Many properties of elements depend on 1 Electron














- Slides: 27

Many properties of elements depend on 1. Electron configuration 2. How strongly outer electrons (-) are attracted to the nucleus (+) This attraction is described by Coulomb’s Law which defines the force of attraction (F) between charged particles (Q)

Zeff • Core electrons shield or screen valence electrons from the charge of the nucleus • Nuclear charge is not experienced the same by all electrons. • Outer electrons experience it less, so Z is reduced. The new Z is called Zeff = Z – S where Z is actual nuclear charge (#p in nucleus) and S is shielding constant (# core e-)

Compare Li to Be to B • Write the electron configuration of Li, Be, and B. • Determine Z, S, and Zeff for each. • What is the trend for Zeff across a period? Explain the trend.

As go across, Zeff increases because atomic number increases: more p in nucleus, so Z increases while n and # core e- remains same: S same

Compare Ne to Na • Write the electron configuration of Na and Ne. • Determine Z, S, and Zeff for each. • What is the trend for Zeff when you start a new period? Explain the trend.

As start a new period, Zeff decreases because there are more protons in the nucleus so Z increases (pull inward is stronger) but there are more core electrons so value of S gets closer to value of Z (more shielding so attraction is lower) The two counter balance one another

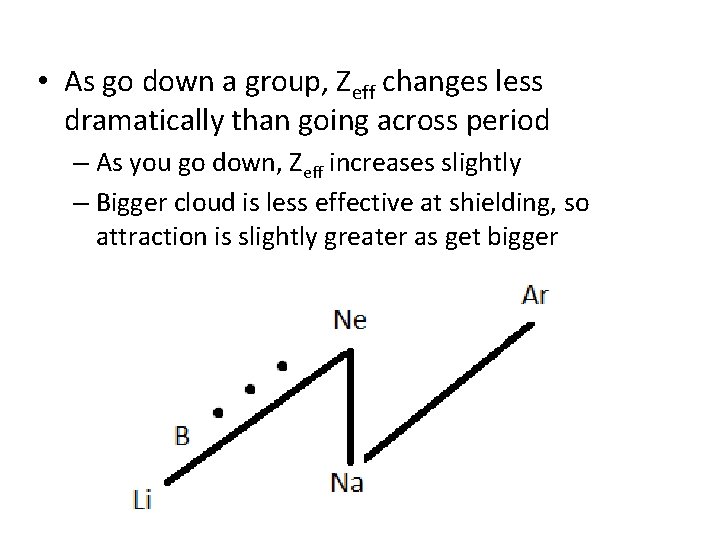
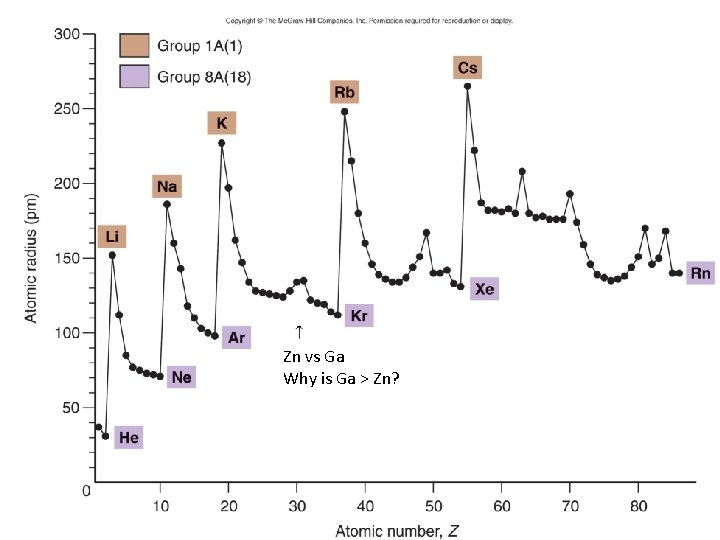
• As go down a group, Zeff changes less dramatically than going across period – As you go down, Zeff increases slightly – Bigger cloud is less effective at shielding, so attraction is slightly greater as get bigger

Zeff and Periodic Trends • Atomic size – Greatly influences other atomic properties and is critical to understanding element behavior • Ionization energy – Energy to remove electrons • Electron affinity/electronegativity – Energy to add electrons

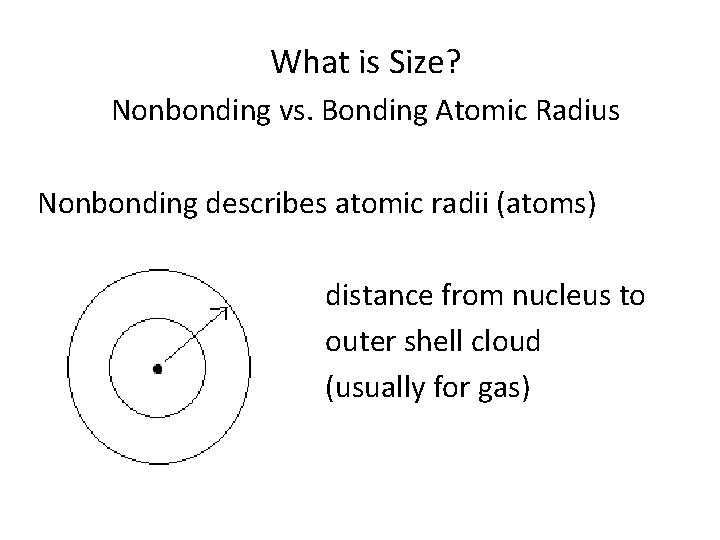
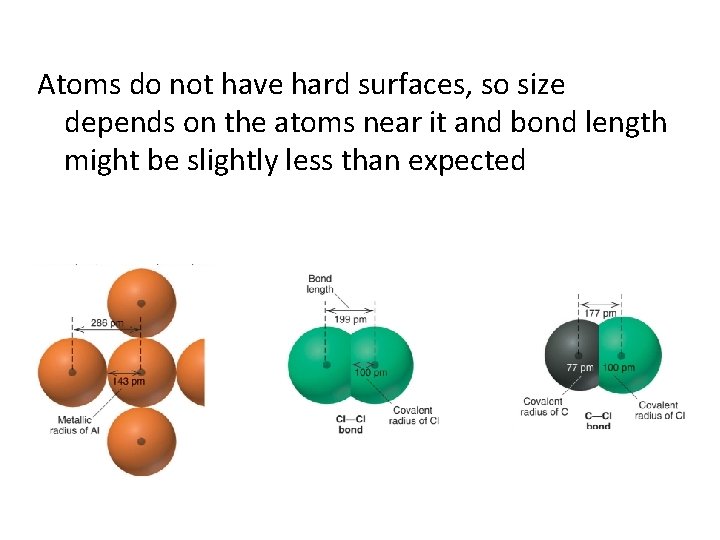
What is Size? Nonbonding vs. Bonding Atomic Radius Nonbonding describes atomic radii (atoms) distance from nucleus to outer shell cloud (usually for gas)

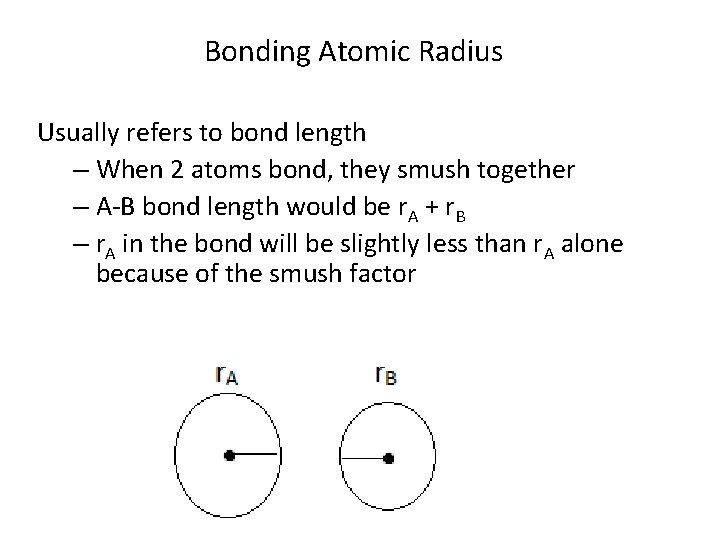
Bonding Atomic Radius Usually refers to bond length – When 2 atoms bond, they smush together – A-B bond length would be r. A + r. B – r. A in the bond will be slightly less than r. A alone because of the smush factor

Atoms do not have hard surfaces, so size depends on the atoms near it and bond length might be slightly less than expected
What happens to size (atomic radii) as you go Down a group? Across a period? WHY?

Zn vs Ga Why is Ga > Zn?

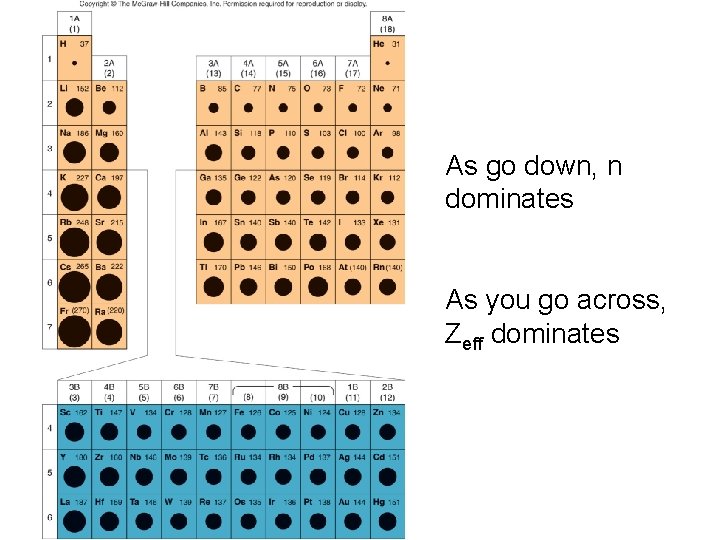
As go down, n dominates As you go across, Zeff dominates

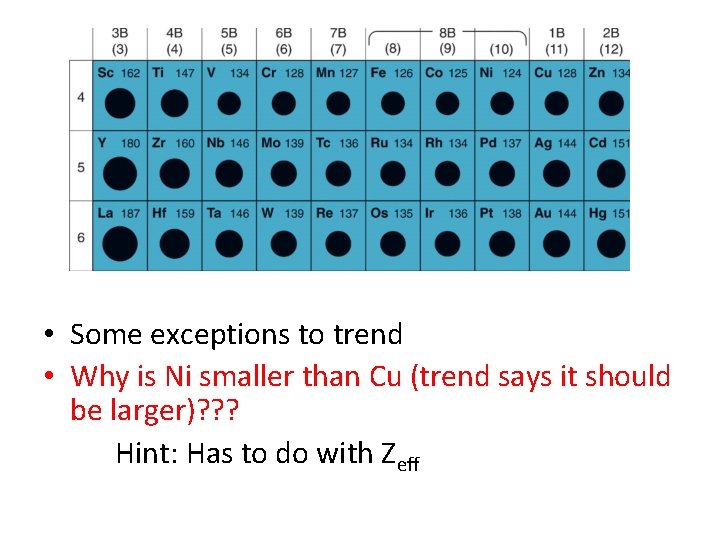
• Some exceptions to trend • Why is Ni smaller than Cu (trend says it should be larger)? ? ? Hint: Has to do with Zeff

Size of Ions • How does size of cation compare to parent atom? Explain. M vs. Mn+ • How does size of anion compare to parent atom? Explain. NM vs. NMn-

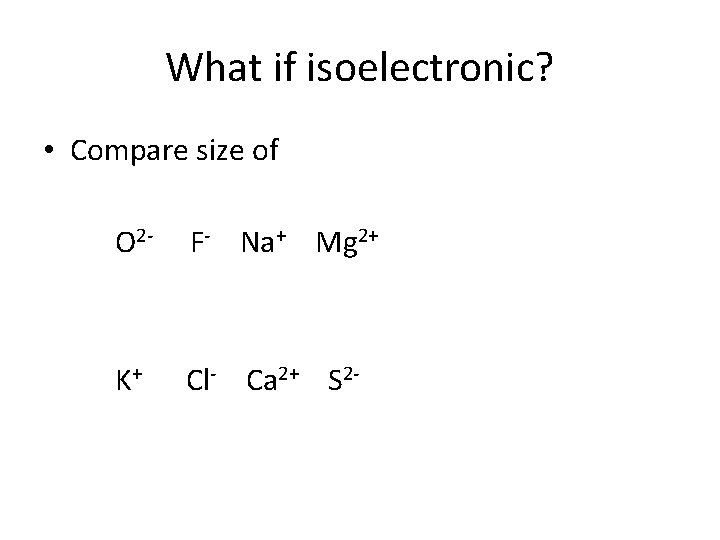
What if isoelectronic? • Compare size of O 2 - F- Na+ Mg 2+ K+ Cl- Ca 2+ S 2 -

Ionization Energy • Energy to ? ? ? • Endo or exo? +∆H or -∆H? • Do all elements have only 1 Eion? • Trend going across a period and down a group?


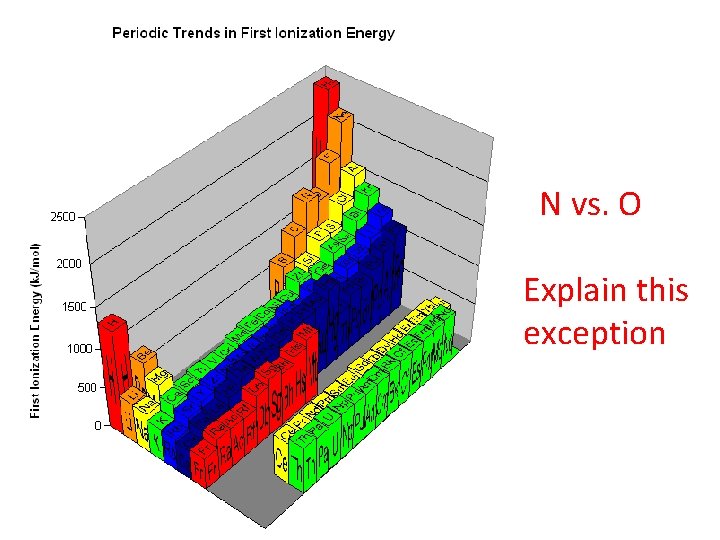
N vs. O Explain this exception

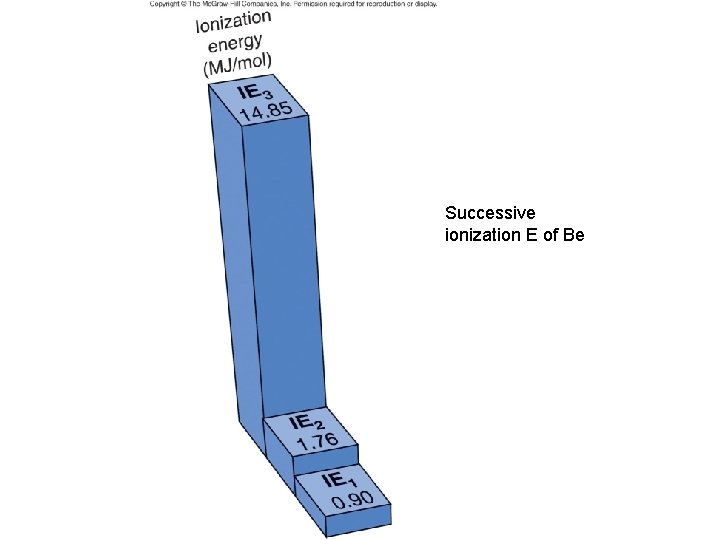
Successive ionization E of Be


Electronegativity and e- Affinity • Do you remember what electronegativity is? • What is the trend across a period and down a group?


Electron Affinity • Energy to ? ? ? • Endo or exo? +∆H or -∆H? • Trend going across a period and down a group?

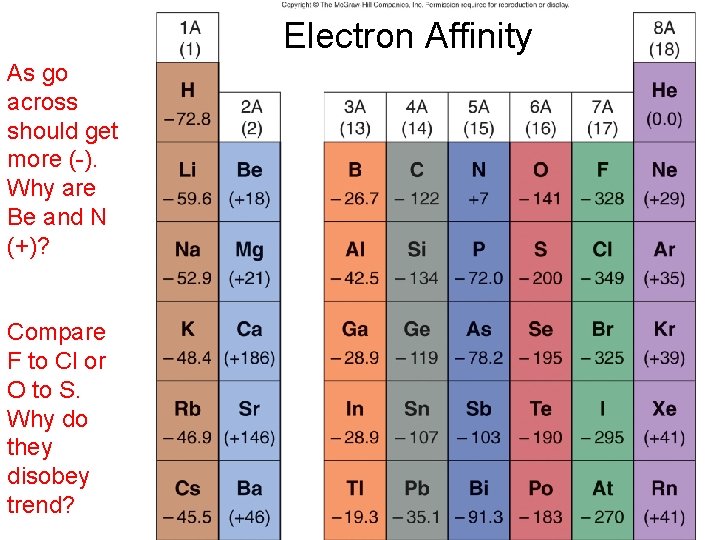
Electron Affinity As go across should get more (-). Why are Be and N (+)? Compare F to Cl or O to S. Why do they disobey trend?
