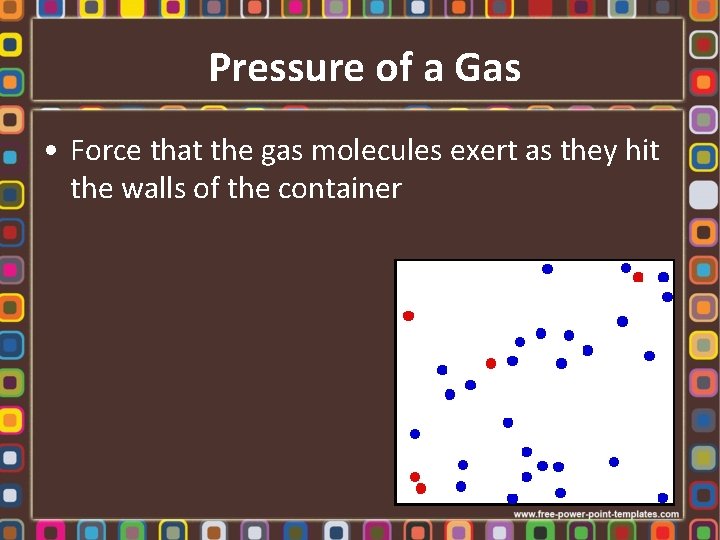
Pressure of a Gas • Force that the gas molecules exert as they hit the walls of the container

Energies and Molecules • Potential Energy • Distance between molecules • Kinetic Energy • Related to temperature of the substance • Internal Energy • The sum of the kinetic and potential energies of a substance.

Melting and Boiling • Heat is added to break bonds between molecules • Some part of the energy is used to work against the environment • Both occur at a specific constant temperature • Let us look at the energies involved

Boiling and Evaporation • Both involve changes from liquid to gas • Boiling occurs at a specific temperature • Evaporation occurs at any temperature • Boiling occurs inside the liquid • Evaporation occurs at the surface of the liquid