STEM CELL TRANSPLANTATION IN SICKLE CELL DISEASE IN



















- Slides: 38

STEM CELL TRANSPLANTATION IN SICKLE CELL DISEASE IN NIGERIA? PROPOSED SELECTION CRITERIA Dr. T. T. Wakama Presentation to the 36 th Annual Scientific and General Meeting of the NSHBT 21 st August 2008

Collaboration

Round Table Discussion • What Next? ü Overwhelming need for BMT in SCA in Nigeria ü ? Entry point for BMT in Nigeria through collaboration with the Italian team ü Individual hospitals can go ahead with their initiatives ü Constitution of two committees

1. Committee on Bone Marrow Transplantation constituting of i. Microbiogist ii. National Blood Transfusion Service iii. Histopathologist iv. Chemical Pathologist v. Haematologist vi. Health planner vii. Finance viii. Paediatrician ix. Rep: NABDA

C’ttee Contd 2. Committee on review of Patient selection criteria • 5 – man committee 1. Dr. TT Wakama (Haematologist NHA) 2. Dr. Seyi Oniyangi (Paediatrician NHA) 3. Dr. PO Ladipo (Obstetrician NHA) 4. Dr. Sunday Ocheni (Haematologist UNTH) 5. Dr. C. Nwauche (Haematologist UPTH)

Mandate! Ø Review Selection Criteria for BMT in patients with SCA in Nigeria Ø Present report to NSHBT at Ife 2008 meeting

INTRODUCTION • • Successful allogeneic haematopoietic stem cell transplantation (SCT) is currently the only cure for sickle cell disease The results of transplantation are best when performed in children with a sibling donor who is HLA-identical Parkman R. The application of bone marrow transplantation to the treatment of genetic diseases. Science 1986; 232: 1373 -1378 Lane PA, Buchanan GR, Hutter JJ, Austin RF, Britton HA, Rogers ZR et al. Sickle cell disease in children and adolescents : Diagnosis, Guidelines for comprehensive care, and care paths and protocols for management of acute and chronic complications. November 2001

INTRODUCTION • The goal of SCT in SCD is to eliminate the sickle erythrocyte and its cellular progenitors and replace them with donor haematopoietic pluripotent stem cells that produce erythrocytes expressing at best no sickle haemoglobin or at worse quantities similar to the trait condition.

INTRODUCTION • The risk of severe adverse events from SCT should be weighed against the possibility of preventing serious complications from sickle cell disease • Published experience in children less than 16 years of age shows that: ü ≈ 80% with Hb SS who undergo SCT from an HLAidentical sibling donor will have sustained engraftment and elimination of all sickle-related symptoms. ü Ten to 15% of patients will reject the stem cell graft, ü ≈ 5% will succumb from complications of the procedure including graft versus host disease

INTRODUCTION • Wide scale implementation of SCT for SCD has been limited by the inability to predict the clinical severity of SCD for a given child and often by the lack of an HLA -identical sibling without SCD

INTRODUCTION • The first published reports of SCT for SCD involved patients who had other more deadly hematological or genetic disorders that were the primary indication for SCT Johnson FL et al. N Engl J Med 1984; 311: 780 -3, Milpied NHJ et al. Lancet 1988; ii: 328 -9 • This initial experience showed elimination of SCD with engraftment of donor hematopoietic stem cells. • In the following decade or more, considerable discussion centered on who should be considered and when they should be referred for transplantation Nagel RL. Seminars in Hematology 1991; 28: 233 -4, Davies SC. Archives of Disease in Childhood 1993; 69: 176 -7, Platt OS et al. N Engl J Med 1996; 335: 426 -8

INTRODUCTION • The first limited series of patients who underwent transplantation as specific therapy for sickle cell disease comprised a group of families from Africa living at the time in Belgium Vermylen C et al. Lancet 1988; 1: 1427 -8. • Based in part on the very good outcome experienced by these initial patients, several multicenter phase II studies for children with symptomatic sickle cell disease were conducted in North America and Europe. Walters MC et al. N Engl J Med 1996; 335: 369 -76, Vermylen C et al. BMT 1998; 22: 1 -6.

INTRODUCTION • Children were felt to be better candidates for SCT than adult pts bc of their lower risk of transplant-related complications such as graft-versus-host disease (GVHD), and bc of a presumed lower burden of sicklerelated organ damage.

Summary of Results of SCT from HLAidentical sibling donors After a period of follow up extending up to 11 years: • > 90% of pts survive • ≈ 85% survive free from sickle cell disease • Sickle cell disease recurred in ≈ 10% of patients • Neurologic complications such as seizures occurred frequently after transplantation, requiring development of preventative measures Abboud MR et al. BMT 1996; 17: 405 -7

Summary of Results of SCT from HLAidentical sibling donors • Among pts who had stable engraftment of donor cells, no subsequent sickle cellrelated clinical events occurred and their pre-existing sickle cell-related organ damage stabilized Vermylen C et al. BMT 1998; 22: 1 -6, Walters MC et al. Blood 2000; 95: 1918 -24, Hernigou P et al. Journal of Bone & Joint Surgery - American Volume 1997; 79: 1726 -30 • Splenic function also recovered Ferster A et al. Blood 1993; 81: 1102 -5

Summary of Results of SCT from HLAidentical sibling donors • Some patients developed mixed donor-host hematopoietic chimerism after transplantation that was stable Sullivan K et al. BMT 1997; 19, Suppl 2: 102 -105 Interestingly, these patients had no symptoms from sickle cell disease. • Primary and secondary amenorrhea were common among females after transplantation • Most patients likely will be infertile. Vermylen C et al. BMT 1998; 22: 1 -6, Walters MC et al. Blood 2000; 95: 1918 -24

Summary of Results of SCT from HLAidentical sibling donors • The risk of secondary cancers after SCT remains uncertain but it is estimated to be less than 5 percent. • Linear growth was normal or accelerated after transplantation in most patients Curtis RE et al. N Engl J of Med 1997; 336: 897 -904. Vermylen C et al. Bone Marrow Transplantation 1998; 22: 1 -6, Walters MC et al. Blood 2000; 95: 1918 -24

Transplantation from alternative sources of stem cells • Umbilical Cord Blood (UCB) and hematopoietic cells from volunteer donors are alternative sources of hematopoietic stem cells that could increase the number of donors for sickle cell disease patients • UCB transplantation for sickle cell disease has been successful. No published report exists of transplantation from volunteer unrelated donors. Brichard B et al. Journal of Pediatrics 1996; 128: 241 -3

• UCB possibly produces less GVHD than does standard SCT • A disadvantage of UCB transplantation is slower hematopoietic engraftment and perhaps a higher rate of graft rejection Rocha V et al. BMT 1998; 21 Suppl 3: S 59 -62 Gluckman E et al. N Engl J of Med 1997; 337: 373 -81 • The relatively low number of hematopoietic stem cells recovered from UCB effectively limits the procedure to children • Strategies for transplantation from unrelated volunteer stem cell donors must surmount the histocompatibility barriers associated with higher rates of GVHD and graft rejection to become viable options

HCT for adults with sickle cell disease • Very little information exists concerning the outcome after SCT among adults • The risk of death with the procedure may be higher, due in part to the higher frequency of GVHD. • Non-myeloablative preparation before SCT could clinically correct sickle cell disease with lower risk of severe complications. Sullivan KM et al. Seminars in Hematology 1991; 28: 250 -9 Storb R et al. Annals of the New York Academy of Sciences 1999; 872: 372 -5

• The aim of non-myeloablative preparation before SCT is to establish stable donorhost hematopoietic chimerism after transplantation, which might provide a significant clinical benefit to patients. • If successful, this approach might improve the safety profile of SCT for older people with advanced organ damage from sickle cell disease

INCLUSION/EXCLUSION CRITERIA IN SELECTING SCD PTS FOR SCT • • Wide scale implementation of SCT for SCD has been limited by the inability to predict the clinical severity of SCD for a given child and often by the lack of an HLA-identical sibling without SCD. Most current studies in SCT in SCD base their Inclusion/Exclusion Criteria on the publication by Walters MC et al in 1996. Walters MC, Patience M, Leisenring W, et al. Bone Marrow Transplantation for sickle cell disease N Engl J Med 1996 ; 335: 369 -376

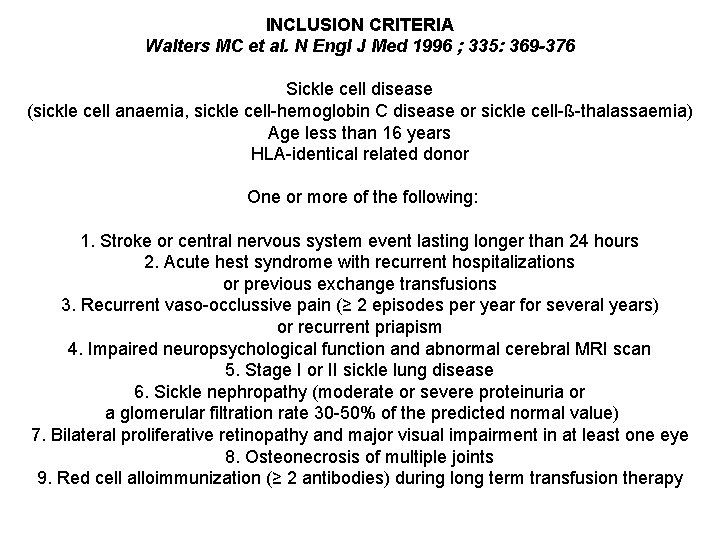
INCLUSION CRITERIA Walters MC et al. N Engl J Med 1996 ; 335: 369 -376 Sickle cell disease (sickle cell anaemia, sickle cell-hemoglobin C disease or sickle cell-ß-thalassaemia) Age less than 16 years HLA-identical related donor One or more of the following: 1. Stroke or central nervous system event lasting longer than 24 hours 2. Acute hest syndrome with recurrent hospitalizations or previous exchange transfusions 3. Recurrent vaso-occlussive pain (≥ 2 episodes per year for several years) or recurrent priapism 4. Impaired neuropsychological function and abnormal cerebral MRI scan 5. Stage I or II sickle lung disease 6. Sickle nephropathy (moderate or severe proteinuria or a glomerular filtration rate 30 -50% of the predicted normal value) 7. Bilateral proliferative retinopathy and major visual impairment in at least one eye 8. Osteonecrosis of multiple joints 9. Red cell alloimmunization (≥ 2 antibodies) during long term transfusion therapy

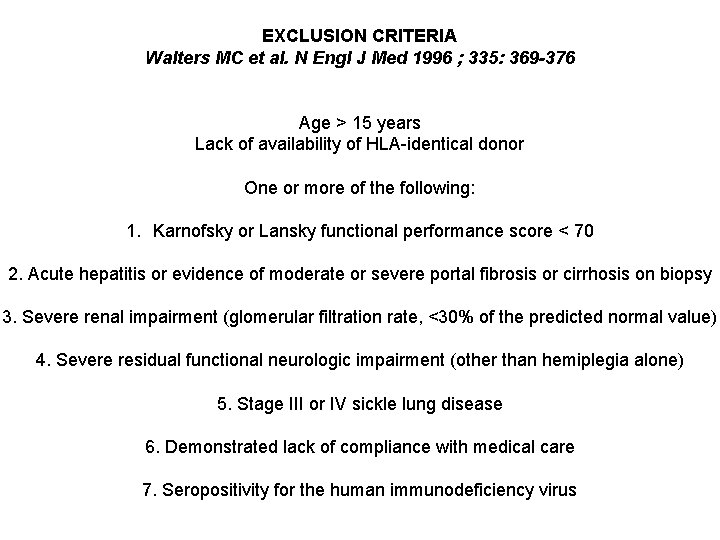
EXCLUSION CRITERIA Walters MC et al. N Engl J Med 1996 ; 335: 369 -376 Age > 15 years Lack of availability of HLA-identical donor One or more of the following: 1. Karnofsky or Lansky functional performance score < 70 2. Acute hepatitis or evidence of moderate or severe portal fibrosis or cirrhosis on biopsy 3. Severe renal impairment (glomerular filtration rate, <30% of the predicted normal value) 4. Severe residual functional neurologic impairment (other than hemiplegia alone) 5. Stage III or IV sickle lung disease 6. Demonstrated lack of compliance with medical care 7. Seropositivity for the human immunodeficiency virus

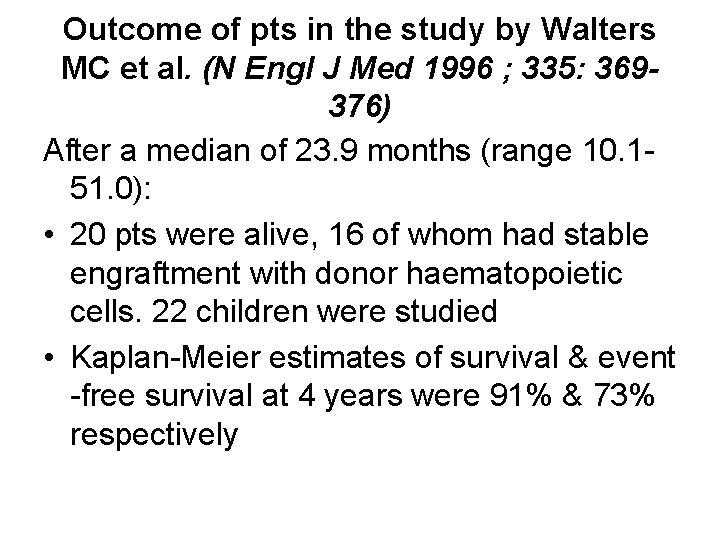
Outcome of pts in the study by Walters MC et al. (N Engl J Med 1996 ; 335: 369376) After a median of 23. 9 months (range 10. 151. 0): • 20 pts were alive, 16 of whom had stable engraftment with donor haematopoietic cells. 22 children were studied • Kaplan-Meier estimates of survival & event -free survival at 4 years were 91% & 73% respectively

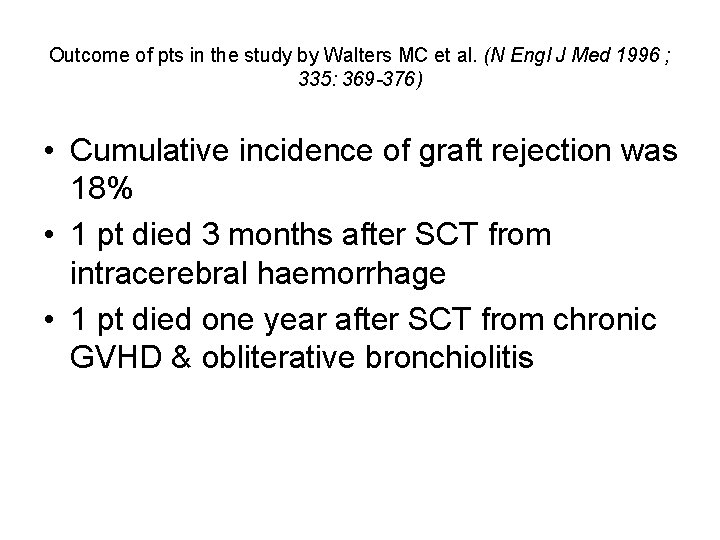
Outcome of pts in the study by Walters MC et al. (N Engl J Med 1996 ; 335: 369 -376) • Cumulative incidence of graft rejection was 18% • 1 pt died 3 months after SCT from intracerebral haemorrhage • 1 pt died one year after SCT from chronic GVHD & obliterative bronchiolitis

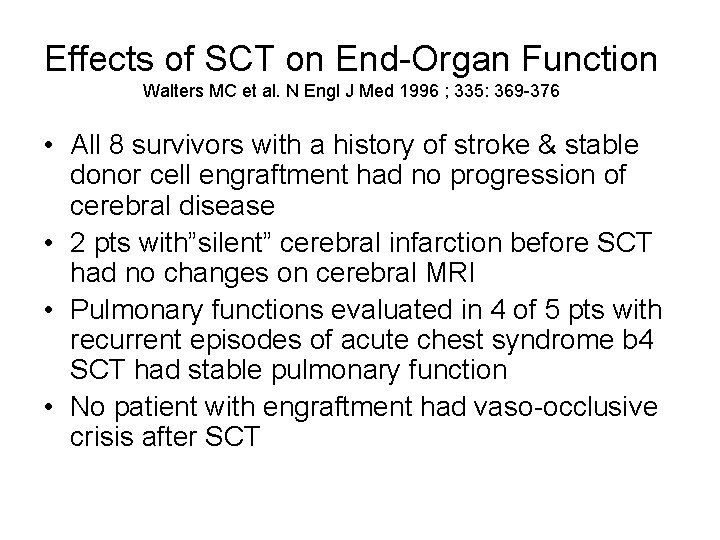
Effects of SCT on End-Organ Function Walters MC et al. N Engl J Med 1996 ; 335: 369 -376 • All 8 survivors with a history of stroke & stable donor cell engraftment had no progression of cerebral disease • 2 pts with”silent” cerebral infarction before SCT had no changes on cerebral MRI • Pulmonary functions evaluated in 4 of 5 pts with recurrent episodes of acute chest syndrome b 4 SCT had stable pulmonary function • No patient with engraftment had vaso-occlusive crisis after SCT

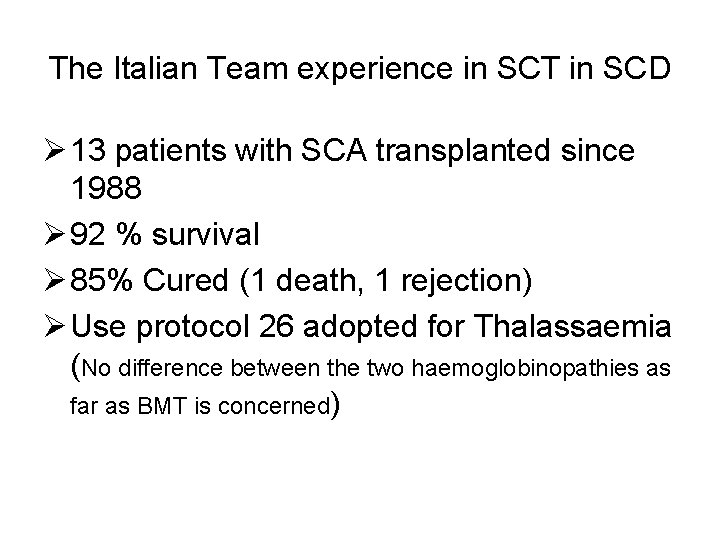
The Italian Team experience in SCT in SCD Ø 13 patients with SCA transplanted since 1988 Ø 92 % survival Ø 85% Cured (1 death, 1 rejection) Ø Use protocol 26 adopted for Thalassaemia (No difference between the two haemoglobinopathies as far as BMT is concerned)

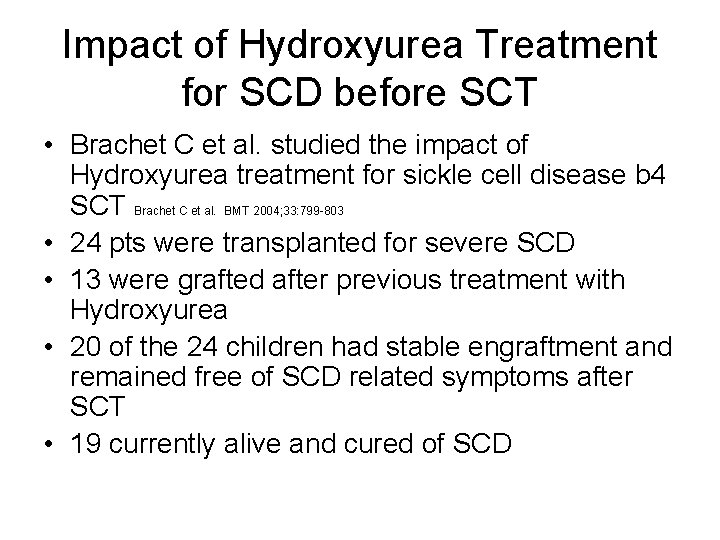
Impact of Hydroxyurea Treatment for SCD before SCT • Brachet C et al. studied the impact of Hydroxyurea treatment for sickle cell disease b 4 SCT Brachet C et al. BMT 2004; 33: 799 -803 • 24 pts were transplanted for severe SCD • 13 were grafted after previous treatment with Hydroxyurea • 20 of the 24 children had stable engraftment and remained free of SCD related symptoms after SCT • 19 currently alive and cured of SCD

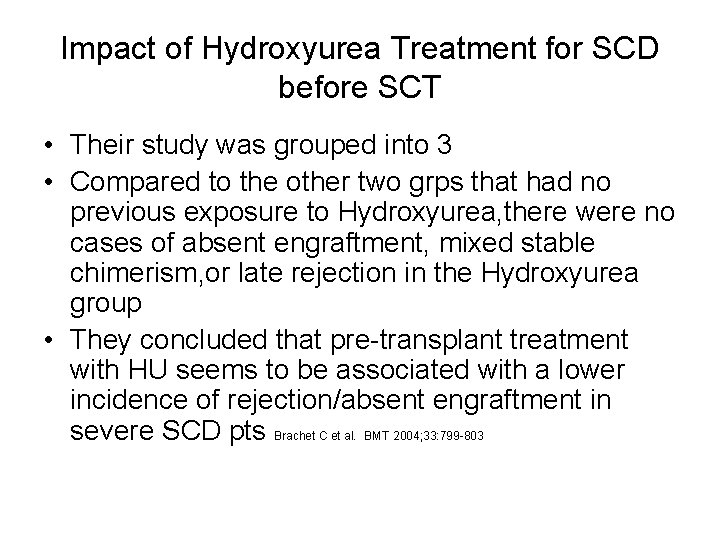
Impact of Hydroxyurea Treatment for SCD before SCT • Their study was grouped into 3 • Compared to the other two grps that had no previous exposure to Hydroxyurea, there were no cases of absent engraftment, mixed stable chimerism, or late rejection in the Hydroxyurea group • They concluded that pre-transplant treatment with HU seems to be associated with a lower incidence of rejection/absent engraftment in severe SCD pts Brachet C et al. BMT 2004; 33: 799 -803

Recommendations of the Abuja Committee for Inclusion/Exclusion Criteria for SCT in SCD in Nigeria • a) INCLUSION CRITERIA (a + b + c + at least one under d Established diagnosis of Sickle Cell Disease (Hb. SS, Hb. SC, Hb. SB Thalassemia) b) Availability of HLA- matched sibling or unrelated Stem Cell donor (preferably: Hb. AA >Hb. AC > Hb. AS). c) Age of patient ≤ sixteen years. (17 -35 yrs could be enlisted in a latter trial group) d) At least one of the following clinical complications: I) Sickle Cell Disease-related neurological deficits, stroke or impaired neuropsychiatric function or abnormal cerebral MRI or cerebral arteriogram or MRI angiographic study. II) Recurrent acute sickle chest syndrome (> two episodes) which has failed to respond to trial of Hydroxycarbamide of at least six months or where Hydroxycarbamide is contraindicated.

INCLUSION CRITERIA (a + b + c + at least one under d) d) continues: III) Recurrent severe debilitating pain (including priapism) due to vaso-occlusive crisis which has failed to respond to trial of Hydroxycarbamide of at least six months or where Hydroxycarbamide is contraindicated. IV) Sickle Cell Nephropathy (moderate-severe proteinuria or Glomerular Filtration Rate of 30%-50% of predicted normal value) V) Bilateral proliferative retinopathy or major visual impairment of at least one eye. VI) Osteonecrosis of multiple joints. VII) Transfusion dependence and alloimmunisation to Red cell antibodies VIII) Sickle Cell Disease relapsing after previous Haematopoietic stem cell transplantation

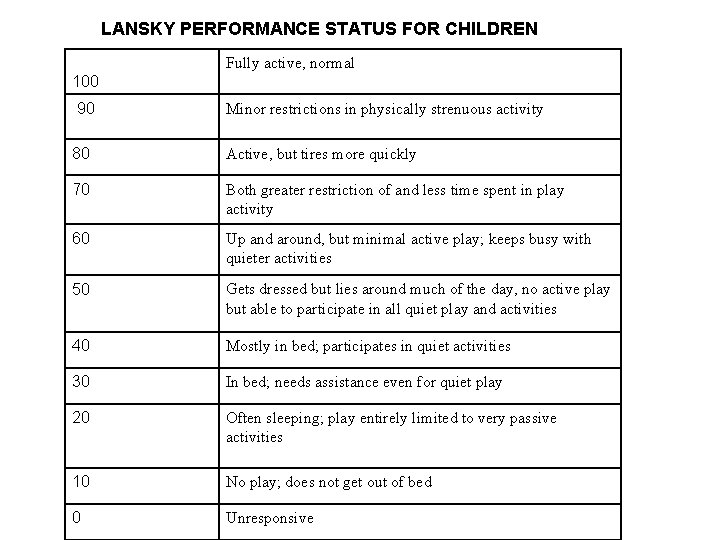
Recommendations of the Abuja Committee for Inclusion/Exclusion Criteria for SCT in SCD in Nigeria EXCLUSION CRITERIA (Any one or combinations of the following) I) Age of patient > sixteen years II) Non-availability of HLA- matched stem cell Donor III) Acute hepatitis, Liver cirrhosis or severe portal fibrosis on liver biopsy IV) Severe renal impairment (GFR less than 30% of predicted normal value) V) HIV / AIDS in potential recipient. VI) Lansky performance score < 70% VII) Pulmonary hypertension VIII) Severe residual functional neurologic impairment (other than hemiplegia alone)

LANSKY PERFORMANCE STATUS FOR CHILDREN Fully active, normal 100 90 Minor restrictions in physically strenuous activity 80 Active, but tires more quickly 70 Both greater restriction of and less time spent in play activity 60 Up and around, but minimal active play; keeps busy with quieter activities 50 Gets dressed but lies around much of the day, no active play but able to participate in all quiet play and activities 40 Mostly in bed; participates in quiet activities 30 In bed; needs assistance even for quiet play 20 Often sleeping; play entirely limited to very passive activities 10 No play; does not get out of bed 0 Unresponsive

Further Reading/References 1. Management & Therapy of Sickle Cell Disease: Hematopoietic Cell Transplantation. Mark Walters, M. D. Oakland Children's Hospital Oakland, California www. sickle. bwh. harvard. edu/sickle_bmt. html Accessed 16 th August, 2008 2. Walters MC, Patience M, Leisenring W, et al. Bone Marrow Transplantation for sickle cell disease N Engl J Med 1996 ; 335: 369 -376 3. The European Blood & Marrow Transplantation (EBMT) Handbook, 5 th edition (2008 Revised Edition)

Appreciation ü Dr. Sunday Ocheni ü Dr. OP Ogbe ü The Italian team led by Prof Lucarelli ü All the local resource persons ü All participants

THANK YOU FOR BEING AN ATTENTIVE AUDIENCE

Thank you for listening and God Bless
 Stem cell or bone marrow transplantation thailand
Stem cell or bone marrow transplantation thailand Gene therapy for sickle cell disease
Gene therapy for sickle cell disease Codominance-
Codominance- Types of sickle cell disease
Types of sickle cell disease Sickle cell symptoms
Sickle cell symptoms Hemoglobin electrophoresis
Hemoglobin electrophoresis Bone marrow transplantation sri lanka
Bone marrow transplantation sri lanka Law of transplantation
Law of transplantation Patrick evrard mont godinne
Patrick evrard mont godinne Cultural transplantation examples
Cultural transplantation examples Types of transplant
Types of transplant Genotypic ratio of monohybrid cross
Genotypic ratio of monohybrid cross Sickle cell karyotype
Sickle cell karyotype Life expectancy of sickle cell patients
Life expectancy of sickle cell patients Sickle cell hemoglobin structure
Sickle cell hemoglobin structure Difference between sickle cell anaemia and thalassemia
Difference between sickle cell anaemia and thalassemia Sickle cell anemia lab values
Sickle cell anemia lab values Tiki barber sickle cell
Tiki barber sickle cell Protein synthesis and mutations
Protein synthesis and mutations Pethidine in sickle cell
Pethidine in sickle cell Sickle cell karyotype
Sickle cell karyotype Pedigree of sickle cell anemia
Pedigree of sickle cell anemia Sickle cell hemoglobin structure
Sickle cell hemoglobin structure Sickle cell punnett square
Sickle cell punnett square Is sickle cell anemia codominant
Is sickle cell anemia codominant Sickle cell osteomyelitis
Sickle cell osteomyelitis Film
Film Sickle blood cell
Sickle blood cell Sickle cell anemia
Sickle cell anemia Sickle cell anemia codominance
Sickle cell anemia codominance Old world flycatchers
Old world flycatchers Nata sickle cell
Nata sickle cell Sickle cell anemia
Sickle cell anemia Sickle cell pain
Sickle cell pain Sickle cell anemia symptoms
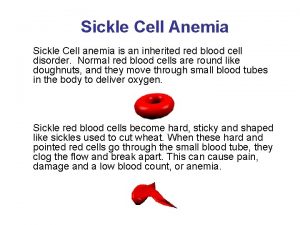
Sickle cell anemia symptoms Anemia
Anemia Everardo cobos
Everardo cobos Inheritance pattern of sickle cell anemia
Inheritance pattern of sickle cell anemia Sickle cell anemia dna sequence
Sickle cell anemia dna sequence