Marcos de Lima MD Stem Cell Transplantation Program
























- Slides: 36

异基因干�胞移植后�防复�的 �先和�持治�策略 Marcos de Lima, MD Stem Cell Transplantation Program Case Western Reserve University Hospitals Seidman Cancer Center Cleveland - OH

Disclosures Celgene : research grant





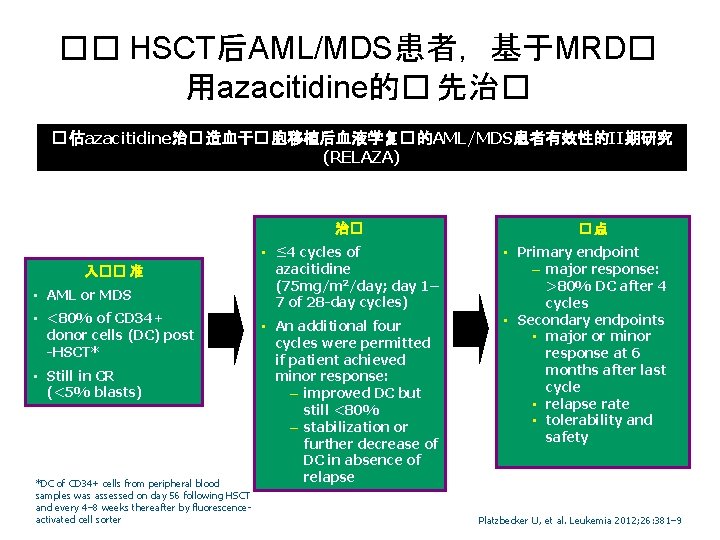
�� HSCT后AML/MDS患者,基于MRD� 用azacitidine的� 先治� � 估azacitidine治� 造血干� 胞移植后血液学复� 的AML/MDS患者有效性的II期研究 (RELAZA) 治� 入�� 准 • AML or MDS • <80% of CD 34+ donor cells (DC) post -HSCT* • Still in CR (<5% blasts) *DC of CD 34+ cells from peripheral blood samples was assessed on day 56 following HSCT and every 4– 8 weeks thereafter by fluorescenceactivated cell sorter • ≤ 4 cycles of azacitidine (75 mg/m 2/day; day 1– 7 of 28 -day cycles) • An additional four cycles were permitted if patient achieved minor response: – improved DC but still <80% – stabilization or further decrease of DC in absence of relapse �点 • Primary endpoint – major response: >80% DC after 4 cycles • Secondary endpoints • major or minor response at 6 months after last cycle • relapse rate • tolerability and safety Platzbecker U, et al. Leukemia 2012; 26: 381– 9

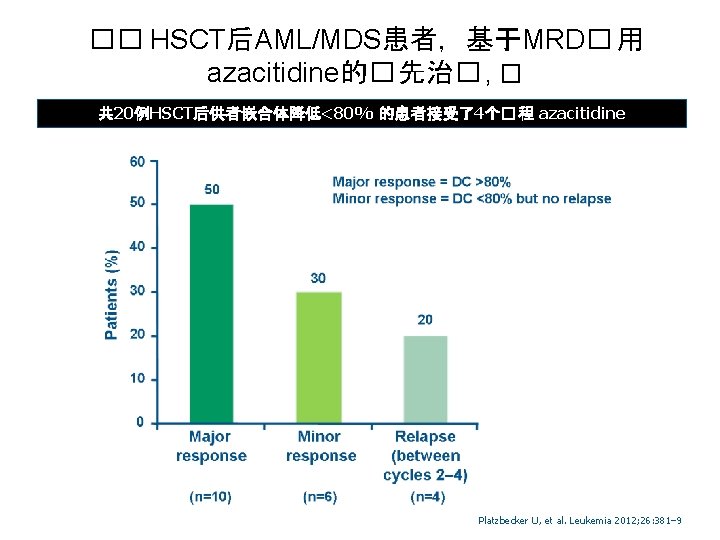
�� HSCT后AML/MDS患者,基于MRD� 用 azacitidine的� 先治� , � 共 20例HSCT后供者嵌合体降低<80% 的患者接受了4个� 程 azacitidine Platzbecker U, et al. Leukemia 2012; 26: 381– 9






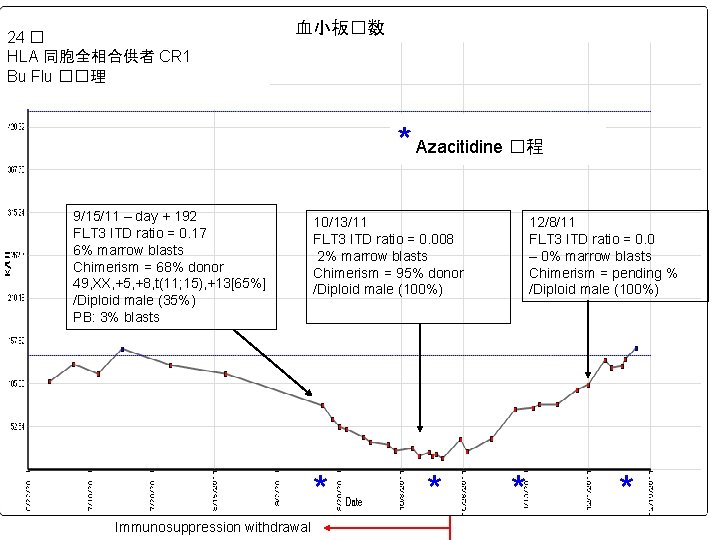
24 � HLA 同胞全相合供者 CR 1 Bu Flu ��理 血小板�数 * Azacitidine �程 9/15/11 – day + 192 FLT 3 ITD ratio = 0. 17 6% marrow blasts Chimerism = 68% donor 49, XX, +5, +8, t(11; 15), +13[65%] /Diploid male (35%) PB: 3% blasts 10/13/11 FLT 3 ITD ratio = 0. 008 2% marrow blasts Chimerism = 95% donor /Diploid male (100%) * Immunosuppression withdrawal * 12/8/11 FLT 3 ITD ratio = 0. 0 – 0% marrow blasts Chimerism = pending % /Diploid male (100%) * *


去甲基化�物 �典的�想 : 异基因HSCT者 (�用 Bu. Cy): - decitabine 400 mg / m 2, 600 mg / m 2 and 800 mg / m 2 de Lima. Cancer. 2003 Mar 1; 97(5): 1242 -7. 低�量�期�用 decitabine 治�血液�瘤的I 期研究. 5 -20 mg/m 2 5 天/周 x 2 周 15 mg/m 2 最佳 - 30 次 < MTD Issa JP et al. Blood. 2004 Mar 1; 103(5): 1635 -40. �用持��� - 越�越好. �量 – 低者好、相同或差 ? ?


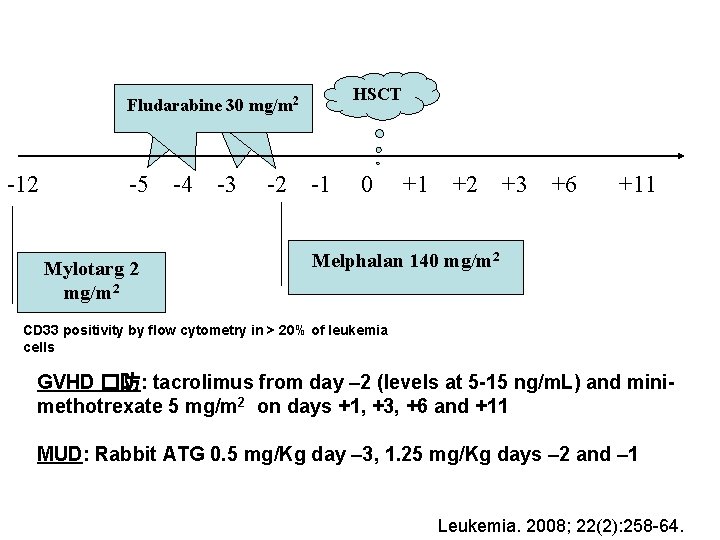
2 2 2 Fludarabine 30 mg/m 2 Fludarabine 30 mg/m HSCT -12 -5 -4 -3 -2 -1 0 +1 +2 +3 +6 +11 Mylotarg 2 mg/m 2 Melphalan 140 mg/m 2 CD 33 positivity by flow cytometry in > 20% of leukemia cells GVHD �防: tacrolimus from day – 2 (levels at 5 -15 ng/m. L) and minimethotrexate 5 mg/m 2 on days +1, +3, +6 and +11 MUD: Rabbit ATG 0. 5 mg/Kg day – 3, 1. 25 mg/Kg days – 2 and – 1 Leukemia. 2008; 22(2): 258 -64.


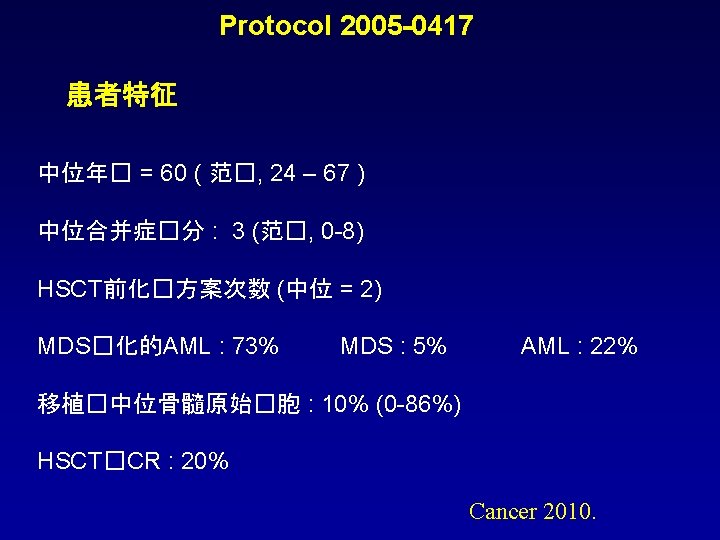
Azacitidine�持治� – MTD : 32 mg/m 2 Median follow-up = 16 months 50% �无关供者 HSCT �用了96 个�程 – 安全.

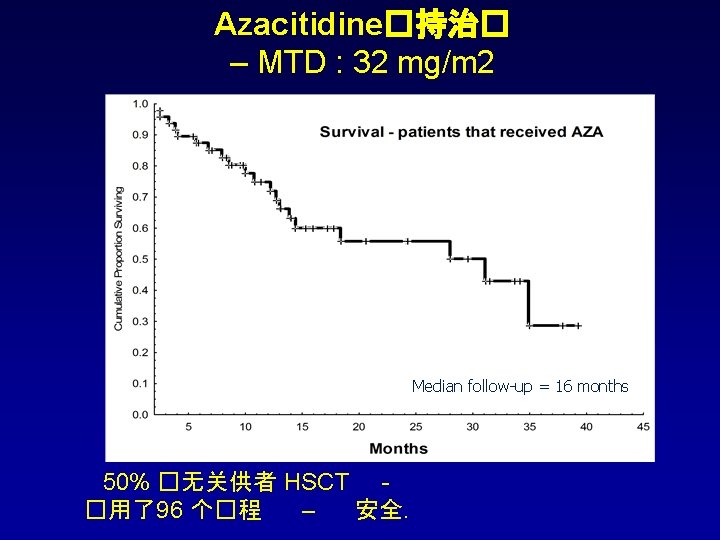
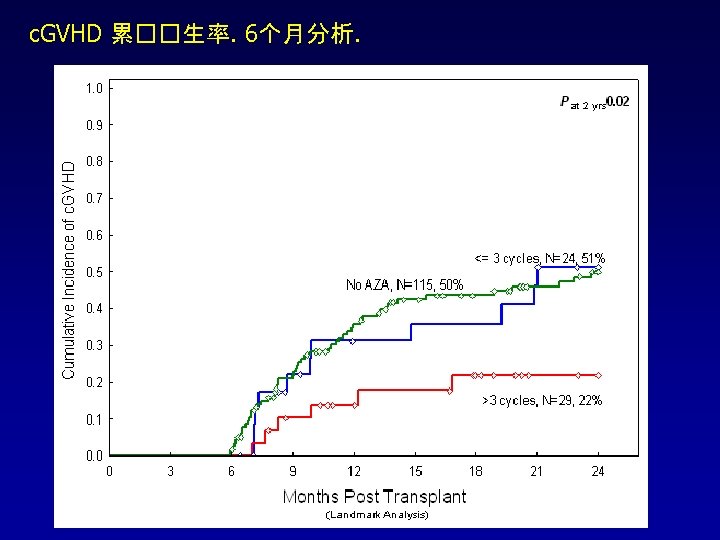
Fitted Bayesian logistic 回� 模型 : 随着用��� 延� ,c. GVHD 并未增加, 与� 量无关 �量 均� SD Posterior 95% CI 有益效� 的 可能性 2. 50% 97. 50% 截距 0. 582 0. 779 -0. 887 2. 111 - Azacitidine �量 0. 014 0. 036 5 -0. 083 0. 057 0. 658 � 程数 -0. 439 0. 311 -1. 073 0. 159 0. 928



去甲基化�物 – 潜在的作用 • 增加�瘤相关抗原如 CTA (Roman-Gomez, 2007) Tatjana Stankovic 等的表达. Goodyear et al. • 增加造血�胞 KIR 配体的表达 (Liu, 2009) • 恢复降低的�瘤�胞上 HLA I, II和 III �抗原表达 (Campoli & Ferrone, 2008) (Pinto et al – 1984) • 增加已知的次要抗原的表达 (Hambach, 2009) • 影响 micro. RNA 功能 - 抑制�瘤基因 • 增加 Fox. P 3 表达和 Treg generation (Polansky, 2008) (Choi et al. 2010) (Sanchez-Abarca et al. 2010) John Di. Persio et al. Goodyear et al. Blood 2011. ? GVL 耐受性 不影响复�? ↑GVL

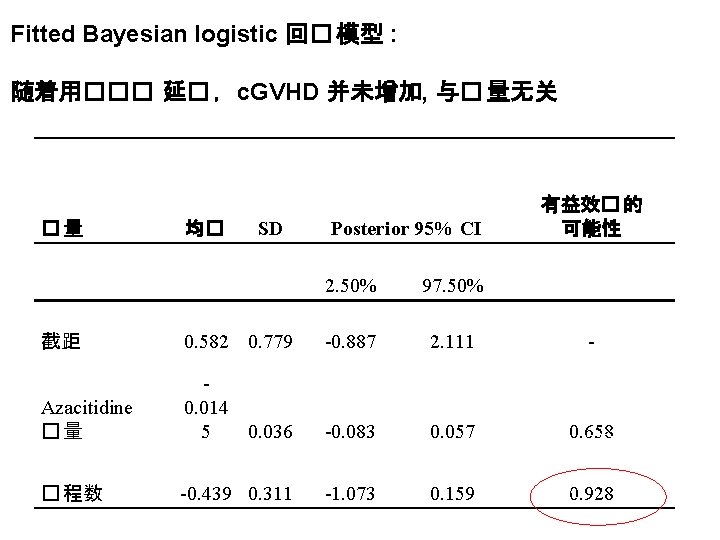
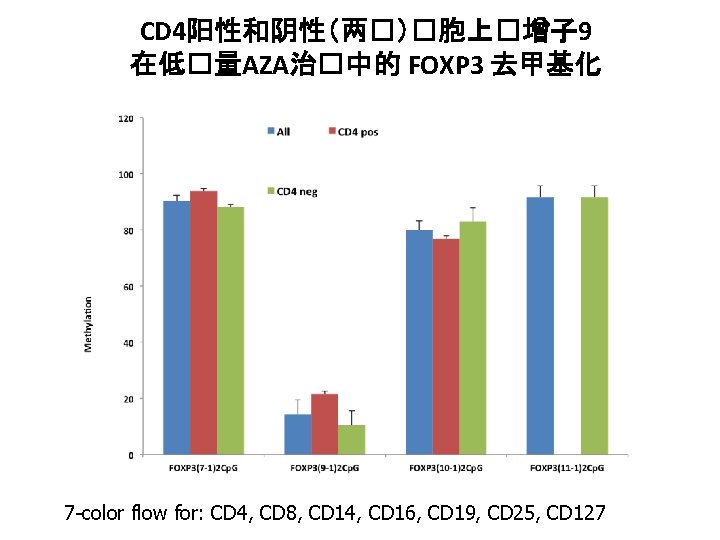
CD 4阳性和阴性(两�)�胞上�增子 9 在低�量AZA治�中的 FOXP 3 去甲基化 7 -color flow for: CD 4, CD 8, CD 14, CD 16, CD 19, CD 25, CD 127


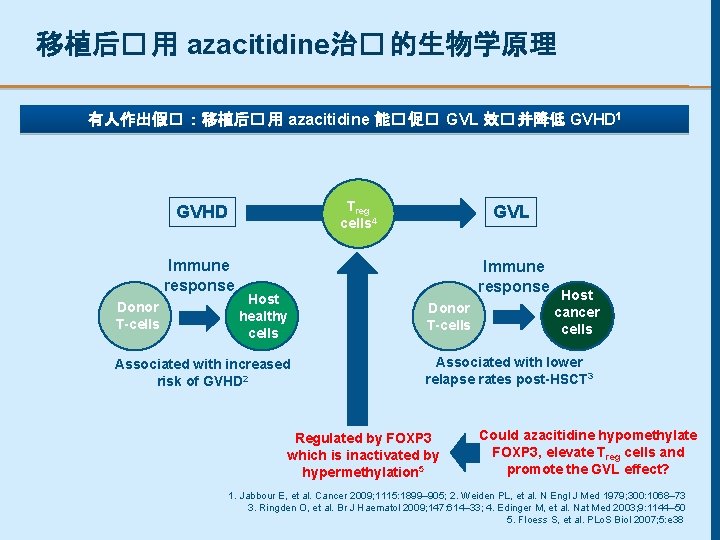
移植后� 用 azacitidine治� 的生物学原理 有人作出假� :移植后� 用 azacitidine 能� 促� GVL 效� 并降低 GVHD 1 Treg cells 4 GVHD Immune response Donor T-cells Host healthy cells Associated with increased risk of GVHD 2 GVL Immune response Donor T-cells Host cancer cells Associated with lower relapse rates post-HSCT 3 Regulated by FOXP 3 which is inactivated by hypermethylation 5 Could azacitidine hypomethylate FOXP 3, elevate Treg cells and promote the GVL effect? 1. Jabbour E, et al. Cancer 2009; 1115: 1899– 905; 2. Weiden PL, et al. N Engl J Med 1979; 300: 1068– 73 3. Ringden O, et al. Br J Haematol 2009; 147: 614– 33; 4. Edinger M, et al. Nat Med 2003; 9: 1144– 50 5. Floess S, et al. PLo. S Biol 2007; 5: e 38


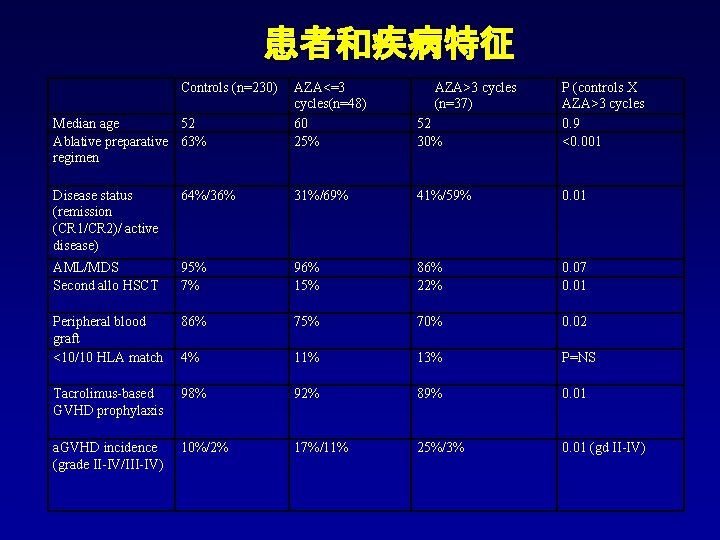
患者和疾病特征 Controls (n=230) Median age 52 Ablative preparative 63% regimen AZA<=3 cycles(n=48) 60 25% AZA>3 cycles (n=37) 52 30% P (controls X AZA>3 cycles 0. 9 <0. 001 Disease status (remission (CR 1/CR 2)/ active disease) 64%/36% 31%/69% 41%/59% 0. 01 AML/MDS Second allo HSCT 95% 7% 96% 15% 86% 22% 0. 07 0. 01 Peripheral blood graft <10/10 HLA match 86% 75% 70% 0. 02 4% 11% 13% P=NS Tacrolimus-based GVHD prophylaxis 98% 92% 89% 0. 01 a. GVHD incidence (grade II-IV/III-IV) 10%/2% 17%/11% 25%/3% 0. 01 (gd II-IV)



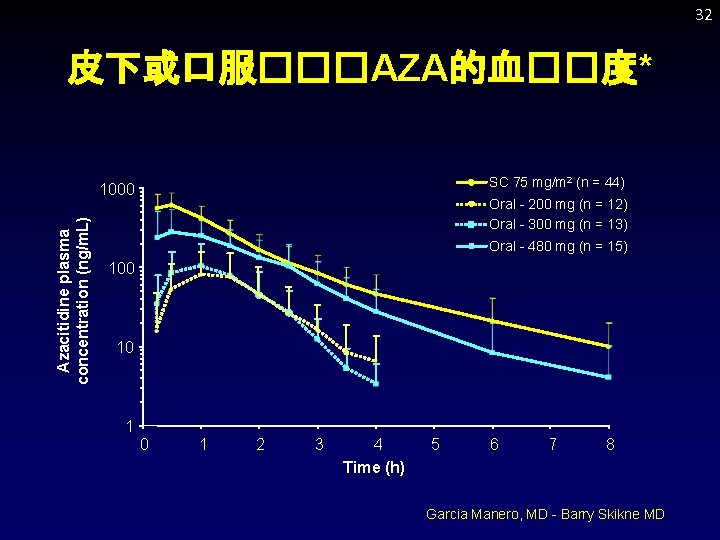
32 皮下或口服���AZA的血��度* SC 75 mg/m 2 (n = 44) Azacitidine plasma concentration (ng/m. L) 1000 Oral - 200 mg (n = 12) Oral - 300 mg (n = 13) Oral - 480 mg (n = 15) 100 10 1 2 3 4 Time (h) 5 6 7 8 Garcia Manero, MD - Barry Skikne MD



M D Anderson 癌症中心 干�胞移植部 Richard Champlin Borje Andersson Uday Popat Roy Jones Yago Nieto Elizabeth Shpall Jeffrey Molldrem Paolo Anderlini John Mc. Mannis Stefan Ciurea Chitra Hosing Martin Korbling Partow Kebriaei Issa Khouri Michael Andreef Steven Kornblau Betul Oran Muzaffar Qazilbash Amin Alousi Laurence Cooper Laura Worth Susan Staba Demetrios Petropoulos Simrit Parmar Image Credit: NASA/JPL/Space Science Institute

Marcos. delima@uhhospitals. org Sao Joao del Rey - Brasil