Limportanza di far sapere cosa si fa e















































- Slides: 99

L’importanza di far sapere cosa si fa e per chi. Informare sui risultati Paolo Cravedi Istituto di Ricerche Farmacologiche Mario Negri, Bergamo Seriate, 16 Dicembre 2010 1

Chi stabilisce in medicina cosa è efficace e cosa no? Una volta c’era qualche conoscenza sulle cause delle malattie (non molte, fino a 60 anni fa) e soprattutto “l’esperienza” del medico Esperienza era aver visto altri casi simili e cercare di ricordarsi chi, in seguito a quale cura, stava meglio o era guarito e chi no

I nuovi ammalati si curavano con quello che si pensava avesse fatto bene agli altri Molti medici le loro esperienze le scrivevano su libri o riviste Altri ne parlavano con altri medici, si creava una tradizione ma questo metodo era molto pericoloso


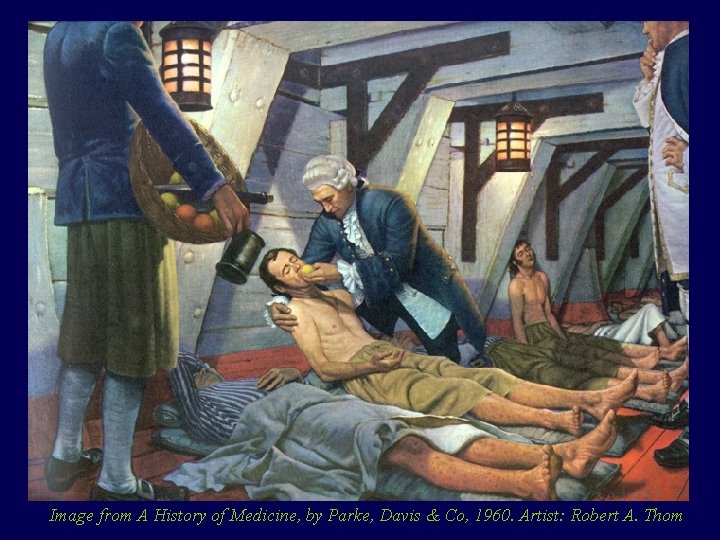
A metà del ‘ 700 James Lind dottore di Edimburgo a bordo di una delle navi della regina era in un bel guaio, i marinai sanguinavano dalle gengive e perdevano i denti: scorbuto

Image from A History of Medicine, by Parke, Davis & Co, 1960. Artist: Robert A. Thom

Lind divise 12 marinai in sei gruppi di due Ciascuno dei due della coppia avrebbe avuto un trattamento diverso da quello delle altre coppie

Solo i due marinai che avevano avuto succo di agrumi (di due arance e un limone al giorno) guarirono, gli altri no Lind lo scrisse Fu probabilmente il primo studio clinico controllato


SE SI VUOLE SPERIMENTARE SULL’UOMO UN FARMACO SERVONO (IN ITALIA E IN TUTTO IL MONDO) 1 - Dati di laboratorio che suggeriscano un meccanismo d’azione plausibile 2 - Dati sull’animale che indichino che funziona 3 - Studi sul volontario sano che dimostrino che non fa male

PLACEBO Placebo e’ una qualsiasi sostanza innocua o un qualsiasi intervento non farmacologico privi di efficacia terapeutica. E’ deliberatamente somministrato alla persona che acconsente di assumerlo come alternativa a un trattamento attivo di cui si voglia sperimentare l’efficacia o la sicurezza.

L’uso del placebo quindi e’ legittimo solo a scopo sperimentale e solo in presenza del consenso informato del paziente e, secondo la Dichiarazione di Helsinki, solo se non vi siano trattamenti di provata efficacia per la situazione clinica soggetta a sperimentazione. Somministrare un nuovo farmaco potenzialmente efficace al di fuori di un contesto sperimentale significherebbe esporre il paziente al rischio di una sua tossicita’ sconosciuta; negarglielo a priori significherebbe privarlo della possibilita’ di goderne il possibile effetto benefico.

L’unica soluzione eticamente e scientificamente valida e’ la sperimentazione: il caso (la randomizzazione) distribuira’ trattamento potenzialmente efficace o placebo a una popolazione di pazienti e il confronto dell’esito clinico nei due gruppi di trattamento consentira’ di concludere se il farmaco sperimentale e’ superiore al placebo.

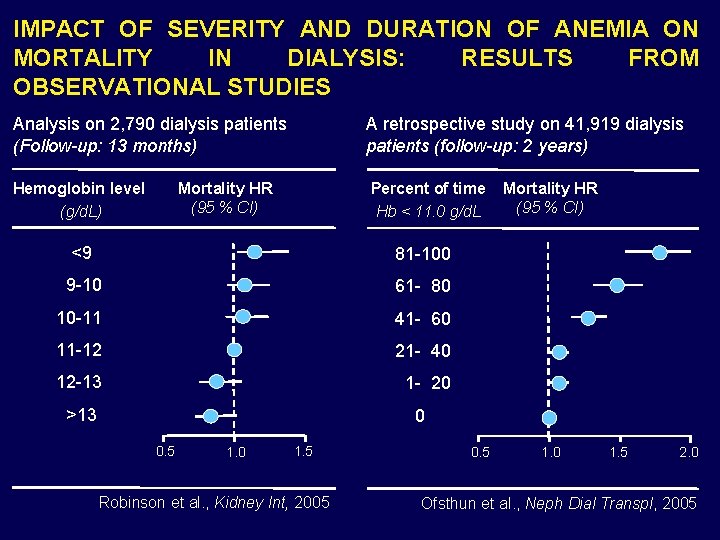
IMPACT OF SEVERITY AND DURATION OF ANEMIA ON MORTALITY IN DIALYSIS: RESULTS FROM OBSERVATIONAL STUDIES Analysis on 2, 790 dialysis patients (Follow-up: 13 months) A retrospective study on 41, 919 dialysis patients (follow-up: 2 years) Hemoglobin level (g/d. L) Percent of time Hb < 11. 0 g/d. L Mortality HR (95 % CI) <9 81 -100 9 -10 61 - 80 10 -11 41 - 60 11 -12 21 - 40 12 -13 1 - 20 >13 Mortality HR (95 % CI) 0 0. 5 1. 0 1. 5 Robinson et al. , Kidney Int, 2005 0. 5 1. 0 1. 5 2. 0 Ofsthun et al. , Neph Dial Transpl, 2005

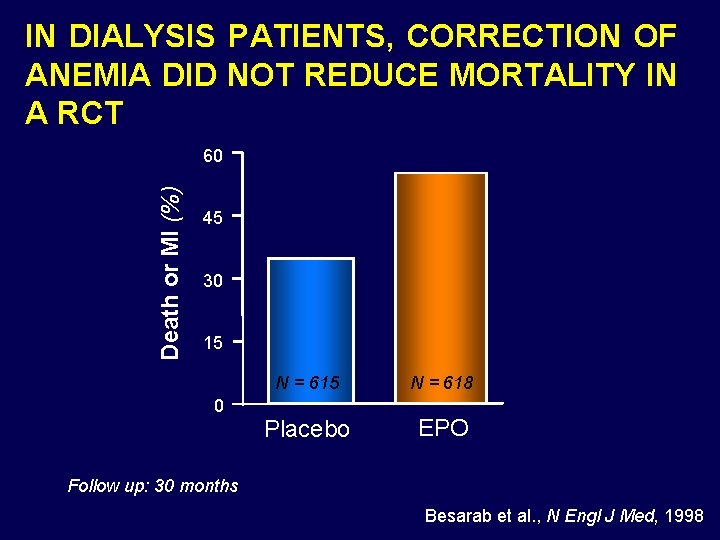
IN DIALYSIS PATIENTS, CORRECTION OF ANEMIA DID NOT REDUCE MORTALITY IN A RCT Death or MI (%) 60 45 30 15 0 N = 615 N = 618 Placebo EPO Follow up: 30 months Besarab et al. , N Engl J Med, 1998

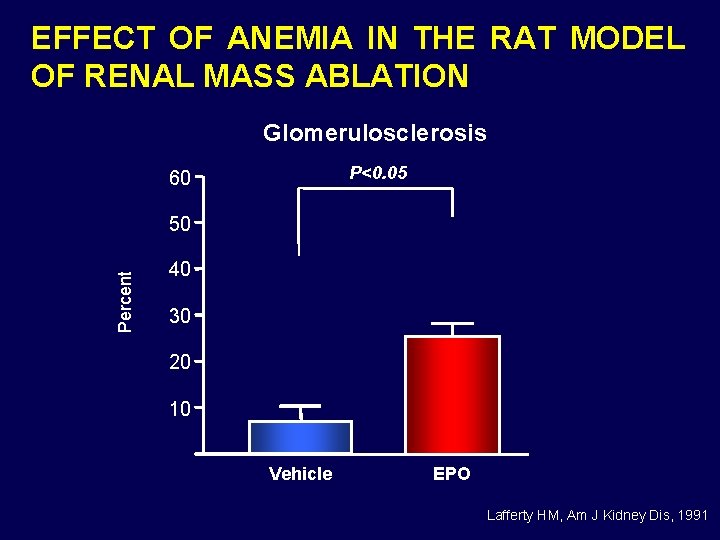
EFFECT OF ANEMIA IN THE RAT MODEL OF RENAL MASS ABLATION Glomerulosclerosis P<0. 05 60 Percent 50 40 30 20 10 Vehicle EPO Lafferty HM, Am J Kidney Dis, 1991

Randomized controlled trials: are they always really worth?

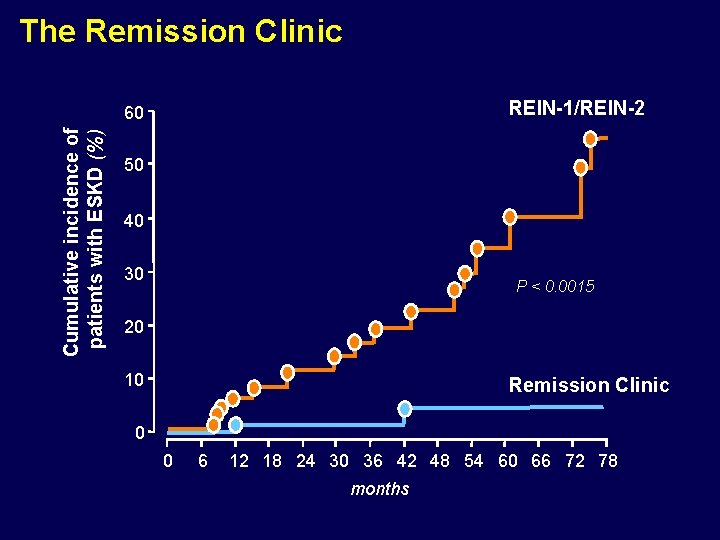
The Remission Clinic REIN-1/REIN-2 Cumulative incidence of patients with ESKD (%) 60 50 40 30 P < 0. 0015 20 10 Remission Clinic 0 0 6 12 18 24 30 36 42 48 54 60 66 72 78 months

Reviewer ## (from New England Journal of Medicine) “Though the results of this study are of interests, it can be argued that findings essentially provide very good rational for a randomized clinical trial comparing this Remission Clinic approach versus conventional therapy”

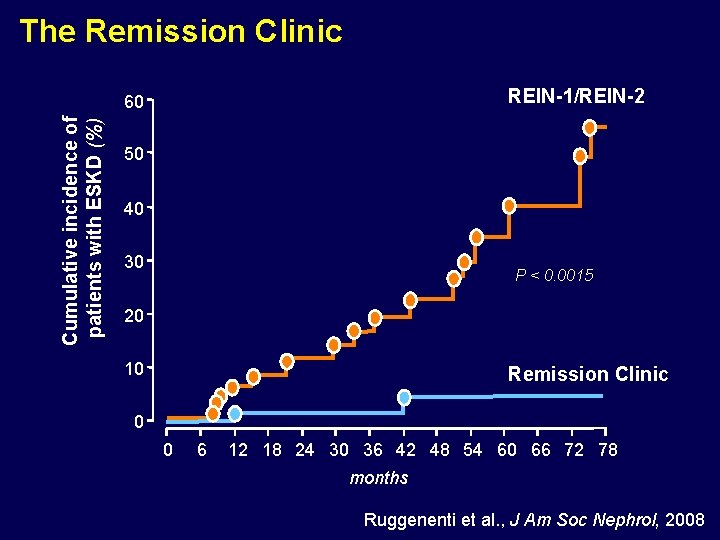
The Remission Clinic REIN-1/REIN-2 Cumulative incidence of patients with ESKD (%) 60 50 40 30 P < 0. 0015 20 10 Remission Clinic 0 0 6 12 18 24 30 36 42 48 54 60 66 72 78 months Ruggenenti et al. , J Am Soc Nephrol, 2008


Design: Systematic review of randomized controlled trials Data sources: Medline, Web of Science, Embase, and the Cochrane Library databases Study selection: Studies showing the effects of using a parachute during free fall Main outcome measure: Death or major trauma defined as an injury severity score > 15

No randomized controlled trials of parachute use have been undertaken

The basis for parachute use is purely observational The parachute industry has earned billions of dollars depending on belief in the efficacy of their product

Many current treatments - e. g. steroids in many autoimmune disases - are not based on results from RCT. Their efficacy is self evident and it would sound unethical to test them against placebo. On the other hand, we are watching the growth of trials with ‘new’ molecules whose results do not to provide any major advantage (at least not to patients) over current medical practive.

NON INFERIORITY TRIALS The new products offer little innovation. Some analyses indicate that only 10 -20% of the products reaching the market offer substantial advantages over existing ones Use of equivalence or non-inferiority trials rather than superiority designs implies the intention of not trying to prove any additional value of new drugs Non-inferiority allows new products to compete with older ones on the basis of small differences made to seem to benefit patients Garattini S, BJCP, 2004

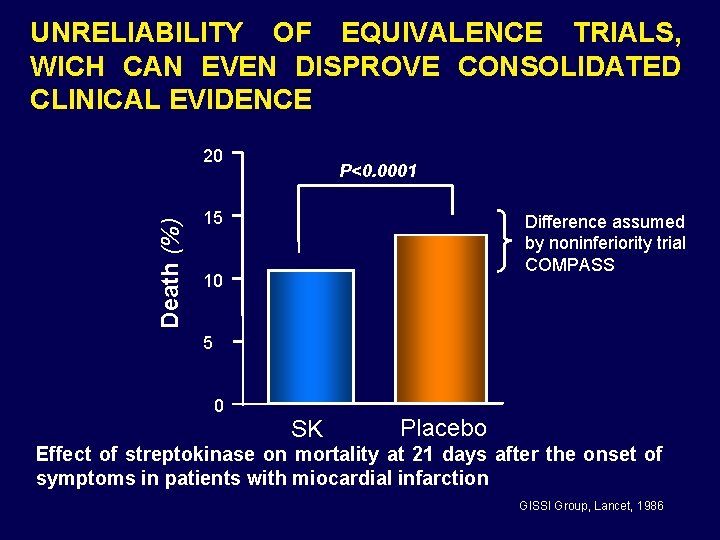
UNRELIABILITY OF EQUIVALENCE TRIALS, WICH CAN EVEN DISPROVE CONSOLIDATED CLINICAL EVIDENCE Death (%) 20 P<0. 0001 15 Difference assumed by noninferiority trial COMPASS 10 5 0 SK Placebo Effect of streptokinase on mortality at 21 days after the onset of symptoms in patients with miocardial infarction GISSI Group, Lancet, 1986

Assuming as equivalent a treatment that can result in 50% excess mortality is open to criticism from both the ethical and the methodological point of view. Equivalence trials should not be accepted as a basis for marketing authorization by the regulatory authorities.

A more convincing approach might be to limit non-inferiority trials for the sake of improved safety

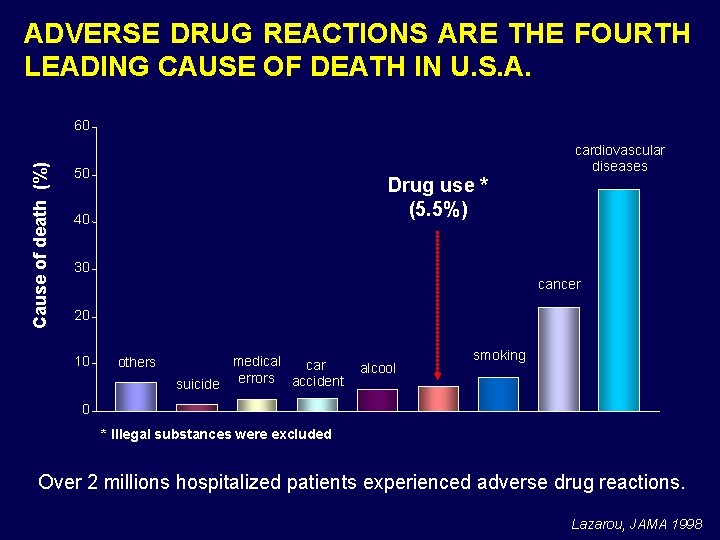
ADVERSE DRUG REACTIONS ARE THE FOURTH LEADING CAUSE OF DEATH IN U. S. A. Cause of death (%) 60 50 Drug use * (5. 5%) 40 cardiovascular diseases 30 cancer 20 10 others medical car errors accident suicide alcool smoking 0 * Illegal substances were excluded Over 2 millions hospitalized patients experienced adverse drug reactions. Lazarou, JAMA 1998

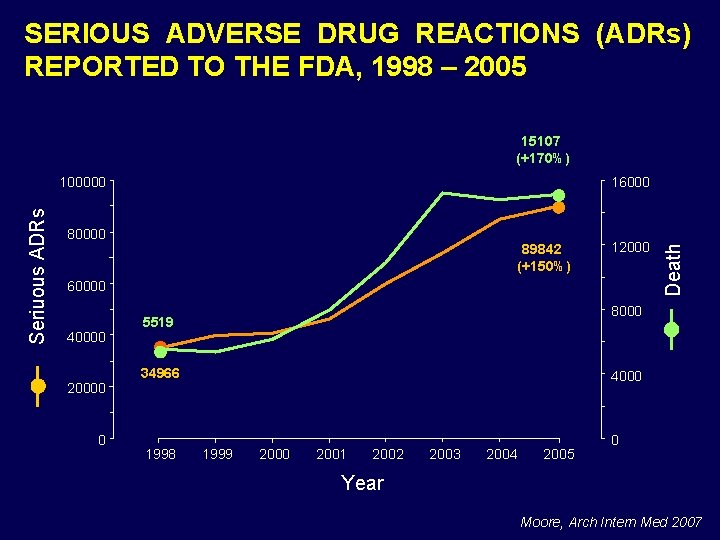
SERIOUS ADVERSE DRUG REACTIONS (ADRs) REPORTED TO THE FDA, 1998 – 2005 15107 (+170%) 16000 80000 89842 (+150%) 12000 60000 40000 20000 0 8000 5519 34966 1998 Death Seriuous ADRs 100000 4000 1999 2000 2001 2002 2003 2004 2005 0 Year Moore, Arch Intern Med 2007

Even to test superior safety profile of a new drug compared to older ones, a superiority trial would be a preferable way to compare effectiveness of two treatments in terms of survival without adverse events.

The Story of Chronic Kidney Disease

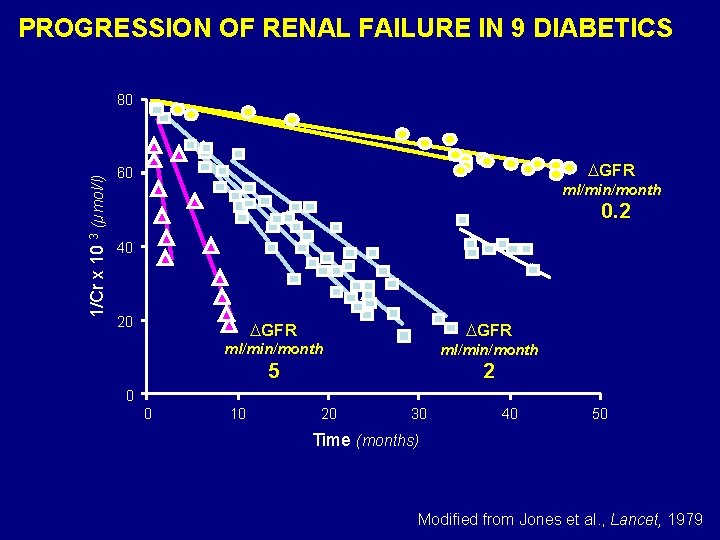
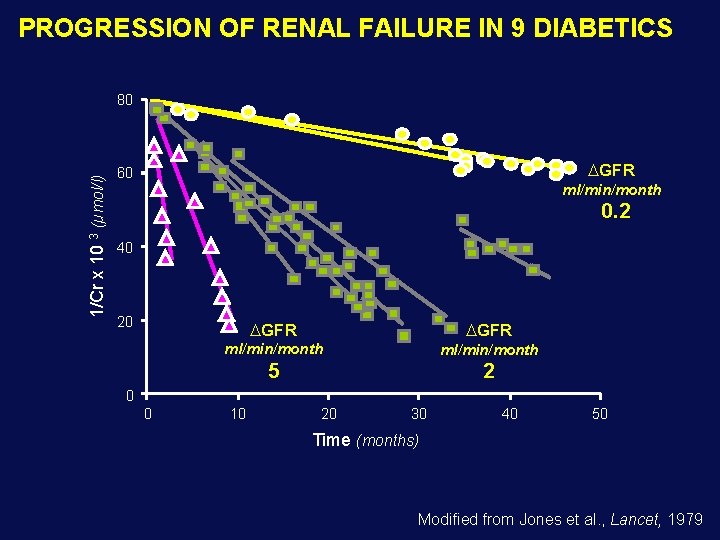
PROGRESSION OF RENAL FAILURE IN 9 DIABETICS 1/Cr x 10 3 (µmol/l) 80 GFR 60 ml/min/month 0. 2 40 20 GFR ml/min/month 5 2 0 0 10 20 30 40 50 Time (months) Modified from Jones et al. , Lancet, 1979

Glomerular hypertension Disease progression

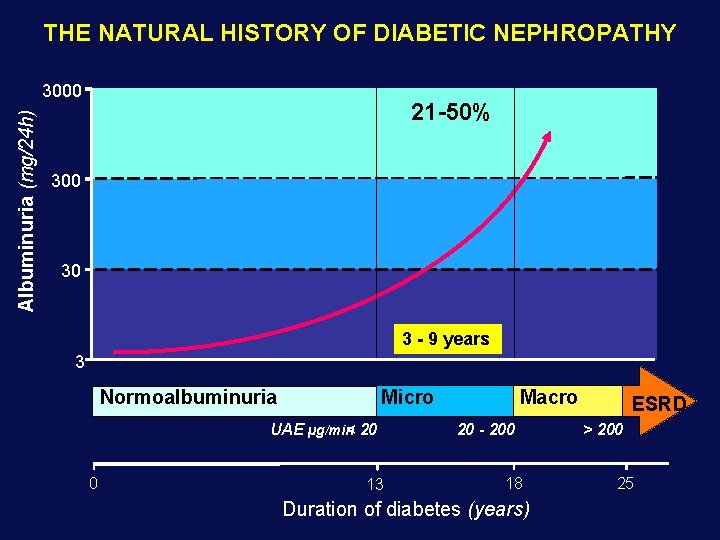
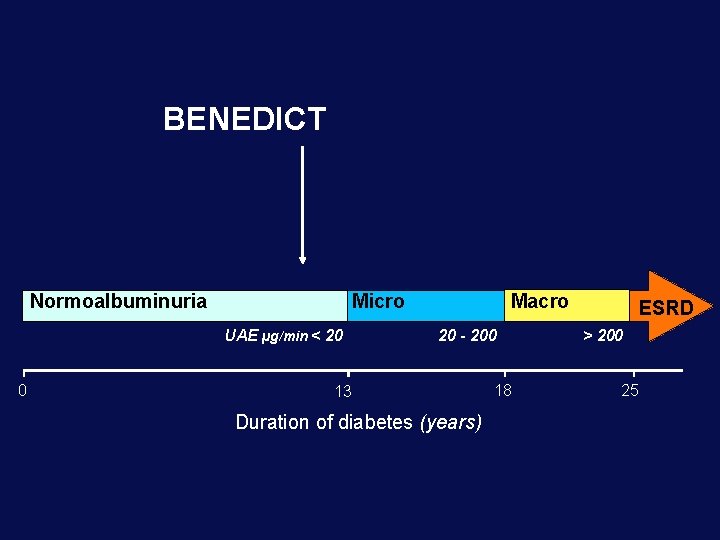
THE NATURAL HISTORY OF DIABETIC NEPHROPATHY Albuminuria (mg/24 h) 3000 21 -50% 300 30 3 - 9 years 3 Normoalbuminuria Micro UAE µg/min< 20 0 13 Macro 20 - 200 18 Duration of diabetes (years) ESRD > 200 25

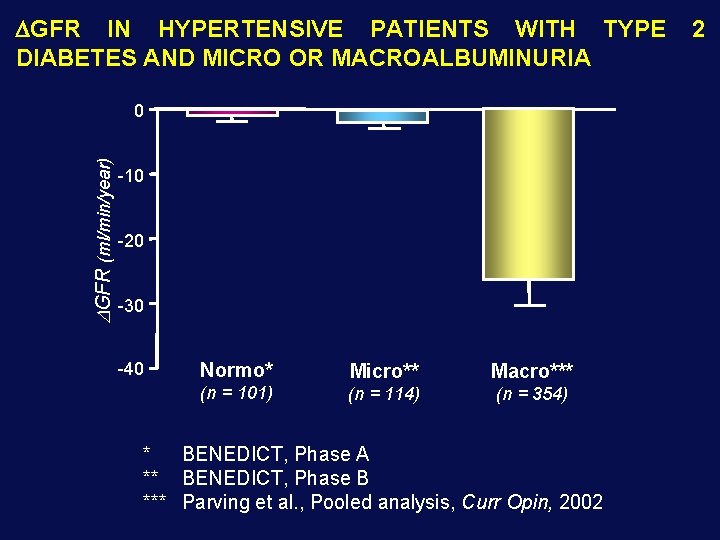
GFR IN HYPERTENSIVE PATIENTS WITH TYPE DIABETES AND MICRO OR MACROALBUMINURIA DGFR (ml/min/year) 0 -10 -20 -30 -40 Normo* Micro** Macro*** (n = 101) (n = 114) (n = 354) * BENEDICT, Phase A ** BENEDICT, Phase B *** Parving et al. , Pooled analysis, Curr Opin, 2002 2

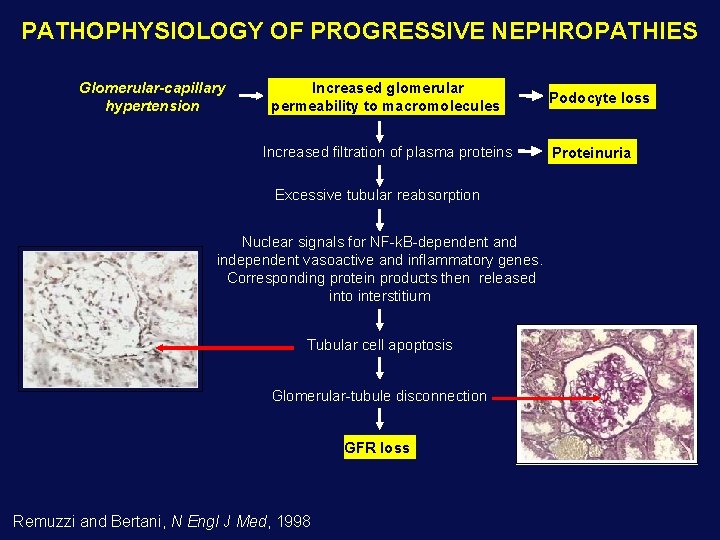
PATHOPHYSIOLOGY OF PROGRESSIVE NEPHROPATHIES Glomerular-capillary hypertension Increased glomerular permeability to macromolecules Increased filtration of plasma proteins Excessive tubular reabsorption Nuclear signals for NF-k. B-dependent and independent vasoactive and inflammatory genes. Corresponding protein products then released into interstitium Tubular cell apoptosis Glomerular-tubule disconnection GFR loss Remuzzi and Bertani, N Engl J Med, 1998 Podocyte loss Proteinuria

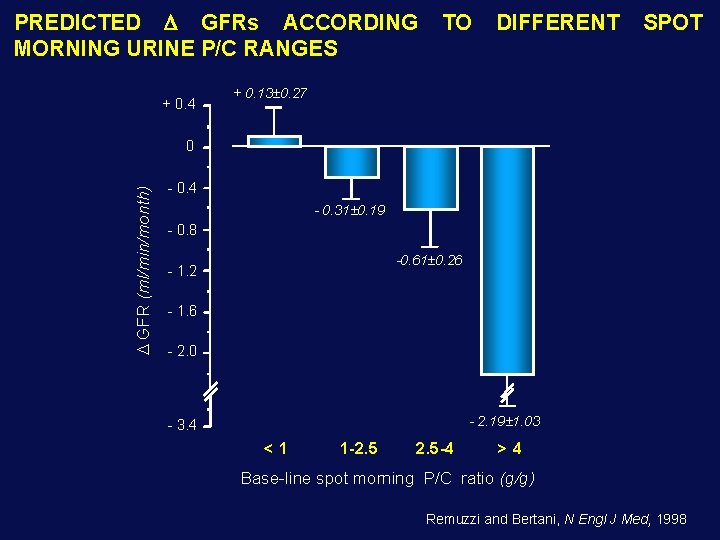
PREDICTED GFRs ACCORDING MORNING URINE P/C RANGES + 0. 4 TO DIFFERENT SPOT + 0. 13± 0. 27 GFR (ml/min/month) 0 - 0. 4 - 0. 31± 0. 19 - 0. 8 -0. 61± 0. 26 - 1. 2 - 1. 6 - 2. 0 - 2. 19± 1. 03 - 3. 4 <1 1 -2. 5 -4 >4 Base-line spot morning P/C ratio (g/g) Remuzzi and Bertani, N Engl J Med, 1998

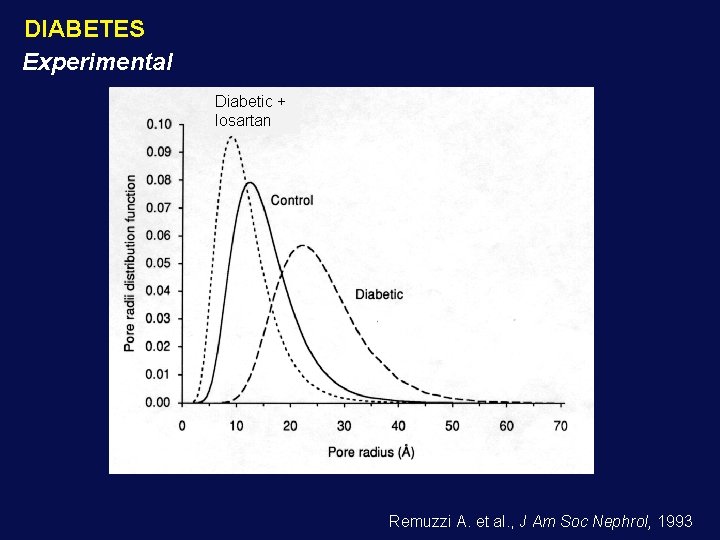
DIABETES Experimental Diabetic + losartan Remuzzi A. et al. , J Am Soc Nephrol, 1993

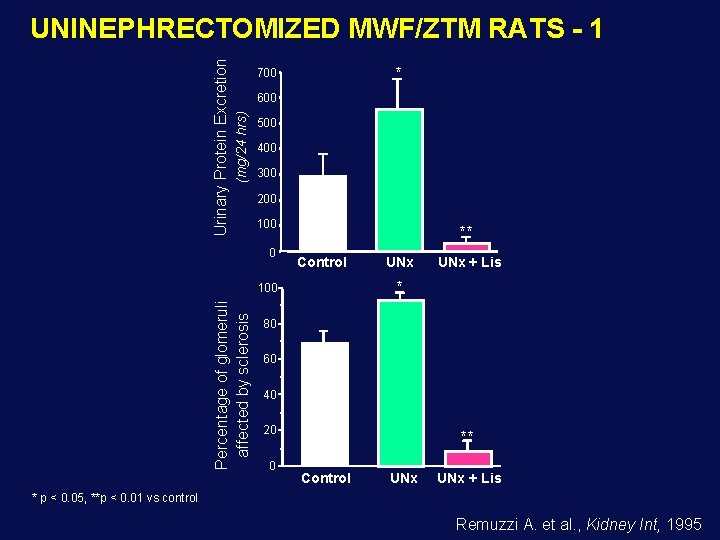
* 700 600 (mg/24 hrs) Urinary Protein Excretion UNINEPHRECTOMIZED MWF/ZTM RATS - 1 500 400 300 200 100 0 ** Control UNx + Lis * 100 Percentage of glomeruli affected by sclerosis UNx 80 60 40 20 0 ** Control UNx + Lis * p < 0. 05, **p < 0. 01 vs control Remuzzi A. et al. , Kidney Int, 1995

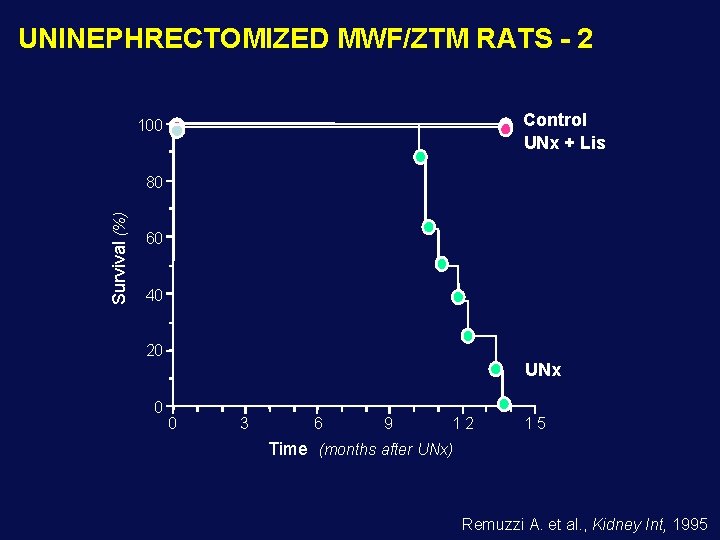
UNINEPHRECTOMIZED MWF/ZTM RATS - 2 Control UNx + Lis 100 Survival (%) 80 60 40 20 UNx 0 0 3 6 9 12 15 Time (months after UNx) Remuzzi A. et al. , Kidney Int, 1995

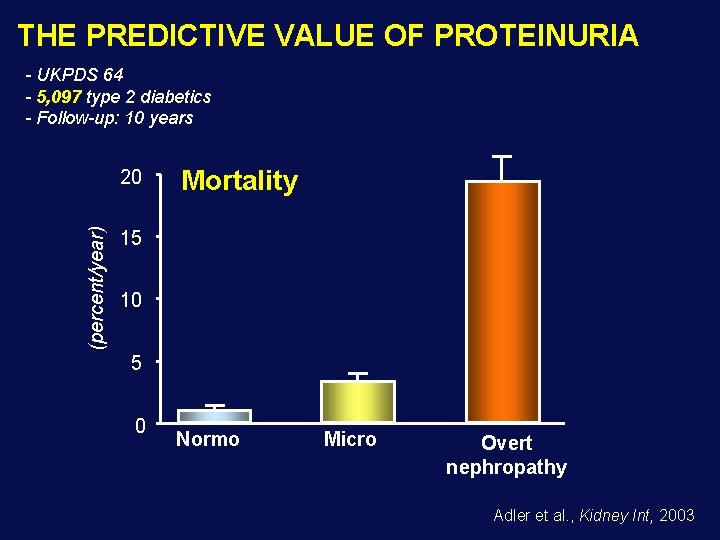
THE PREDICTIVE VALUE OF PROTEINURIA - UKPDS 64 - 5, 097 type 2 diabetics - Follow-up: 10 years (percent/year) 20 Mortality 15 10 5 0 Normo Micro Overt nephropathy Adler et al. , Kidney Int, 2003

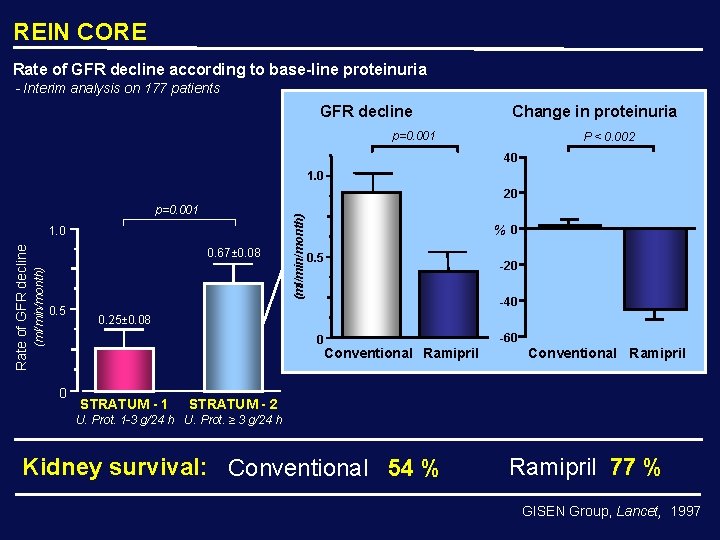
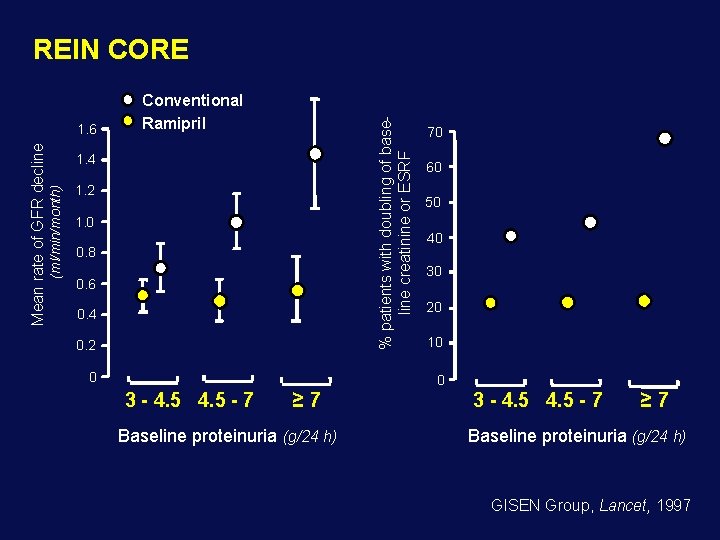
REIN CORE Rate of GFR decline according to base-line proteinuria - Interim analysis on 177 patients GFR decline Change in proteinuria p=0. 001 P < 0. 002 40 1. 0 p=0. 001 0. 67± 0. 08 (ml/min/month) Rate of GFR decline 1. 0 0. 5 (ml/min/month) 20 %0 0. 5 -40 0. 25± 0. 08 0 0 -20 STRATUM - 1 -60 Conventional Ramipril STRATUM - 2 U. Prot. 1 -3 g/24 h U. Prot. ≥ 3 g/24 h Kidney survival: Conventional 54 % Ramipril 77 % GISEN Group, Lancet, 1997

REIN CORE % patients with doubling of baseline creatinine or ESRF 1. 4 (ml/min/month) Mean rate of GFR decline 1. 6 Conventional Ramipril 1. 2 1. 0 0. 8 0. 6 0. 4 0. 2 0 70 60 50 40 30 20 10 0 3 - 4. 5 - 7 ≥ 7 Baseline proteinuria (g/24 h) GISEN Group, Lancet, 1997

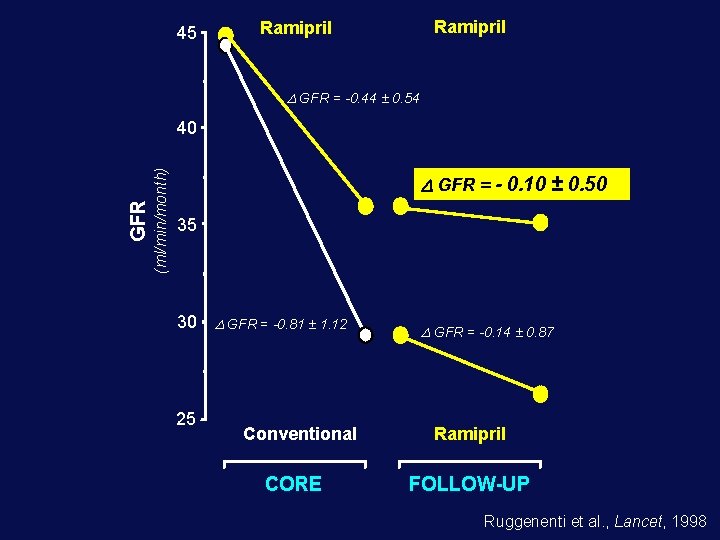
45 Ramipril D GFR = -0. 44 ± 0. 54 (ml/min/month) GFR 40 GFR = - 0. 10 ± 0. 50 35 30 25 D GFR = -0. 81 ± 1. 12 Conventional CORE D GFR = -0. 14 ± 0. 87 Ramipril FOLLOW-UP Ruggenenti et al. , Lancet, 1998

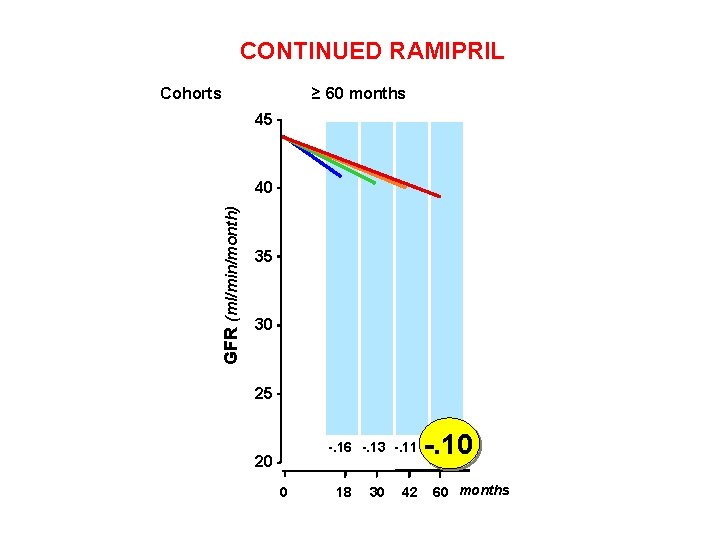
CONTINUED RAMIPRIL Cohorts ≥ 60 months 45 GFR (ml/min/month) 40 35 30 25 -. 16 -. 13 -. 11 20 0 18 30 42 -. 10 60 months

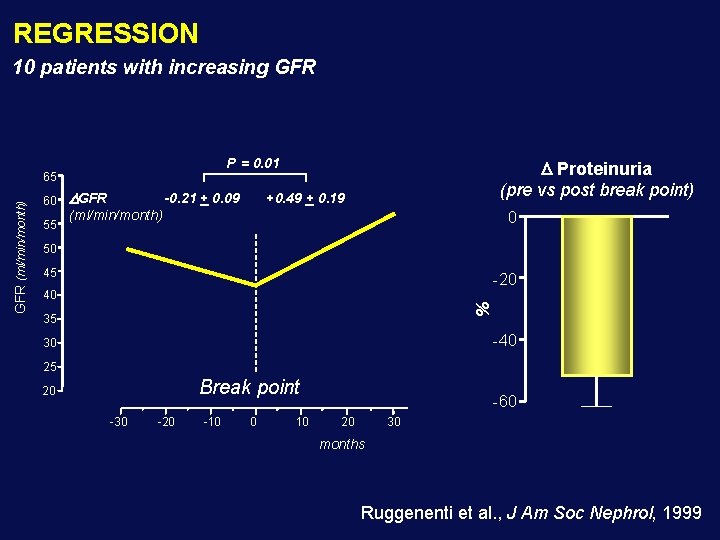
REGRESSION 10 patients with increasing GFR P = 0. 01 60 55 GFR -0. 21 + 0. 09 Proteinuria (pre vs post break point) +0. 49 + 0. 19 (ml/min/month) 0 50 45 -20 40 % GFR (ml/min/month) 65 35 -40 30 25 Break point 20 -30 -20 -10 0 10 -60 20 30 months Ruggenenti et al. , J Am Soc Nephrol, 1999

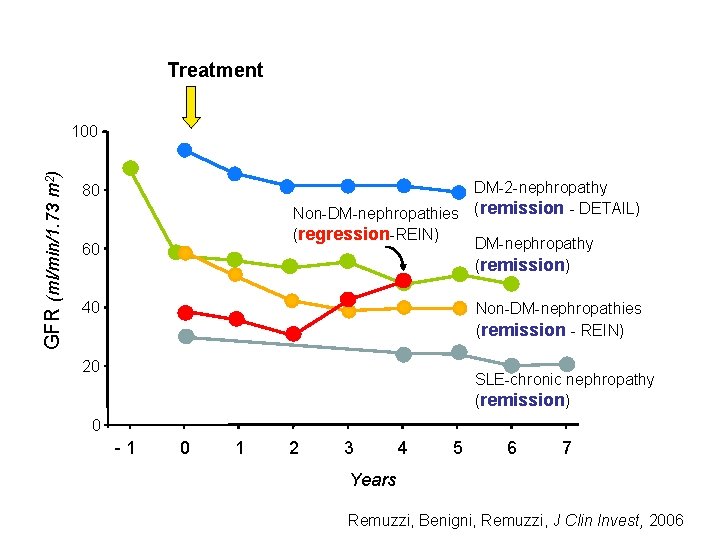
Treatment GFR (ml/min/1. 73 m 2) 100 DM-2 -nephropathy Non-DM-nephropathies (remission - DETAIL) (regression-REIN) DM-nephropathy (remission) 80 60 40 Non-DM-nephropathies (remission - REIN) 20 SLE-chronic nephropathy (remission) 0 -1 0 1 2 3 4 5 6 7 Years Remuzzi, Benigni, Remuzzi, J Clin Invest, 2006

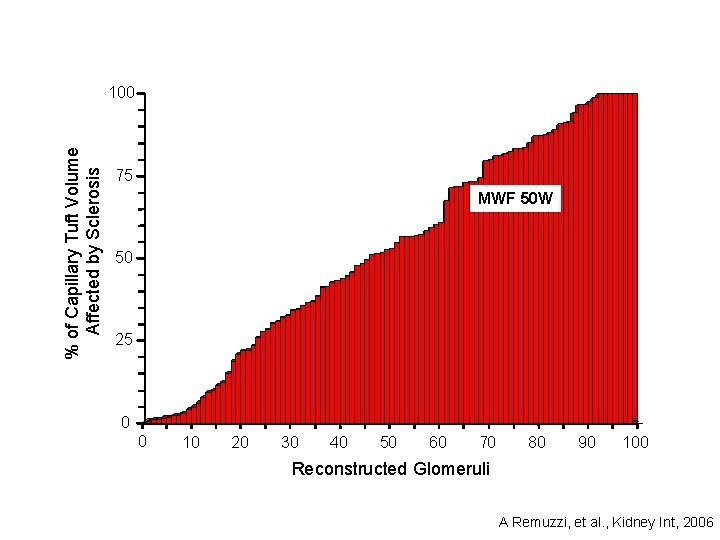
% of Capillary Tuft Volume Affected by Sclerosis 100 75 MWF 50 W 50 25 0 0 10 20 30 40 50 60 70 80 90 100 Reconstructed Glomeruli A Remuzzi, et al. , Kidney Int, 2006

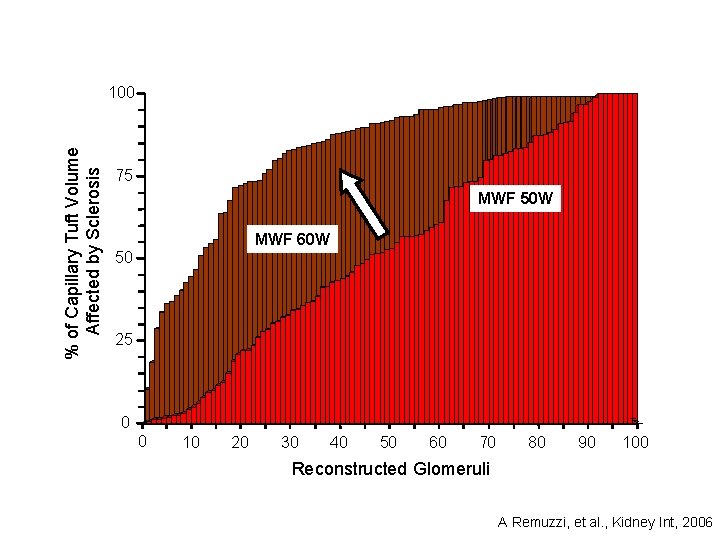
% of Capillary Tuft Volume Affected by Sclerosis 100 75 MWF 50 W MWF 60 W 50 25 0 0 10 20 30 40 50 60 70 80 90 100 Reconstructed Glomeruli A Remuzzi, et al. , Kidney Int, 2006

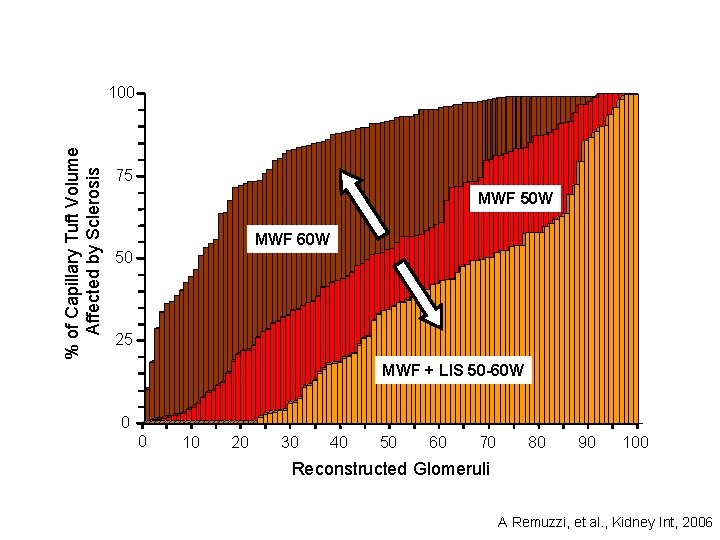
% of Capillary Tuft Volume Affected by Sclerosis 100 75 MWF 50 W MWF 60 W 50 25 MWF + LIS 50 -60 W 0 0 10 20 30 40 50 60 70 80 90 100 Reconstructed Glomeruli A Remuzzi, et al. , Kidney Int, 2006

Sclerosis was effectively reabsorbed and a consistent amount of glomerular tissue regained normal structure This suggests neoformation of glomerular capillary segments

INSIGHT INTO ACE-INDUCED REPAIR/ANGIOGENESIS RENAL Renal cells Adult differentiated Resident progenitor/stem Extra renal cells Endothelial progenitor and/or bone marrowderived stem

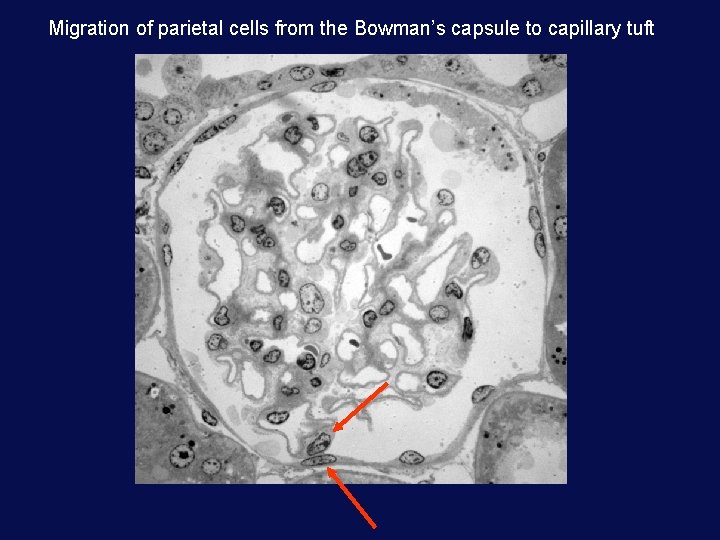
Migration of parietal cells from the Bowman’s capsule to capillary tuft

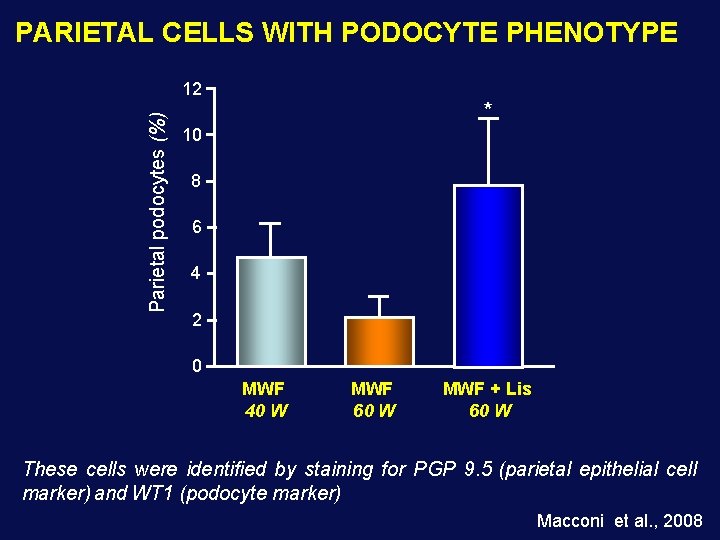
PARIETAL CELLS WITH PODOCYTE PHENOTYPE Parietal podocytes (%) 12 * 10 8 6 4 2 0 MWF 40 W MWF 60 W MWF + Lis 60 W These cells were identified by staining for PGP 9. 5 (parietal epithelial cell marker) and WT 1 (podocyte marker) Macconi et al. , 2008

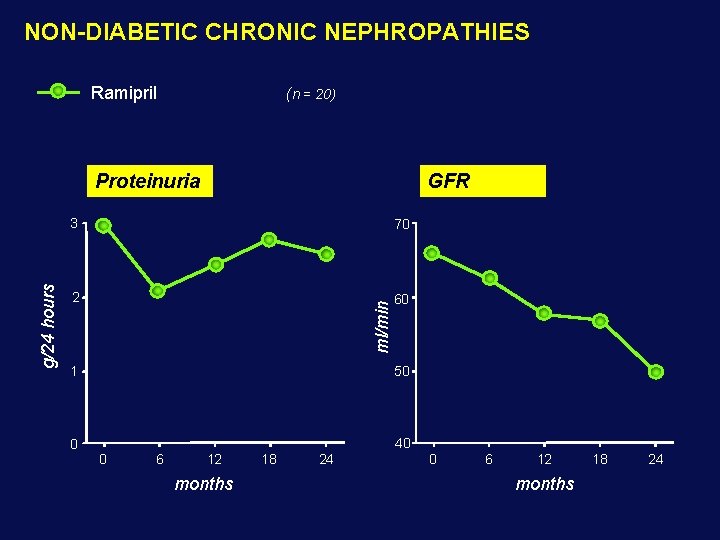
NON-DIABETIC CHRONIC NEPHROPATHIES Ramipril (n = 20) GFR 3 70 2 60 ml/min g/24 hours Proteinuria 1 50 0 40 0 6 12 months 18 24

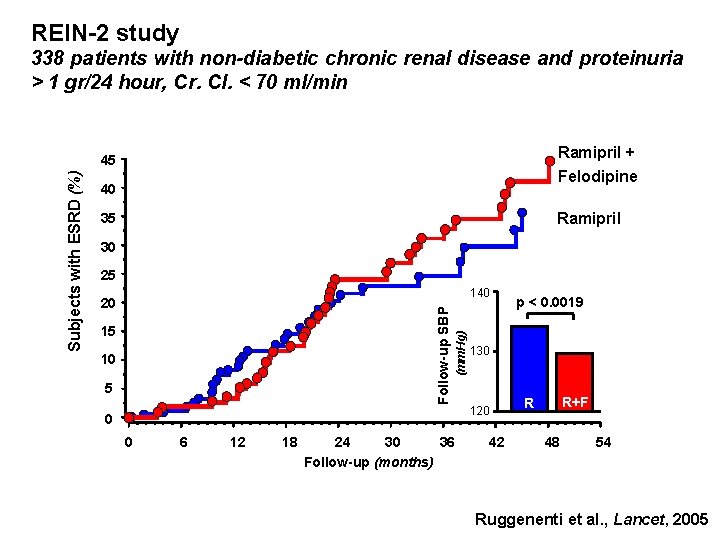
REIN-2 study 338 patients with non-diabetic chronic renal disease and proteinuria > 1 gr/24 hour, Cr. Cl. < 70 ml/min Ramipril + Felodipine 40 Ramipril 35 30 25 15 10 5 (mm. Hg) 140 20 Follow-up SBP Subjects with ESRD (%) 45 0 0 6 12 18 24 30 36 Follow-up (months) p < 0. 0019 130 120 42 R+F R 48 54 Ruggenenti et al. , Lancet, 2005

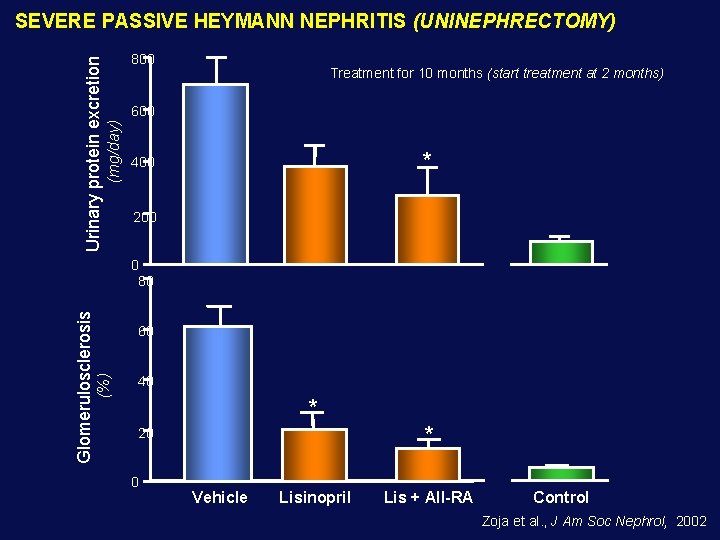
800 Treatment for 10 months (start treatment at 2 months) 600 (mg/day) Urinary protein excretion SEVERE PASSIVE HEYMANN NEPHRITIS (UNINEPHRECTOMY) * 400 200 60 (%) Glomerulosclerosis 0 80 40 * * 20 0 Vehicle Lisinopril Lis + AII-RA Control Zoja et al. , J Am Soc Nephrol, 2002

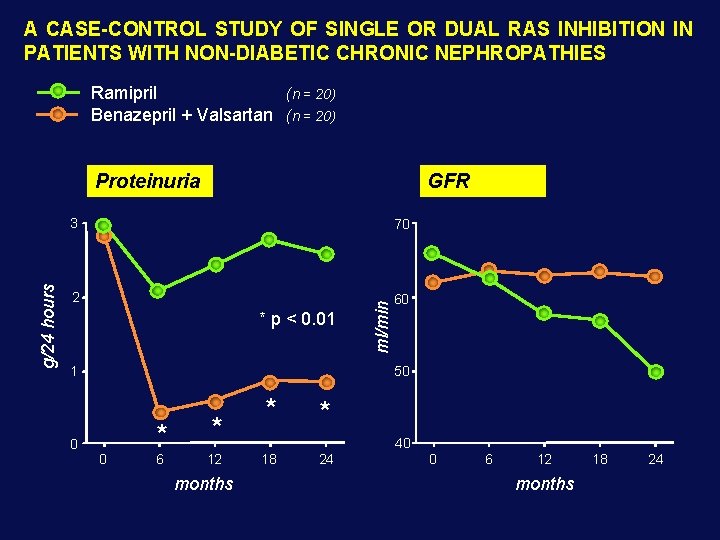
A CASE-CONTROL STUDY OF SINGLE OR DUAL RAS INHIBITION IN PATIENTS WITH NON-DIABETIC CHRONIC NEPHROPATHIES Ramipril (n = 20) Benazepril + Valsartan (n = 20) GFR 3 70 2 60 * p < 0. 01 1 0 ml/min g/24 hours Proteinuria 50 * 0 6 * 12 months * * 40 18 24 0 6 12 months 18 24

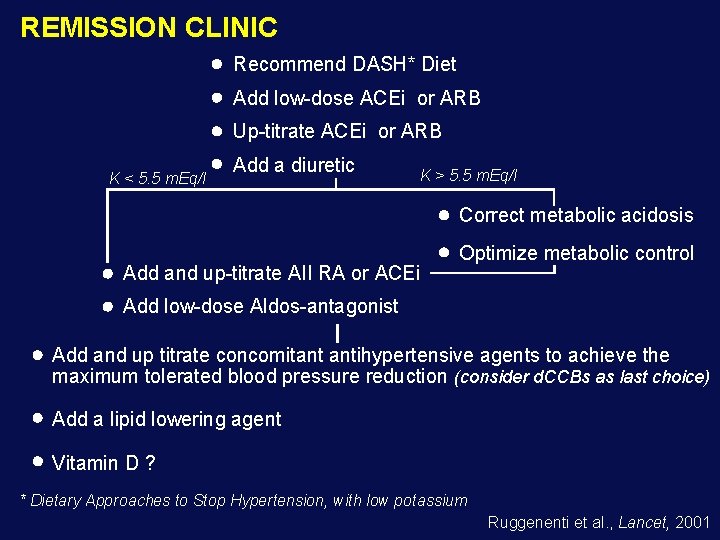
REMISSION CLINIC Recommend DASH* Diet Add low-dose ACEi or ARB Up-titrate ACEi or ARB K < 5. 5 m. Eq/l Add a diuretic K > 5. 5 m. Eq/l Correct metabolic acidosis Add and up-titrate AII RA or ACEi Optimize metabolic control Add low-dose Aldos-antagonist Add and up titrate concomitant antihypertensive agents to achieve the maximum tolerated blood pressure reduction (consider d. CCBs as last choice) Add a lipid lowering agent Vitamin D ? * Dietary Approaches to Stop Hypertension, with low potassium Ruggenenti et al. , Lancet, 2001

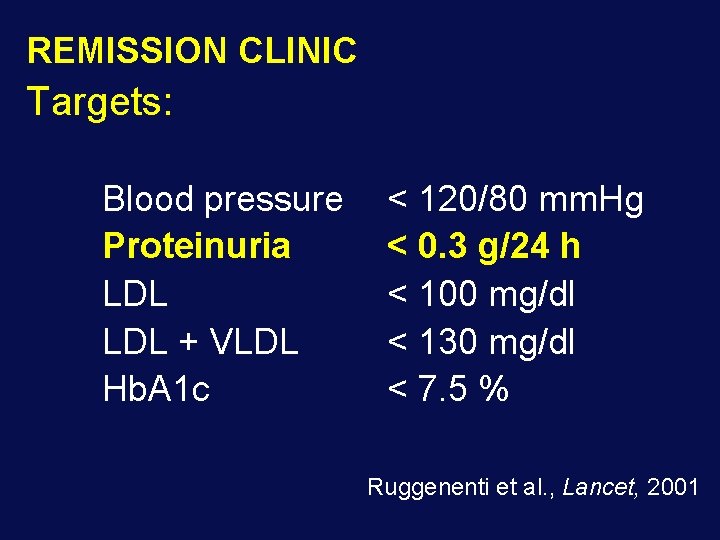
REMISSION CLINIC Targets: Blood pressure Proteinuria LDL + VLDL Hb. A 1 c < 120/80 mm. Hg < 0. 3 g/24 h < 100 mg/dl < 130 mg/dl < 7. 5 % Ruggenenti et al. , Lancet, 2001

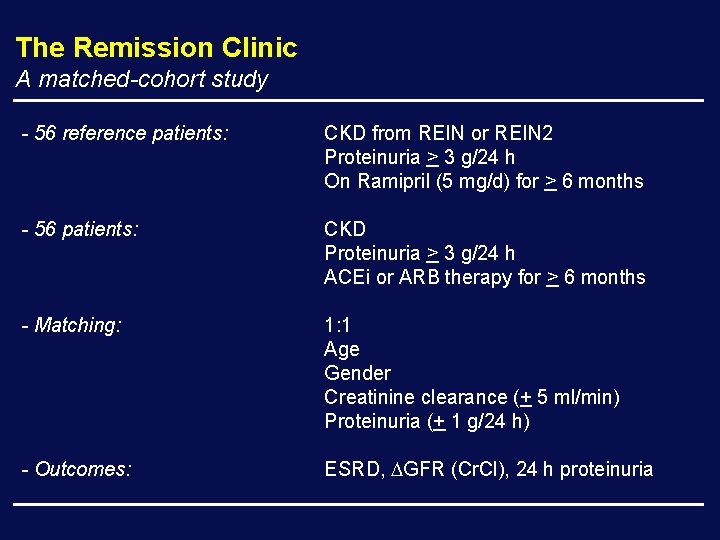
The Remission Clinic A matched-cohort study - 56 reference patients: CKD from REIN or REIN 2 Proteinuria > 3 g/24 h On Ramipril (5 mg/d) for > 6 months - 56 patients: CKD Proteinuria > 3 g/24 h ACEi or ARB therapy for > 6 months - Matching: 1: 1 Age Gender Creatinine clearance (+ 5 ml/min) Proteinuria (+ 1 g/24 h) - Outcomes: ESRD, GFR (Cr. Cl), 24 h proteinuria

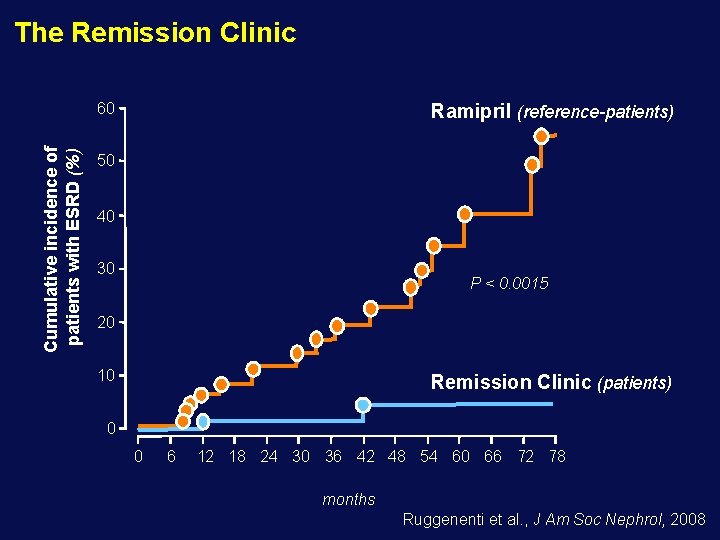
The Remission Clinic Cumulative incidence of patients with ESRD (%) 60 Ramipril (reference-patients) 50 40 30 P < 0. 0015 20 10 Remission Clinic (patients) 0 0 6 12 18 24 30 36 42 48 54 60 66 72 78 months Ruggenenti et al. , J Am Soc Nephrol, 2008

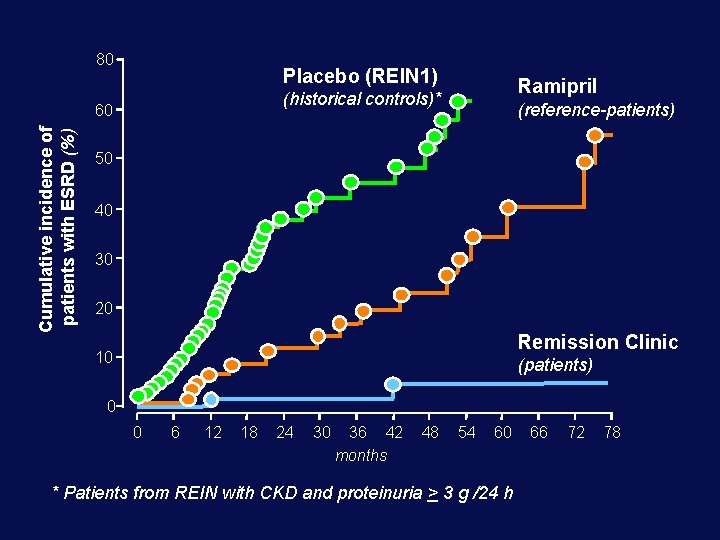
80 Placebo (REIN 1) 60 Cumulative incidence of patients with ESRD (%) Ramipril (historical controls)* (reference-patients) 50 40 30 20 Remission Clinic 10 (patients) 0 0 6 12 18 24 30 36 42 months 48 54 60 * Patients from REIN with CKD and proteinuria > 3 g /24 h 66 72 78


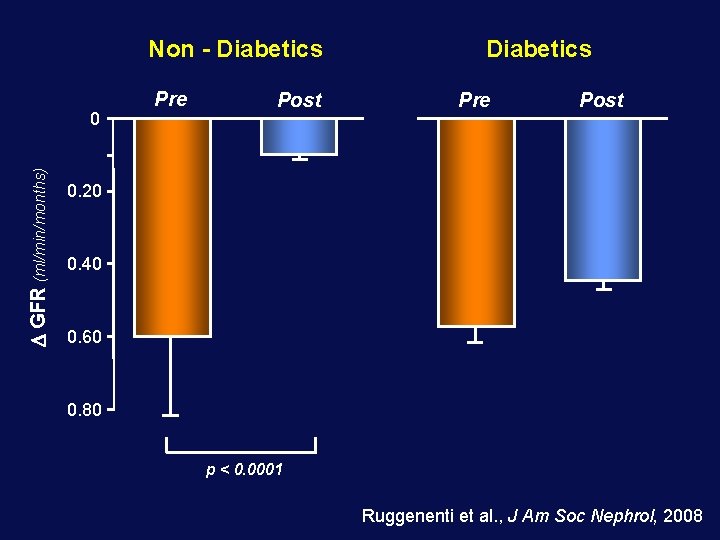
Non - Diabetics GFR (ml/min/months) 0 Pre Post Diabetics Pre Post 0. 20 0. 40 0. 60 0. 80 p < 0. 0001 Ruggenenti et al. , J Am Soc Nephrol, 2008

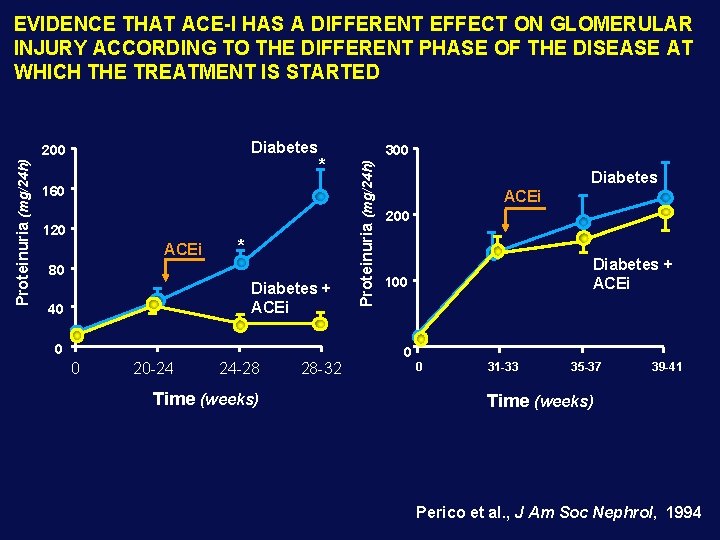
EVIDENCE THAT ACE-I HAS A DIFFERENT EFFECT ON GLOMERULAR INJURY ACCORDING TO THE DIFFERENT PHASE OF THE DISEASE AT WHICH THE TREATMENT IS STARTED Proteinuria (mg/24 h) * 160 120 ACEi * 80 Diabetes + ACEi 40 0 0 20 -24 24 -28 Time (weeks) 28 -32 300 Proteinuria (mg/24 h) Diabetes 200 Diabetes ACEi 200 Diabetes + ACEi 100 0 0 31 -33 35 -37 39 -41 Time (weeks) Perico et al. , J Am Soc Nephrol, 1994

BENEDICT Normoalbuminuria Micro UAE µg/min < 20 0 Macro 20 - 200 13 Duration of diabetes (years) 18 ESRD > 200 25

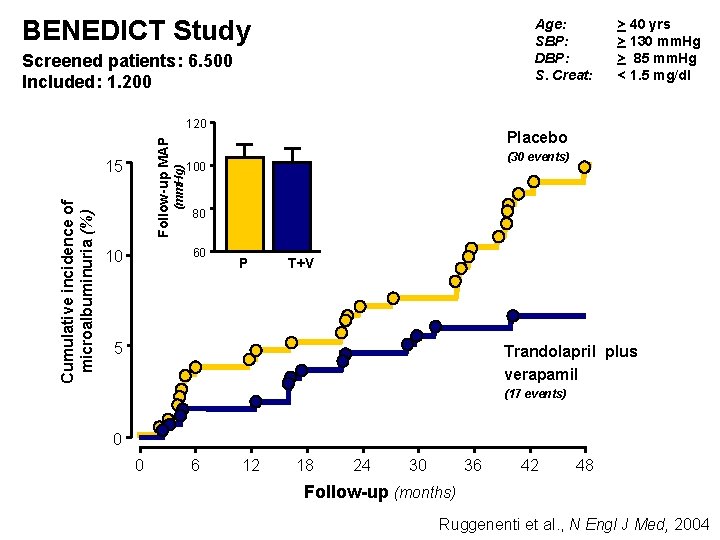
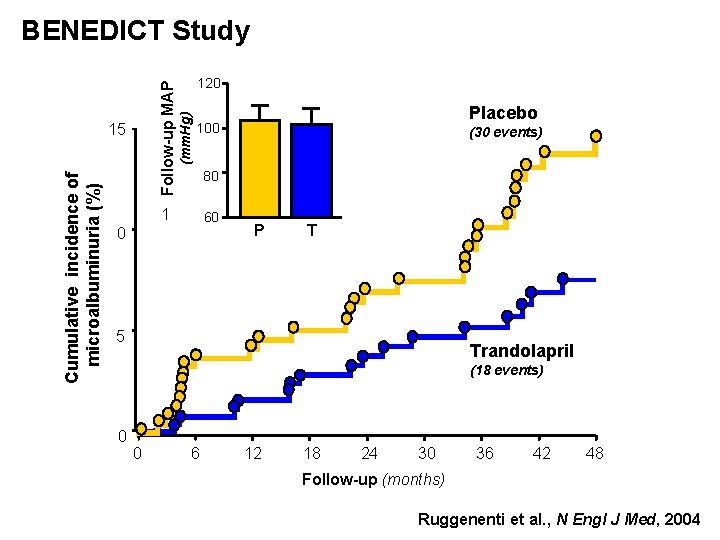
BENEDICT Study Age: SBP: DBP: S. Creat: Screened patients: 6. 500 Included: 1. 200 Cumulative incidence of microalbuminuria (%) 15 (mm. Hg) Follow-up MAP 120 Placebo (30 events) 100 80 60 10 > 40 yrs > 130 mm. Hg > 85 mm. Hg < 1. 5 mg/dl P T+V 5 Trandolapril plus verapamil (17 events) 0 0 6 12 18 24 30 36 42 48 Follow-up (months) Ruggenenti et al. , N Engl J Med, 2004

Cumulative incidence of microalbuminuria (%) 15 120 (mm. Hg) Follow-up MAP BENEDICT Study Placebo 100 (30 events) 80 1 60 0 P T 5 Trandolapril (18 events) 0 0 6 12 18 24 30 36 42 48 Follow-up (months) Ruggenenti et al. , N Engl J Med, 2004

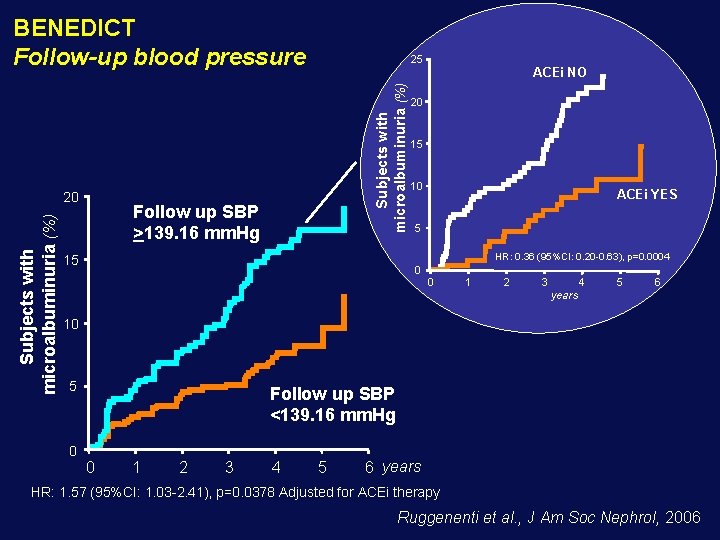
BENEDICT Follow-up blood pressure Subjects with microalbuminuria (%) 20 25 Follow up SBP >139. 16 mm. Hg ACEi NO 20 15 10 ACEi YES 5 HR: 0. 36 (95%CI: 0. 20 -0. 63), p=0. 0004 15 0 0 1 2 3 4 5 6 years 10 5 0 Follow up SBP <139. 16 mm. Hg 0 1 2 3 4 5 6 years HR: 1. 57 (95%CI: 1. 03 -2. 41), p=0. 0378 Adjusted for ACEi therapy Ruggenenti et al. , J Am Soc Nephrol, 2006

Baseline BP did not predict the risk of developing microalbuminuria on follow-up Lower incidence of microalbuminuria observed with more effective BP control, reflected a benefit of treatment

DIABETICS WITH HYPERTENSION 25 should be treated to prevent 1 new case of microalbuminuria over 3 to 4 years

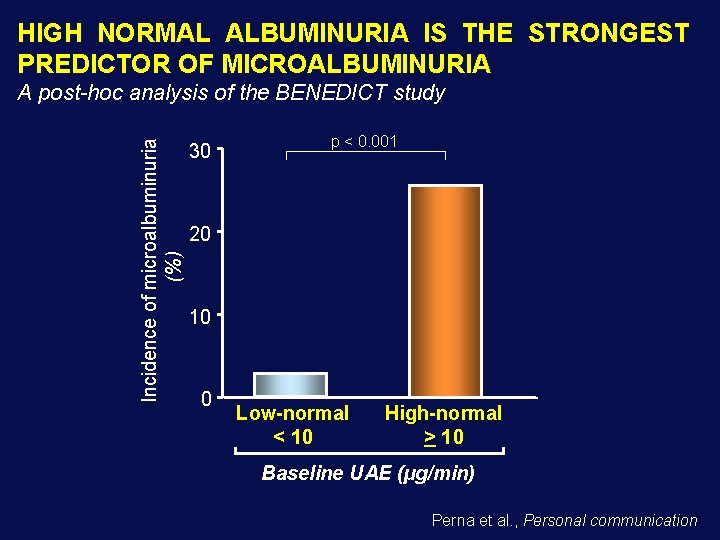
HIGH NORMAL ALBUMINURIA IS THE STRONGEST PREDICTOR OF MICROALBUMINURIA Incidence of microalbuminuria (%) A post-hoc analysis of the BENEDICT study 30 p < 0. 001 20 10 0 Low-normal < 10 High-normal > 10 Baseline UAE (µg/min) Perna et al. , Personal communication

DIABETICS WITH HYPERTENSION AND HIGH-NORMAL ALBUMINURIA 3 should be treated to prevent 1 new case of microalbuminuria over 3 to 4 years

The BENEDICT Network 1. Centro Dacco’ (Ranica) 4 2. Bergamo 3. Alzano Lombardo 7 4. Clusone 2 6 5. Romano di Lombardia 6. Ponte S. Pietro 7. Seriate 8. Treviglio 1 8 5 3

PROGRESSION OF RENAL FAILURE IN 9 DIABETICS 1/Cr x 10 3 (µmol/l) 80 GFR 60 ml/min/month 0. 2 40 20 GFR ml/min/month 5 2 0 0 10 20 30 40 50 Time (months) Modified from Jones et al. , Lancet, 1979

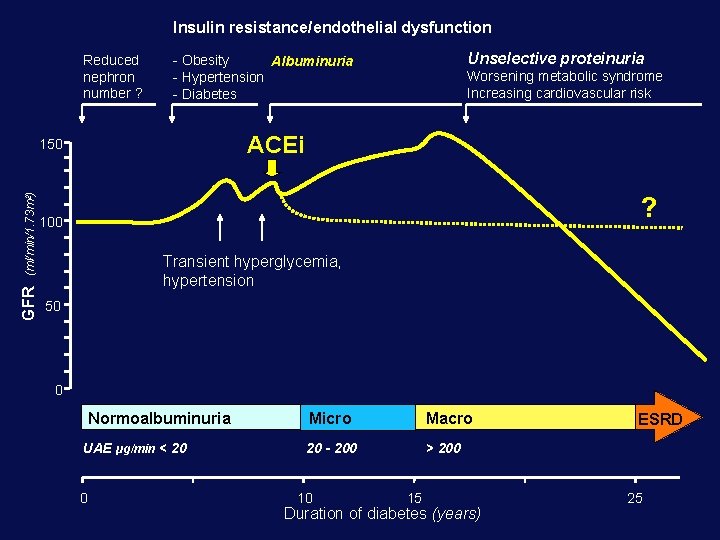
Insulin resistance/endothelial dysfunction Reduced nephron number ? (ml/min/1. 73 m 2) Worsening metabolic syndrome Increasing cardiovascular risk ACEi 150 GFR Unselective proteinuria - Obesity Albuminuria - Hypertension - Diabetes ? 100 Transient hyperglycemia, hypertension 50 0 Normoalbuminuria UAE µg/min < 20 0 Micro Macro 20 - 200 > 200 10 15 Duration of diabetes (years) ESRD 25

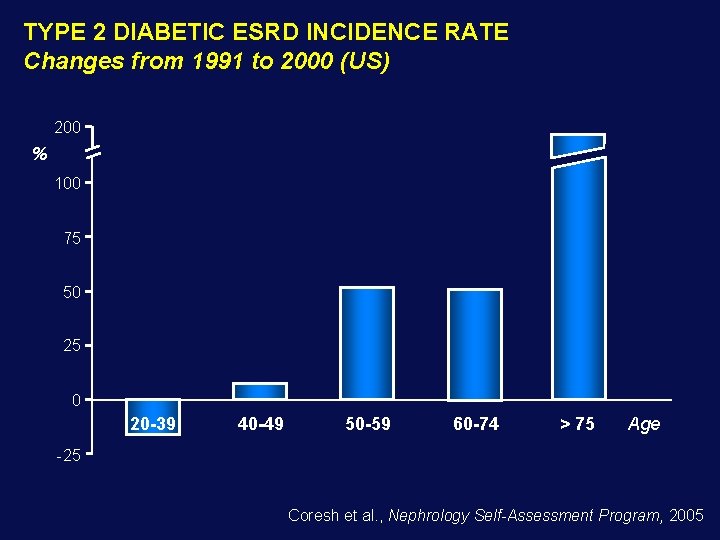
TYPE 2 DIABETIC ESRD INCIDENCE RATE Changes from 1991 to 2000 (US) 200 % 100 75 50 25 0 20 -39 40 -49 50 -59 60 -74 > 75 Age -25 Coresh et al. , Nephrology Self-Assessment Program, 2005

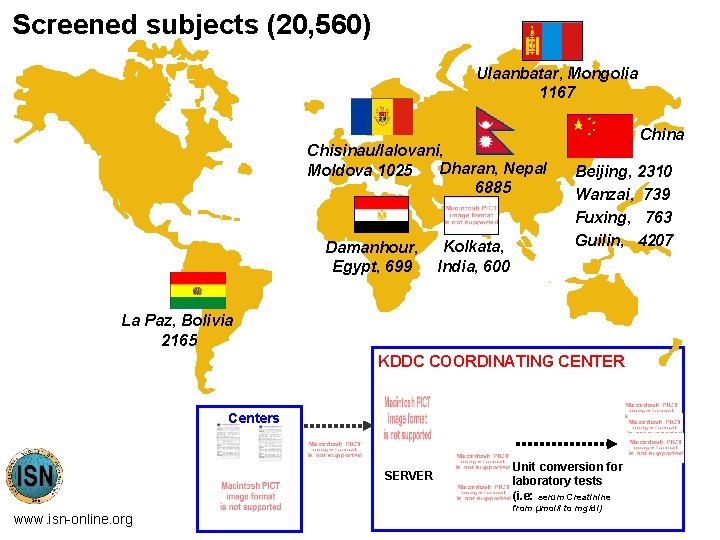
Screened subjects (20, 560) Ulaanbatar, Mongolia 1167 Chisinau/Ialovani, Dharan, Nepal Moldova 1025 6885 Damanhour, Egypt, 699 Kolkata, India, 600 China Beijing, 2310 Wanzai, 739 Fuxing, 763 Guilin, 4207 La Paz, Bolivia 2165 KDDC COORDINATING CENTER Centers SERVER www. isn-online. org Unit conversion for laboratory tests (i. e: serum Creatinine from µmol/l to mg/dl)

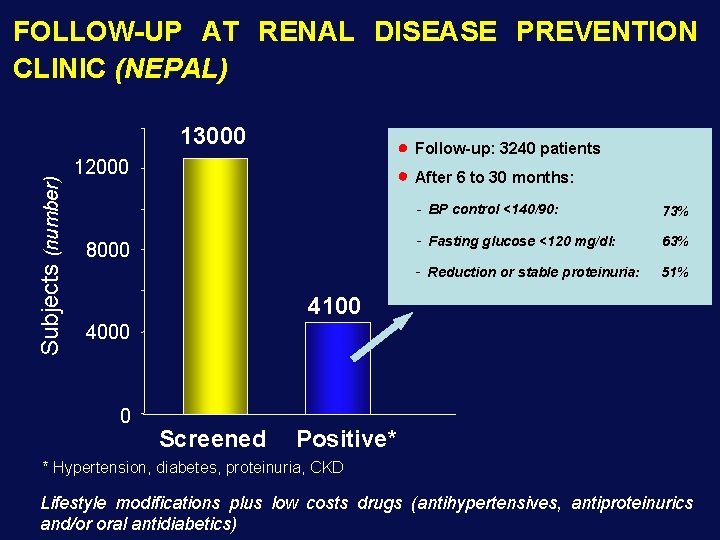
FOLLOW-UP AT RENAL DISEASE PREVENTION CLINIC (NEPAL) Subjects (number) 13000 Follow-up: 3240 patients 12000 After 6 to 30 months: 8000 - BP control <140/90: 73% - Fasting glucose <120 mg/dl: 63% - Reduction or stable proteinuria: 51% 4100 4000 0 Screened Positive* * Hypertension, diabetes, proteinuria, CKD Lifestyle modifications plus low costs drugs (antihypertensives, antiproteinurics and/or oral antidiabetics)

1, 000 deaths


A lot of people think that scientists do not respect ethical and human values and consider science as something in conflict with faith




L’Espresso, 1 ottobre 2009 Il prezzo della vita di Daniela Minerva Farmaci mirati: a volte efficaci, a volte no. Ma che possono allungare anche di pochi mesi un'esistenza. Sono costosissimi. E i medici si chiedono: vale la pena sperimentarli?

Journal of the National Cancer Institute, 2009

We must deal with the escalating price of cancer therapy now If we allow a survival advantage of 1. 2 months to be worth $ 80 000, and by extrapolation survival of 1 year to be valued at $ 800 000, we would need $ 440 billion annually - an amount nearly 100 times the budget of the National Cancer Institute - to extend by 1 year the life of the 550 000 Americans who die of cancer annually And no one would be cured

The current situation cannot continue We cannot ignore the cumulative costs of the tests and treatments we recommend and prescribe As the agents of change, professional societies, including their academic and practicing oncologist members, must lead the way The time to start is now



We politicians - concluded Hillary Clinton - we absolutely need you. What we also need are partnerships between the government and academic and research institutions. But you scientists have to raise your voice and stand up for science, you should participate in the debate personally ‘Thanks for what you are doing’ she told the transplant physicians before leaving ‘and for everything you did in these 50 years for million of people’

Immediately after Alonzo Mourning (famous basketball player from Miami) came on stage Black, about 7 feet tall, speaking like an old actor who everytime when he jokes is waiting for the reaction of the audience. Really funny, you can listen for hours to him Three years ago he received a kidney transplant

This year the Miami Heat won the NBA championship and they won for Alonzo Mourning

Dr. Hardy said “I have done thousands of these, and I can do it with my eyes closed” My response to him “Well, make sure you at least keep one eye open when you are doing my surgery” Alonzo Mourning, World Transplant Congress, 2006
