Legionellosis Risk Management Planning Indiana Chapter Fall Conference































- Slides: 70

Legionellosis: Risk Management Planning Indiana Chapter, Fall Conference of the: Association for Professionals in Infection Control and Epidemiology Michael Coughlin, Ph. D. Weas Engineering, Inc. October 10, 2014

Discussion Topics § § § Legionella Legionellosis Hazard Assessment Risk Mitigation Plan Validation Response to Outbreak

Discussion Topics § § § Legionella Legionellosis Risk Management Plan

Philadelphia: July, 1976 § § Mysterious Illness Affects 221 People 147 Hospitalized 34 Deaths Pneumonia with Flu-like Symptoms § § § § Fever Muscle aches Cough Purulent sputum Fluid in lung Could not visualize any micro-organisms from biopsy Could not culture any micro-organisms from biopsy Did not respond to conventional antibiotic therapy for pneumonia

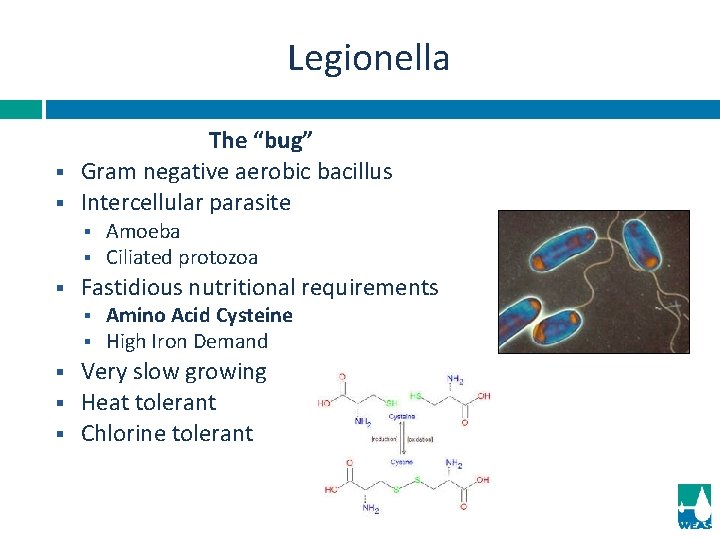
Legionella § § The “bug” Gram negative aerobic bacillus Intercellular parasite § § § Fastidious nutritional requirements § § § Amoeba Ciliated protozoa Amino Acid Cysteine High Iron Demand Very slow growing Heat tolerant Chlorine tolerant

Legionellosis: The Disease § § § § The Infection Acquired via inhalation of microscopic mist particles: < 5 microns Very often found in alveoli Not spread person to person 8, 000 – 18, 000 LD hospitalizations/year (CDC) § 85% Sporadic § 15% Outbreak 8% of diagnosed pneumonias are LD > 90% are of serotype 1 ~ 10% serogroups 2 -14, L. longbeachae, L. bozemanii

Legionellosis: The Disease Infectious Sources § § § § Cooling Towers Ornamental Ponds Vegetable Misters Cooling Misters Nebulizers Stand Alone Humidifiers Shower Heads Tap Aerators Spas Misting Tents Birthing Baths Ice Machines Compost

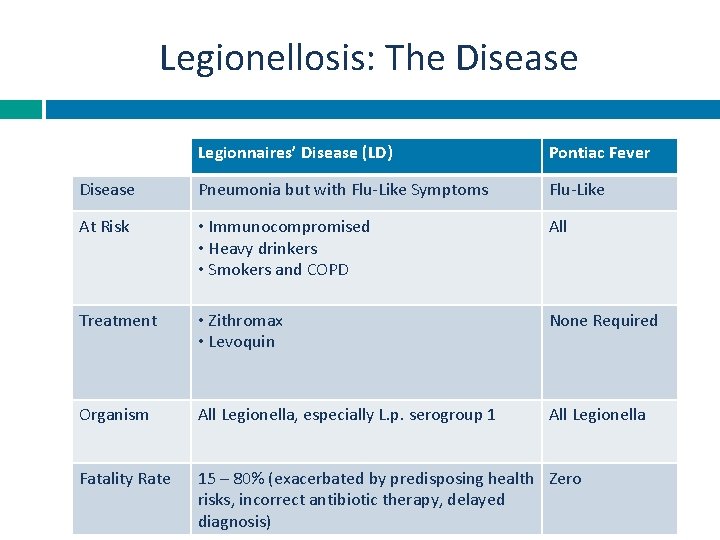
Legionellosis: The Disease Legionnaires’ Disease (LD) Pontiac Fever Disease Pneumonia but with Flu-Like Symptoms Flu-Like At Risk • Immunocompromised • Heavy drinkers • Smokers and COPD All Treatment • Zithromax • Levoquin None Required Organism All Legionella, especially L. p. serogroup 1 All Legionella Fatality Rate 15 – 80% (exacerbated by predisposing health Zero risks, incorrect antibiotic therapy, delayed diagnosis)

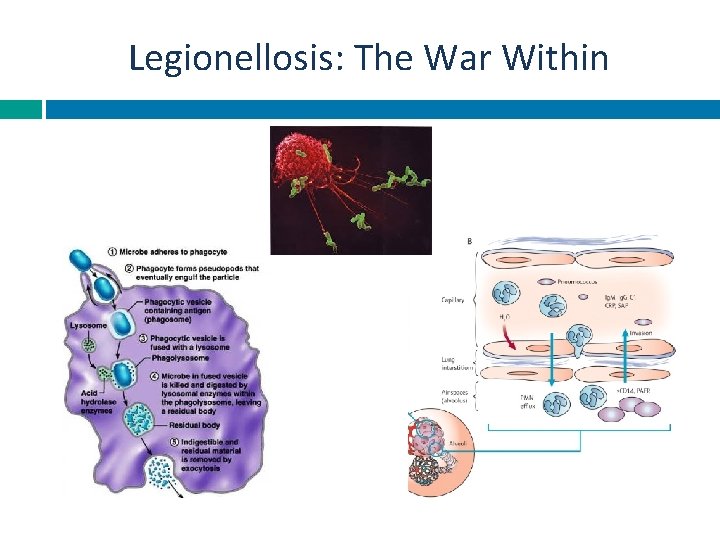
Legionellosis: The War Within

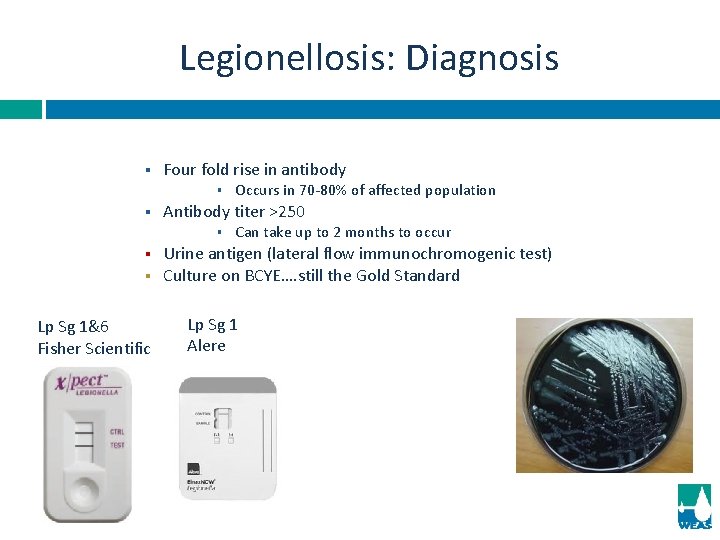
Legionellosis: Diagnosis § Four fold rise in antibody § § Antibody titer >250 § § § Lp Sg 1&6 Fisher Scientific Occurs in 70 -80% of affected population Can take up to 2 months to occur Urine antigen (lateral flow immunochromogenic test) Culture on BCYE…. still the Gold Standard Lp Sg 1 Alere

Legionellosis: Diagnosis Compared to other pneumonias, LD presents same symptoms as other bacterial pneumonias, e. g. , Steptococcus pneumoniae. Why then is it important to identify the causative agent? § Correct antibiotic therapy § Prevent outbreaks § Identify (and eliminate) the source of infection

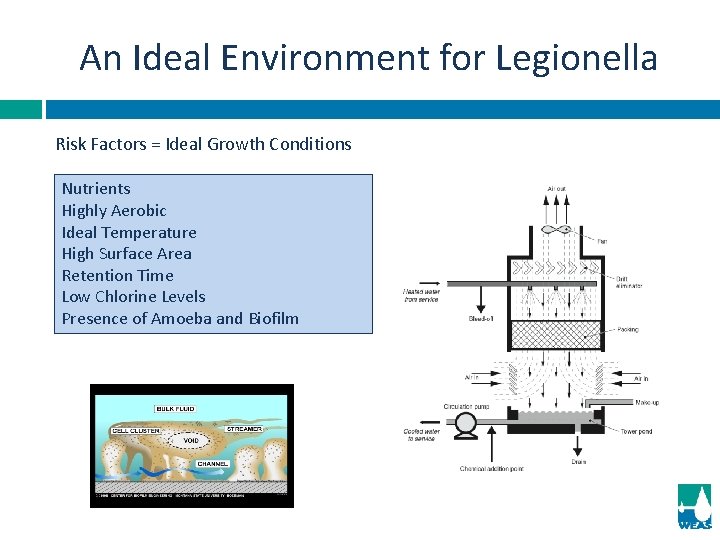
An Ideal Environment for Legionella Risk Factors = Ideal Growth Conditions Nutrients Highly Aerobic Ideal Temperature High Surface Area Retention Time Low Chlorine Levels Presence of Amoeba and Biofilm

Visible Biofilms Condenser Tube Sheets Filter Screens Spray Bars Air Washers Tap Aerators

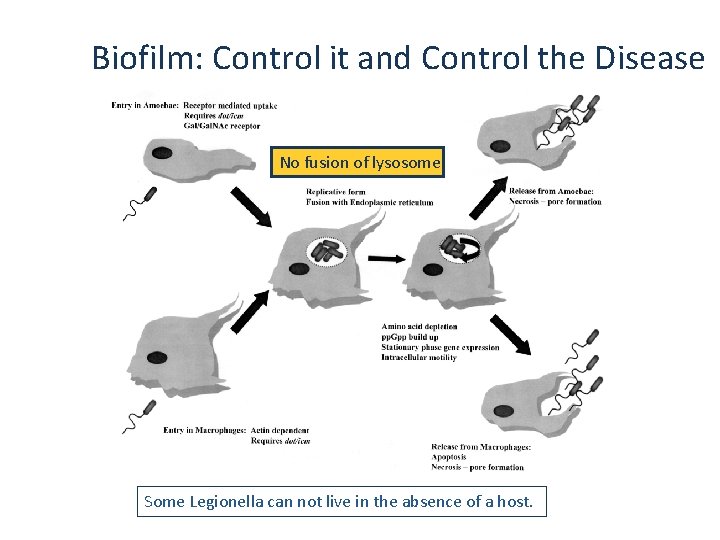
Biofilm: Control it and Control the Disease No fusion of lysosome Some Legionella can not live in the absence of a host.

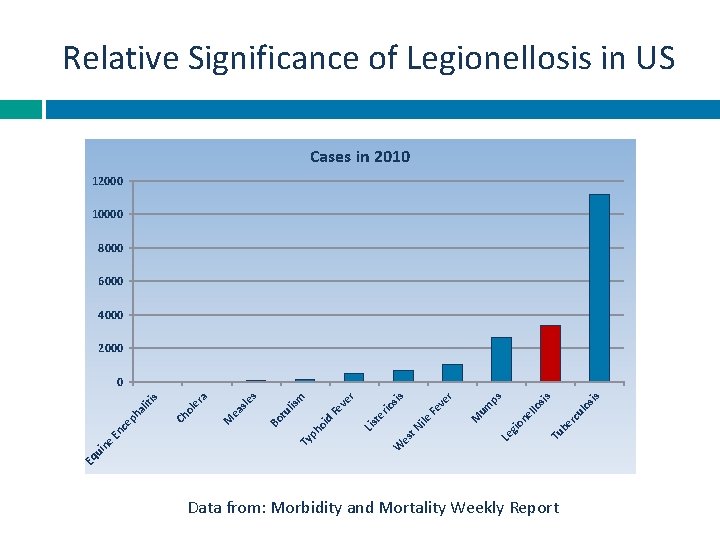
Relative Significance of Legionellosis in US Cases in 2010 12000 10000 8000 6000 4000 2000 os is sis Tu be rc ul llo on e Le gi um ps M r ve Fe es t. N ile Lis te rio sis W Ty ph oi d Fe ve r lis m tu Bo s M ea sle ra ol e Ch Eq u in e En ce ph al iti s 0 Data from: Morbidity and Mortality Weekly Report

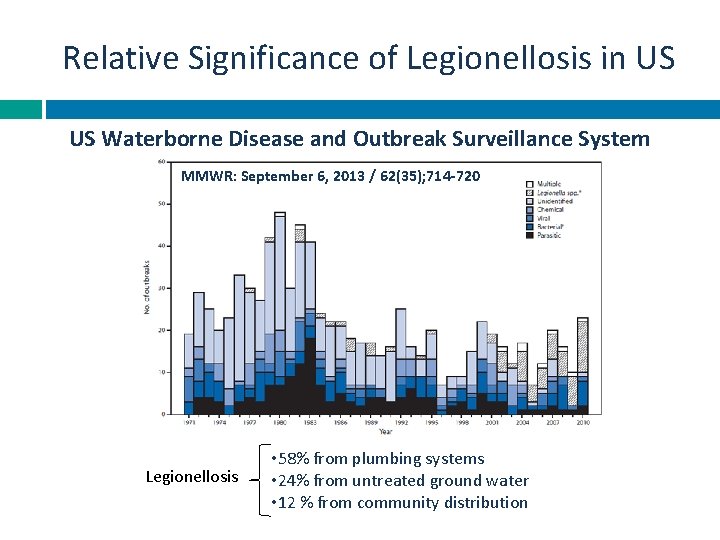
Relative Significance of Legionellosis in US US Waterborne Disease and Outbreak Surveillance System MMWR: September 6, 2013 / 62(35); 714 -720 Legionellosis • 58% from plumbing systems • 24% from untreated ground water • 12 % from community distribution

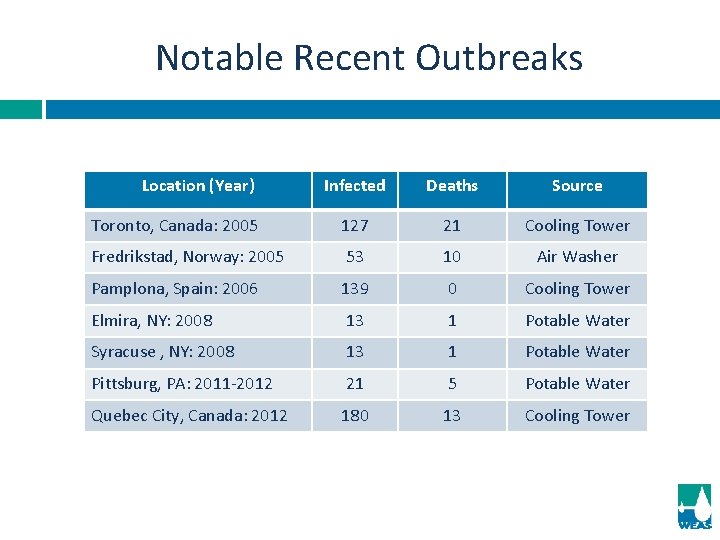
Notable Recent Outbreaks Location (Year) Infected Deaths Source Toronto, Canada: 2005 127 21 Cooling Tower Fredrikstad, Norway: 2005 53 10 Air Washer Pamplona, Spain: 2006 139 0 Cooling Tower Elmira, NY: 2008 13 1 Potable Water Syracuse , NY: 2008 13 1 Potable Water Pittsburg, PA: 2011 -2012 21 5 Potable Water Quebec City, Canada: 2012 180 13 Cooling Tower

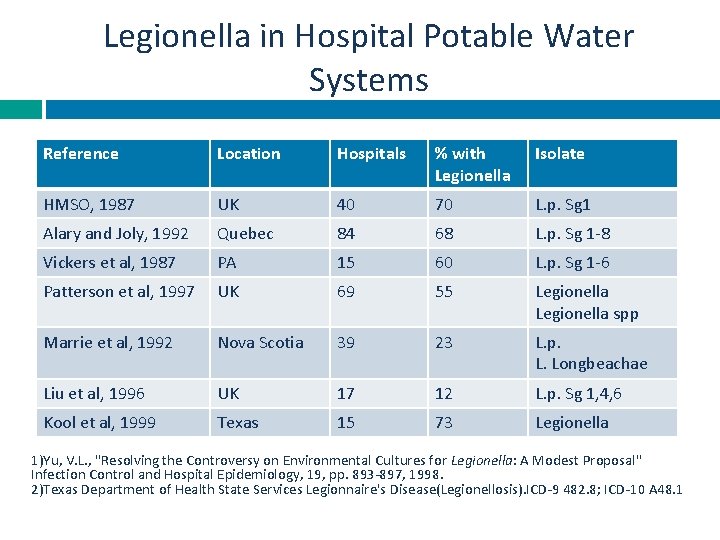
Legionella in Hospital Potable Water Systems Reference Location Hospitals % with Legionella Isolate HMSO, 1987 UK 40 70 L. p. Sg 1 Alary and Joly, 1992 Quebec 84 68 L. p. Sg 1 -8 Vickers et al, 1987 PA 15 60 L. p. Sg 1 -6 Patterson et al, 1997 UK 69 55 Legionella spp Marrie et al, 1992 Nova Scotia 39 23 L. p. L. Longbeachae Liu et al, 1996 UK 17 12 L. p. Sg 1, 4, 6 Kool et al, 1999 Texas 15 73 Legionella 1)Yu, V. L. , "Resolving the Controversy on Environmental Cultures for Legionella: A Modest Proposal" Infection Control and Hospital Epidemiology, 19, pp. 893 -897, 1998. 2)Texas Department of Health State Services Legionnaire's Disease(Legionellosis). ICD-9 482. 8; ICD-10 A 48. 1

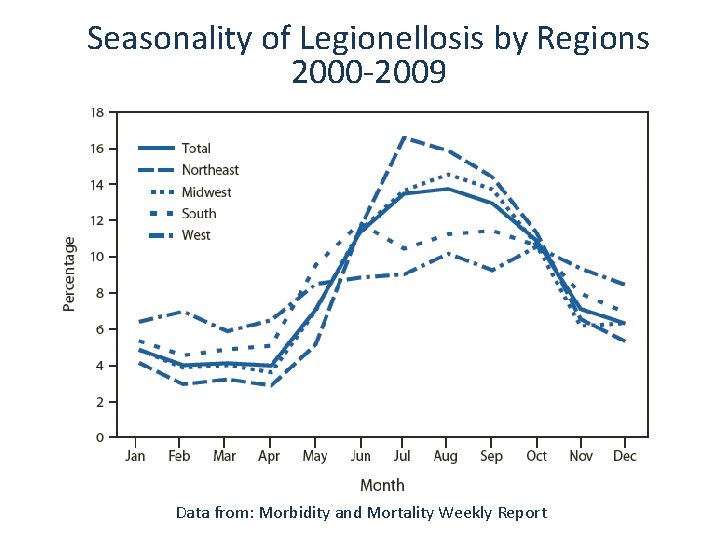
Seasonality of Legionellosis by Regions 2000 -2009 Data from: Morbidity and Mortality Weekly Report

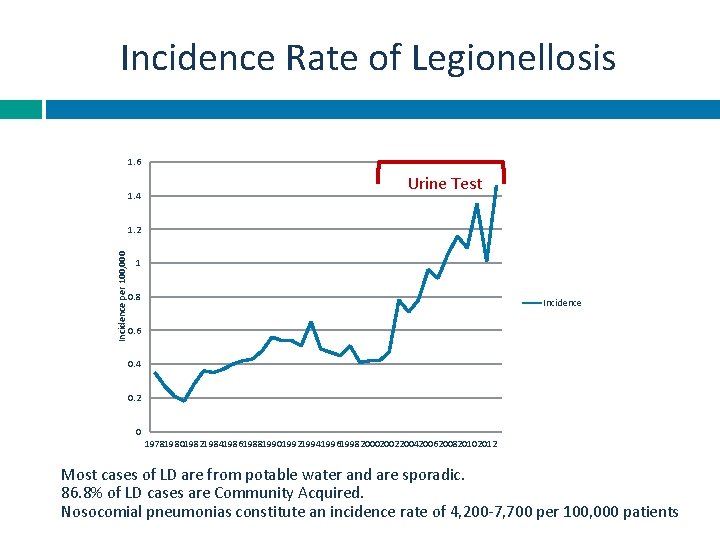
Incidence Rate of Legionellosis 1. 6 1. 4 Urine Test Incidence per 100, 000 1. 2 1 0. 8 Incidence 0. 6 0. 4 0. 2 0 197819801982198419861988199019921994199619982000200220042006200820102012 Most cases of LD are from potable water and are sporadic. 86. 8% of LD cases are Community Acquired. Nosocomial pneumonias constitute an incidence rate of 4, 200 -7, 700 per 100, 000 patients

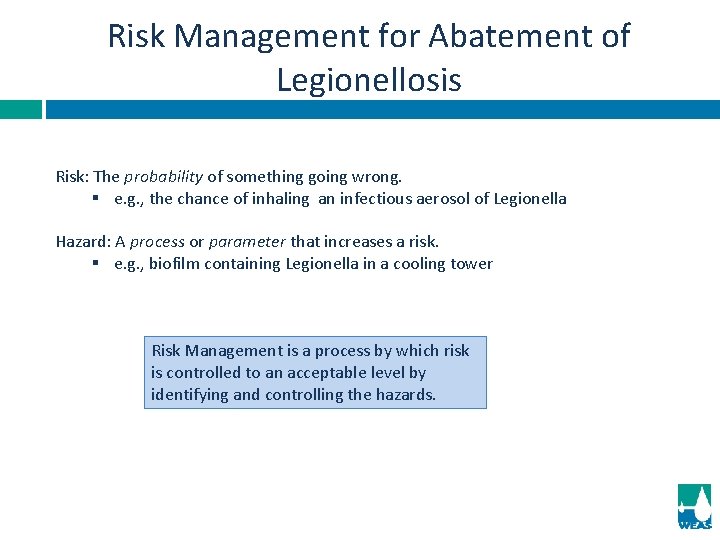
Risk Management for Abatement of Legionellosis Risk: The probability of something going wrong. § e. g. , the chance of inhaling an infectious aerosol of Legionella Hazard: A process or parameter that increases a risk. § e. g. , biofilm containing Legionella in a cooling tower Risk Management is a process by which risk is controlled to an acceptable level by identifying and controlling the hazards.

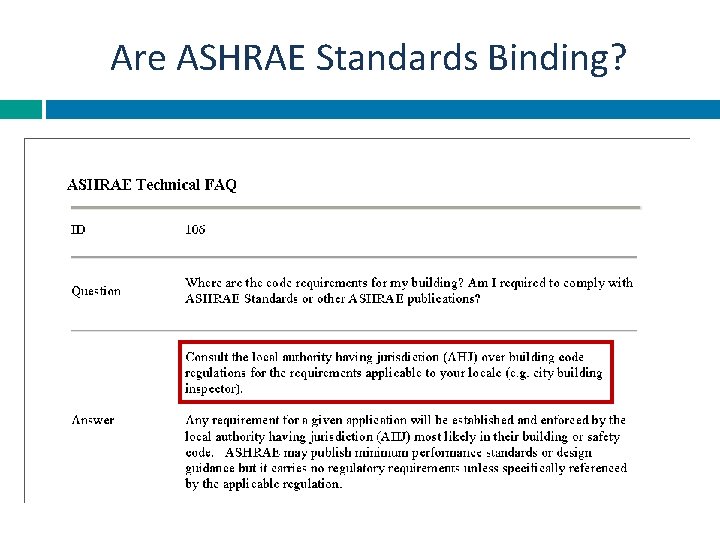
Risk Management: ASHRAE American Society of Heating Refrigeration and Air-Conditioning Engineers ASHRAE is a professional association of engineers that establishes standards and guidelines of performance criteria for institutional and commercial buildings. ASHRAE is accredited by the American National Standards Institute (ANSI) and follows ANSI's requirements for due process and standards development.

Risk Management Oversight: ASHRAE 188 P Will supersede: Guideline 12 -2000 -Minimizing the Risk of Legionellosis Associated with Building Water Systems

Are ASHRAE Standards Binding?

Who Are the AHJ’s? § § § OSHA JCAHO/TJC ASHE

Risk Management Oversight: OSHA Section 5(a)(1) of the Occupational Safety and Health Act: “Each employer shall furnish to each of his employees employment and a place of employment which are free from recognized hazards that are causing or are likely to cause death or serious physical harm to his employees. ” Several conditions must be met for OSHA to issue a General Duty Clause violation: 1. The hazard was recognized. 2. The employer failed to keep the workplace free of a hazard to which his or her employees were exposed. 3. A feasible and useful method was available to correct the hazard. 4. The hazard was causing or likely to cause death or serious injury.

Risk Management Oversight: JCAHO The Joint Commission for the Accreditation of Healthcare Organizations Standard EC 1. 7, requires a management program to: § Reduce the potential for organizational-acquired illness. § Manage pathogenic biological agents in cooling towers, domestic hot water, and other aerosolizing water systems.

Risk Management Oversight: ASHE The American Society for Healthcare Engineering All health care facilities: § Conduct a risk assessment of potential sources of Legionella. § Develop a management plan for maintenance and operation of water systems.

Risk Management Plans Hazard Analysis and Critical Control Point HACCP A Risk Management Program developed by Pillsbury for NASA in 1957.

Hazard Analysis and Critical Control Points HACCP A Risk Management Program developed by Pillsbury for NASA in 1957 because “There’s no room for poop in a NASA suit”

Essentials of a HACCP Plan: “The 7 Principles of HACCP” 1. 2. 3. 4. 5. 6. 7. * Assess the Hazards. *Identify the Critical Hazard and its Critical Control Point (CCP). Establish CCP parameters. Establish monitoring frequency and procedure of CCP. Establish corrective actions when CCP limit is exceeded. Establish record keeping system. Validate the HACCP plan. A CCP is the final step in the process wherein the hazard can be eliminated or adequately mitigated.

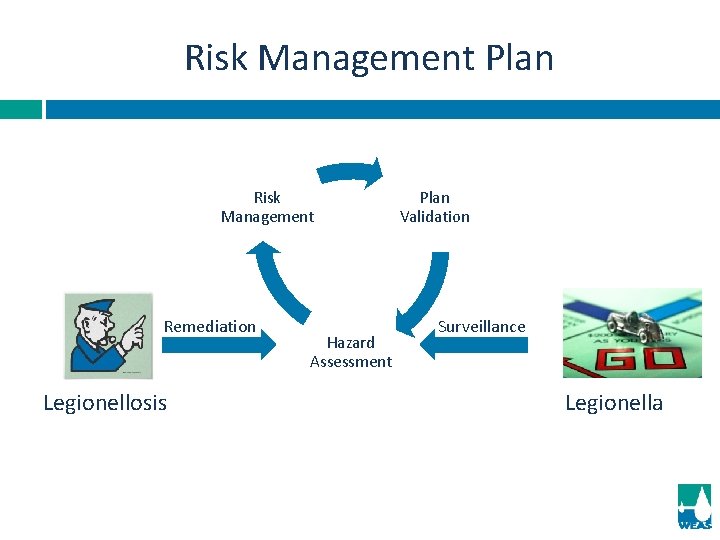
Risk Management Plan by the “Team” Hazard Assessment. Mitigate Management. 1. 2. I. II. III. IV. 3. Establish risk mitigation parameters. Establish test frequency for mitigation procedures. Establish corrective actions when mitigation procedures are not achieved. Establish record keeping system fro mitigation procedures and actions. Plan Validation.

Risk Management Plan Risk Management Remediation Legionellosis Hazard Assessment Plan Validation Surveillance Legionella

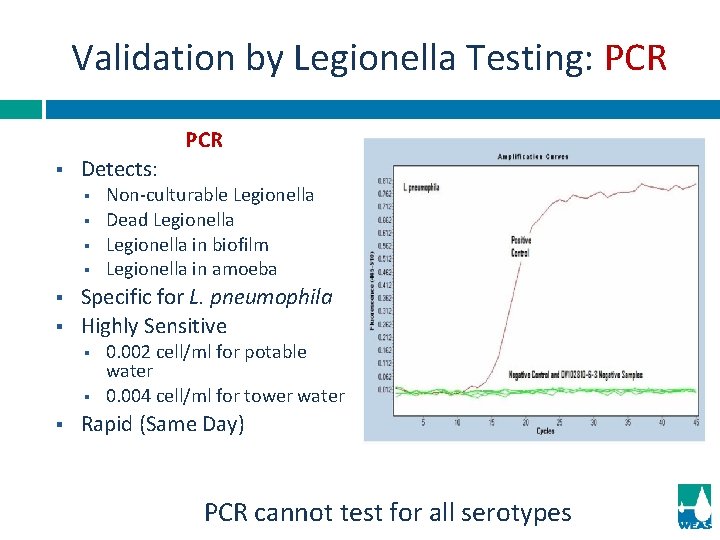
Validation by Legionella Testing: PCR § Detects: § § § Specific for L. pneumophila Highly Sensitive § § § Non-culturable Legionella Dead Legionella in biofilm Legionella in amoeba 0. 002 cell/ml for potable water 0. 004 cell/ml for tower water Rapid (Same Day) PCR cannot test for all serotypes

Validation by Legionella Testing: Culture Methods § Detects only “healthy” Legionella § Sample must be fresh. § Must suppress growth of competitive heterotrophs. § No differentiation of Legionella species § Low Sensitivity § § 0. 05 cell/ml potable water 1 cell/ml tower water Slow amplification (growth)= 3 -4 day incubation done twice Still the CDC Gold Standard

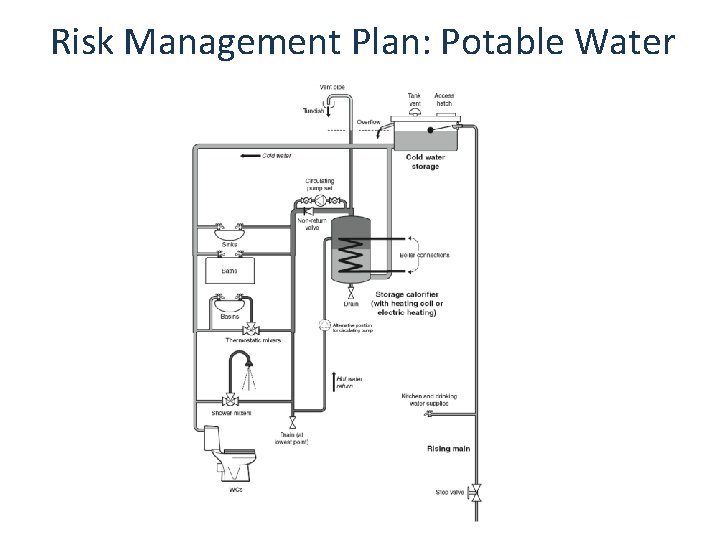
Risk Management Plan: Potable Water

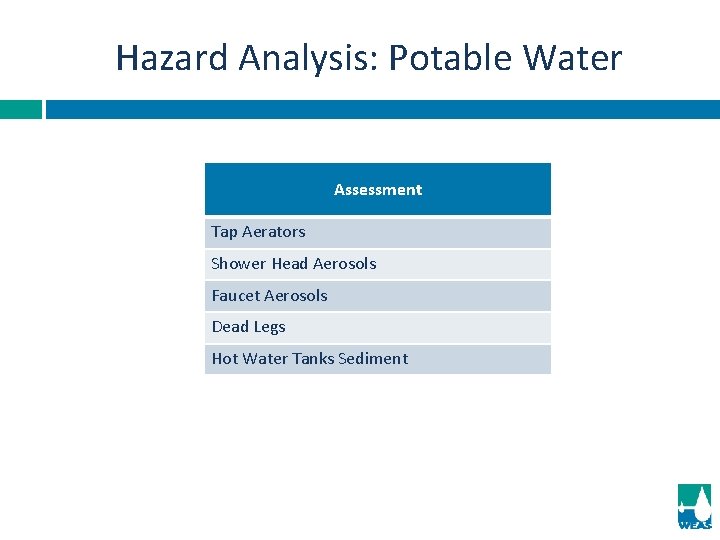
Hazard Analysis: Potable Water Assessment Tap Aerators Shower Head Aerosols Faucet Aerosols Dead Legs Hot Water Tanks Sediment

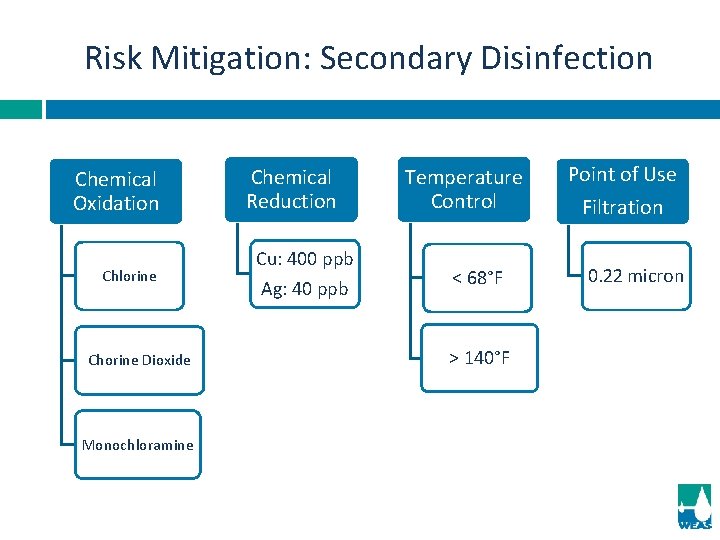
Risk Mitigation: Secondary Disinfection Chemical Oxidation Chlorine Chorine Dioxide Monochloramine Chemical Reduction Cu: 400 ppb Ag: 40 ppb Temperature Control < 68°F > 140°F Point of Use Filtration 0. 22 micron

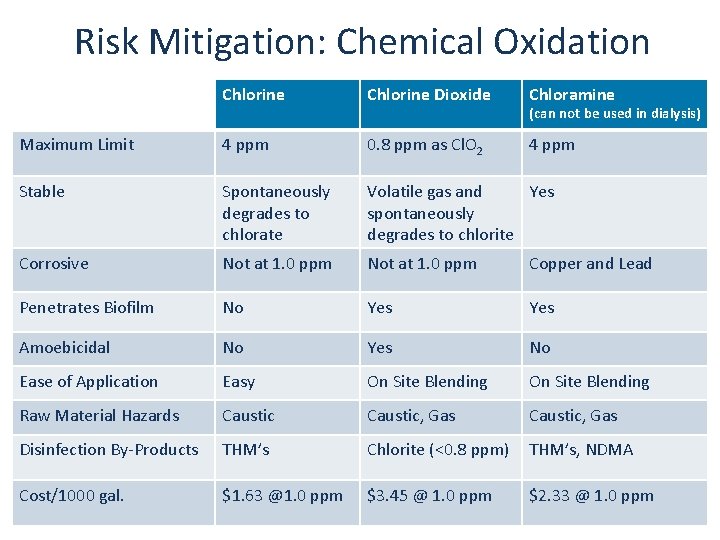
Risk Mitigation: Chemical Oxidation Chlorine Dioxide Chloramine Maximum Limit 4 ppm 0. 8 ppm as Cl. O 2 4 ppm Stable Spontaneously degrades to chlorate Volatile gas and Yes spontaneously degrades to chlorite Corrosive Not at 1. 0 ppm Copper and Lead Penetrates Biofilm No Yes Amoebicidal No Yes No Ease of Application Easy On Site Blending Raw Material Hazards Caustic, Gas Disinfection By-Products THM’s Chlorite (<0. 8 ppm) THM’s, NDMA Cost/1000 gal. $1. 63 @1. 0 ppm $3. 45 @ 1. 0 ppm $2. 33 @ 1. 0 ppm (can not be used in dialysis)

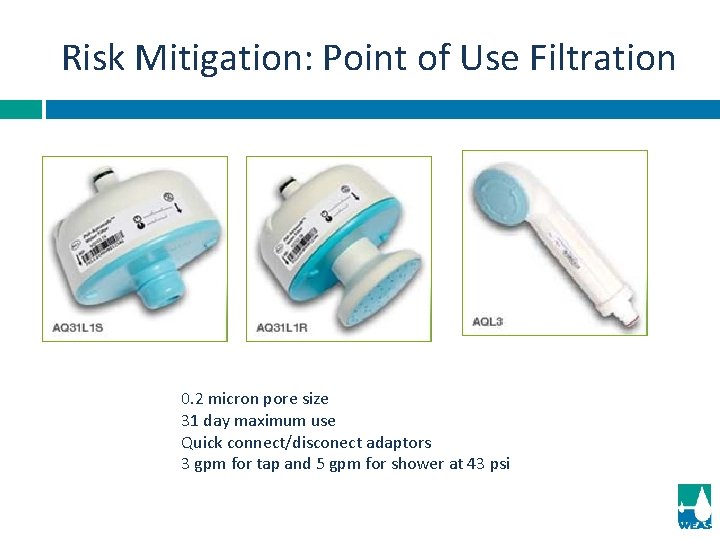
Risk Mitigation: Point of Use Filtration 0. 2 micron pore size 31 day maximum use Quick connect/disconect adaptors 3 gpm for tap and 5 gpm for shower at 43 psi

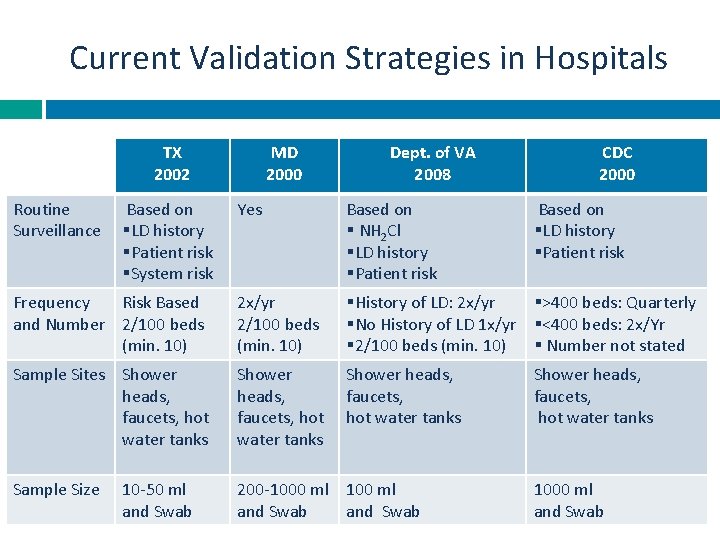
Current Validation Strategies in Hospitals TX 2002 Routine Surveillance Based on §LD history §Patient risk §System risk MD 2000 Dept. of VA 2008 CDC 2000 Yes Based on § NH 2 Cl §LD history §Patient risk Frequency Risk Based and Number 2/100 beds (min. 10) 2 x/yr 2/100 beds (min. 10) §History of LD: 2 x/yr §>400 beds: Quarterly §No History of LD 1 x/yr §<400 beds: 2 x/Yr § 2/100 beds (min. 10) § Number not stated Sample Sites Shower heads, faucets, hot water tanks Sample Size 200 -1000 ml 100 ml and Swab 10 -50 ml and Swab Based on §LD history §Patient risk Shower heads, faucets, hot water tanks 1000 ml and Swab

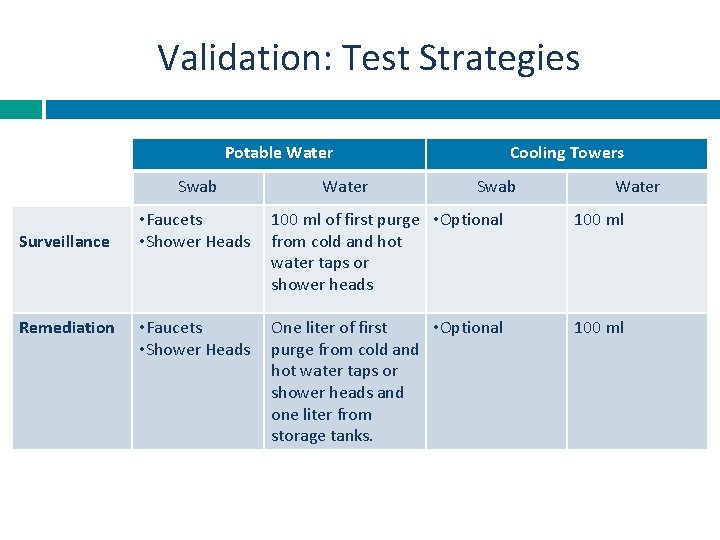
Validation: Test Strategies Potable Water Swab Surveillance Remediation Water Cooling Towers Swab Water • Faucets • Shower Heads 100 ml of first purge • Optional from cold and hot water taps or shower heads 100 ml • Faucets • Shower Heads One liter of first • Optional purge from cold and hot water taps or shower heads and one liter from storage tanks. 100 ml

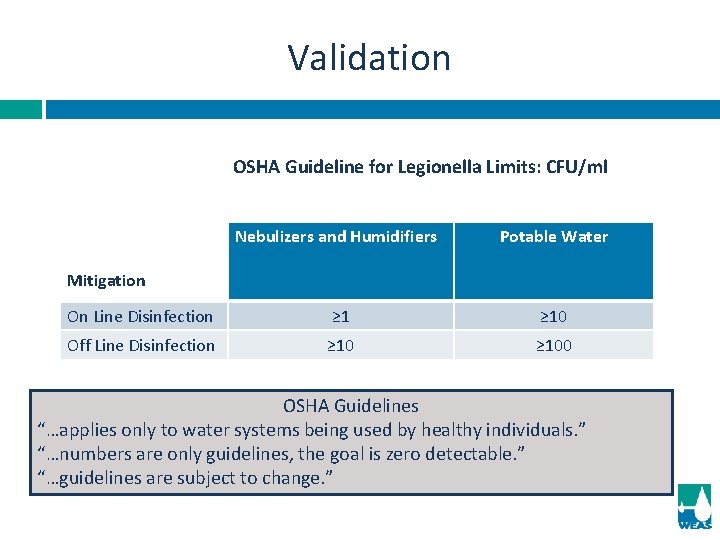
Validation OSHA Guideline for Legionella Limits: CFU/ml Nebulizers and Humidifiers Potable Water On Line Disinfection ≥ 10 Off Line Disinfection ≥ 100 Mitigation OSHA Guidelines “…applies only to water systems being used by healthy individuals. ” “…numbers are only guidelines, the goal is zero detectable. ” “…guidelines are subject to change. ”

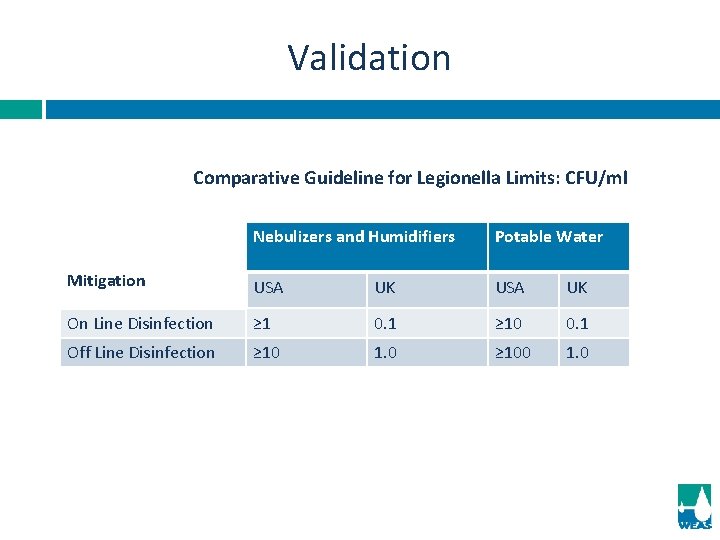
Validation Comparative Guideline for Legionella Limits: CFU/ml Nebulizers and Humidifiers Potable Water Mitigation USA UK On Line Disinfection ≥ 1 0. 1 ≥ 10 0. 1 Off Line Disinfection ≥ 10 1. 0 ≥ 100 1. 0

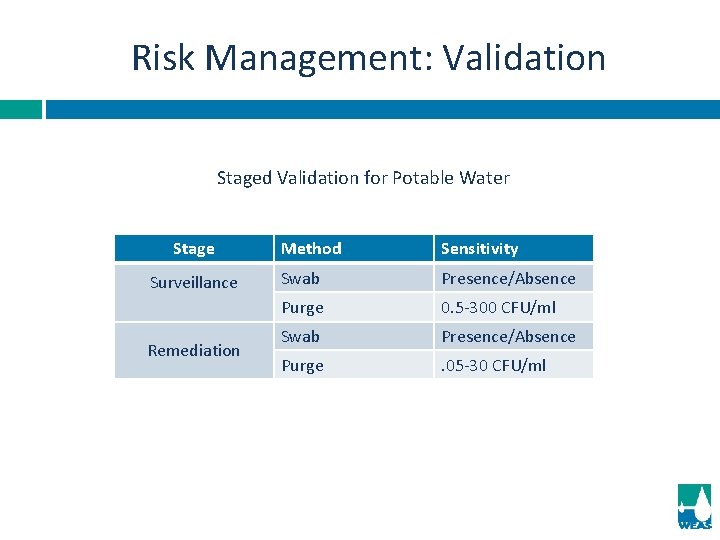
Risk Management: Validation Staged Validation for Potable Water Stage Surveillance Remediation Method Sensitivity Swab Presence/Absence Purge 0. 5 -300 CFU/ml Swab Presence/Absence Purge . 05 -30 CFU/ml

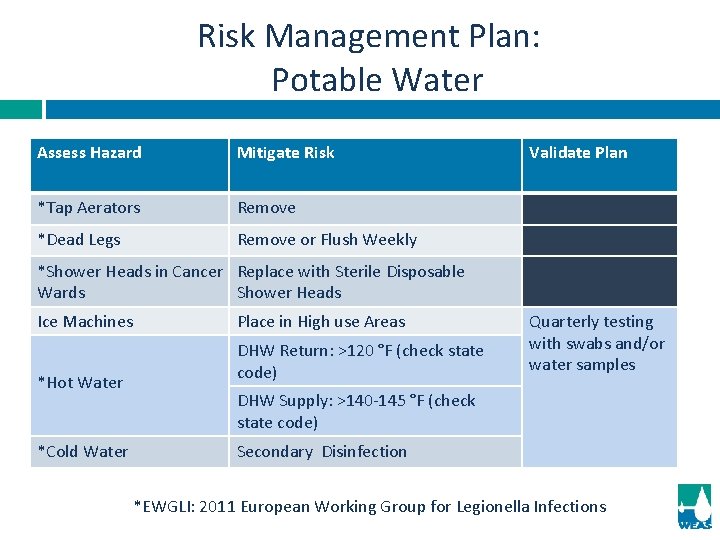
Risk Management Plan: Potable Water Assess Hazard Mitigate Risk *Tap Aerators Remove *Dead Legs Remove or Flush Weekly Validate Plan *Shower Heads in Cancer Replace with Sterile Disposable Wards Shower Heads Ice Machines *Hot Water *Cold Water Place in High use Areas DHW Return: >120 °F (check state code) Quarterly testing with swabs and/or water samples DHW Supply: >140 -145 °F (check state code) Secondary Disinfection *EWGLI: 2011 European Working Group for Legionella Infections

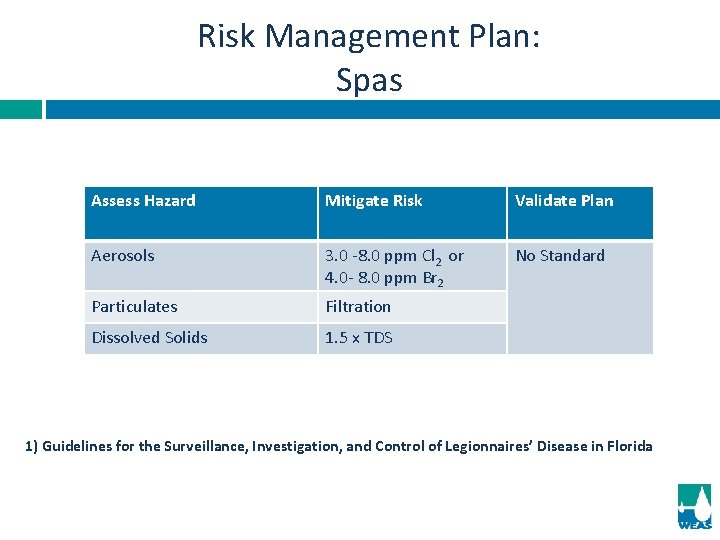
Risk Management Plan: Spas Assess Hazard Mitigate Risk Validate Plan Aerosols 3. 0 -8. 0 ppm Cl 2 or 4. 0 - 8. 0 ppm Br 2 No Standard Particulates Filtration Dissolved Solids 1. 5 x TDS 1) Guidelines for the Surveillance, Investigation, and Control of Legionnaires’ Disease in Florida

Risk Management Plan: Humidifiers and Nebulizers: Use only sterile water Humidification: Use only steam

Remediation of Legionella Contaminated Potable Water Absence of Disease and Low Risk Areas Primary Remediation: §Post signs at each outlet to be flushed warning of potential scald injury. §Flush hot water tanks. §Maintain hot water temperature at 140°F and purge outlets for a minimum of 5 minutes. Secondary Remediation: §Flush hot water tanks. §Maintain a free chlorine residual of 50 ppm for 1 hr or 20 ppm for 2 hrs.

2003 CDC Response to Legionella Detection Absence of Disease and High Risk Areas Decontaminate the water supply. Do not turn on showers or faucets until systems test negative for Legionella Use Sterile water for: 1. 2. 3. i. ii. iii. iv. Sponge baths Showers Tooth brushing Rinsing of nasogastric tubes

2003 CDC Response to Nosocomial Legionellosis When Legionellosis is Confirmed 1. 2. 3. 4. 5. Contact the local or state health department or CDC. Determine source of Legionella with using Infection Control and Engineering resources. § Review historical water records. § Conduct walk-thru and observe personnel duties and operation of equipment. Assess all relevant medical and engineering data. Determine if clinical and environmental samples are a perfect match. Disinfect contaminated systems.

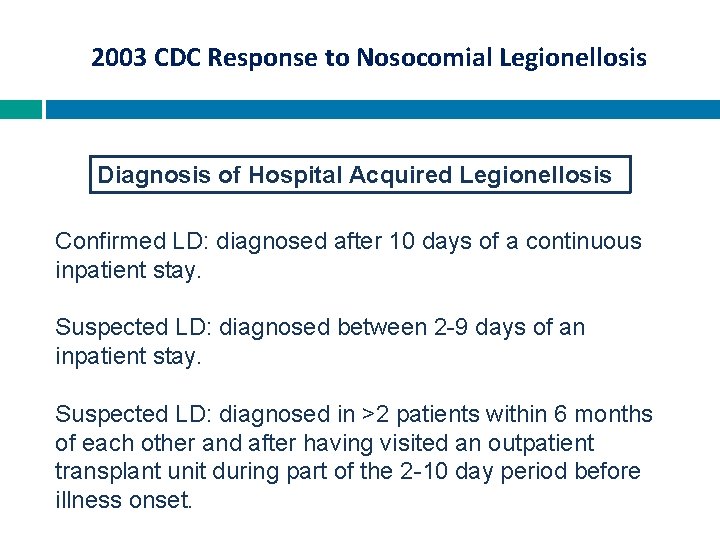
2003 CDC Response to Nosocomial Legionellosis Diagnosis of Hospital Acquired Legionellosis Confirmed LD: diagnosed after 10 days of a continuous inpatient stay. Suspected LD: diagnosed between 2 -9 days of an inpatient stay. Suspected LD: diagnosed in >2 patients within 6 months of each other and after having visited an outpatient transplant unit during part of the 2 -10 day period before illness onset.

2003 CDC Response to Nosocomial Legionellosis Remediation Primary Remediation: Flush hot water tanks. Maintain hot water temperature to 160°F-170 °F and purge outlets for a minimum of 5 minutes. Post warning signs at each outlet being flushed to prevent scald injury to patients, staff, or visitors. Secondary Remediation: Flush hot water tanks. Maintain a free chlorine residual of >2 ppm with or without supplemental heating throughout the system. This might require chlorination of the water heater or tank to levels of 20 -50 ppm. Circulate the water for at least 2 hours and maintain the water p. H between 7. 0 and 8. 0.

2003 CDC Response to Nosocomial Legionellosis Plan Validation • • • Retest every 2 weeks for Legionella every 3 months. If negative after 3 months, continue monthly testing for 3 more months. Maintain all records. If these measures are unsuccessful, seek expert consultation for review of decontamination procedures and assistance with further efforts.

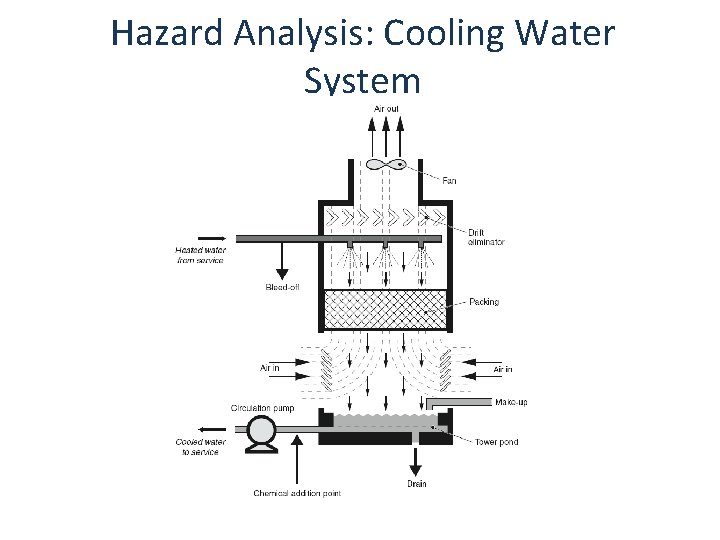
Hazard Analysis: Cooling Water System

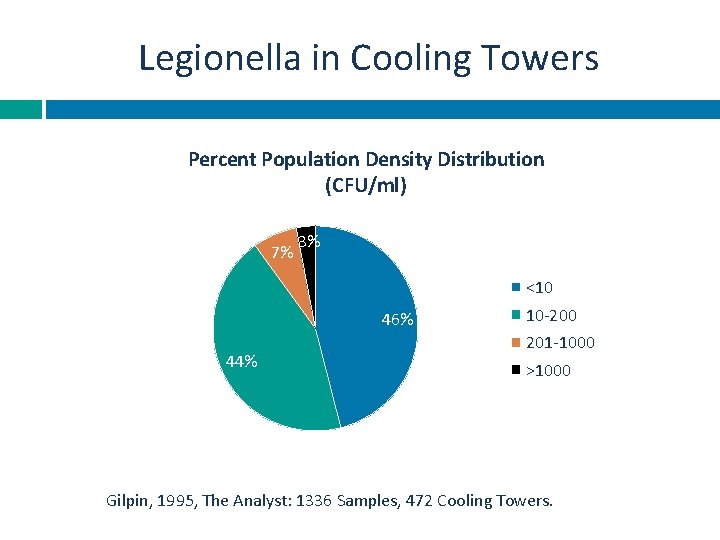
Legionella in Cooling Towers Percent Population Density Distribution (CFU/ml) 7% 3% <10 46% 44% 10 -200 201 -1000 >1000 Gilpin, 1995, The Analyst: 1336 Samples, 472 Cooling Towers.

Cooling Tower: Hazard Analysis § § § Excessive drift Sediment in basin Visible corrosion Visible biofilm Planktonic bacteria Filled with water but not operating

Risk Mitigation: Air Filtration New Slide Air Solution Co.

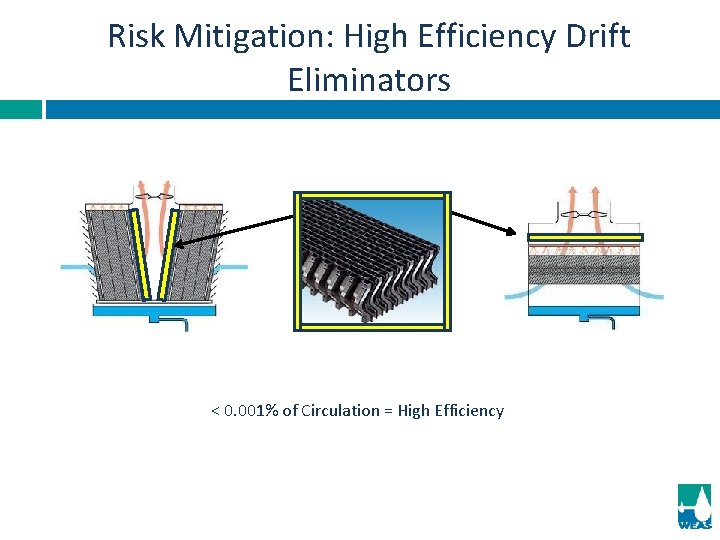
Risk Mitigation: High Efficiency Drift Eliminators < 0. 001% of Circulation = High Efficiency

Risk Mitigation: Minimize Suspended Solids

Risk Mitigation: Minimize Suspended Solids

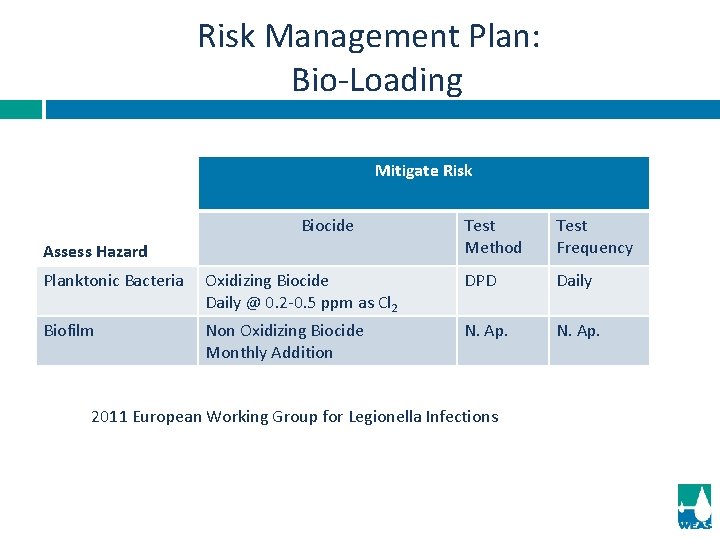
Risk Management Plan: Bio-Loading Mitigate Risk Biocide Assess Hazard Test Method Test Frequency Planktonic Bacteria Oxidizing Biocide Daily @ 0. 2 -0. 5 ppm as Cl 2 DPD Daily Biofilm Non Oxidizing Biocide Monthly Addition N. Ap. 2011 European Working Group for Legionella Infections

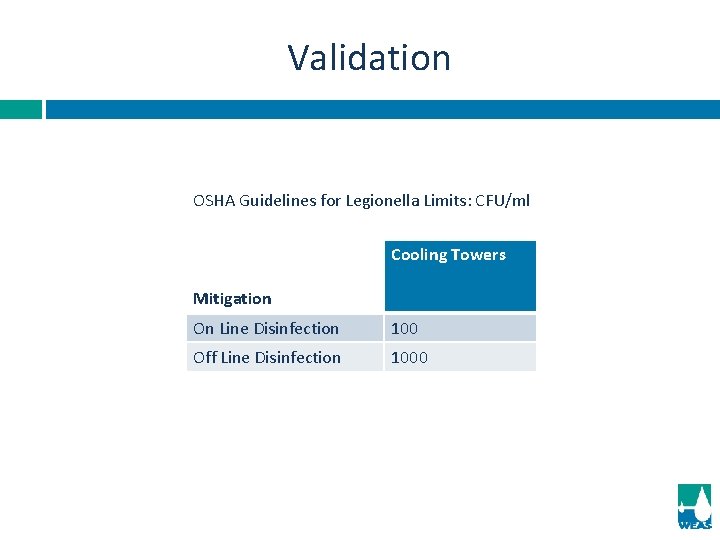
Validation OSHA Guidelines for Legionella Limits: CFU/ml Cooling Towers Mitigation On Line Disinfection 100 Off Line Disinfection 1000

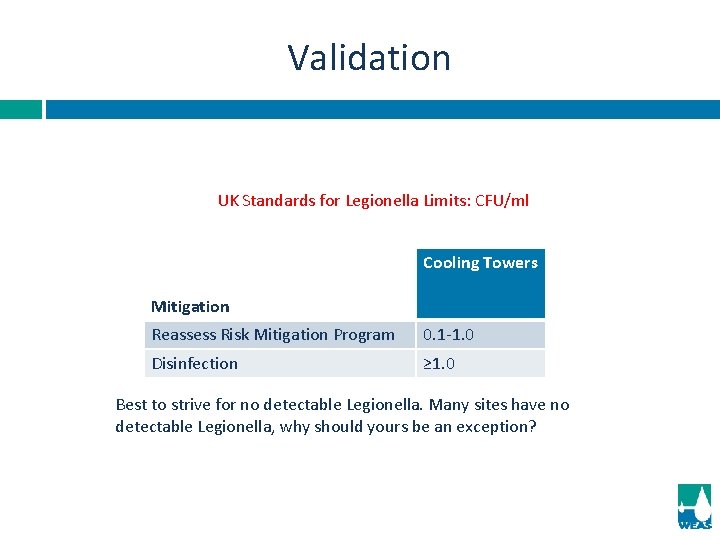
Validation UK Standards for Legionella Limits: CFU/ml Cooling Towers Mitigation Reassess Risk Mitigation Program 0. 1 -1. 0 Disinfection ≥ 1. 0 Best to strive for no detectable Legionella. Many sites have no detectable Legionella, why should yours be an exception?

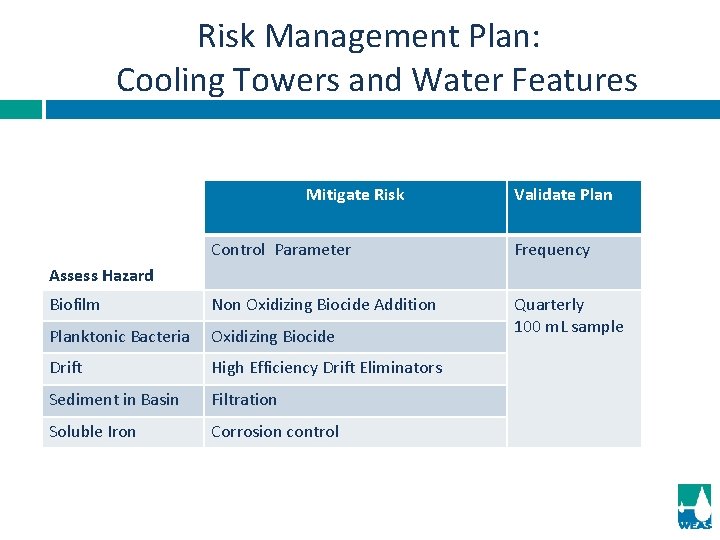
Risk Management Plan: Cooling Towers and Water Features Mitigate Risk Validate Plan Control Parameter Frequency Biofilm Non Oxidizing Biocide Addition Planktonic Bacteria Oxidizing Biocide Quarterly 100 m. L sample Drift High Efficiency Drift Eliminators Sediment in Basin Filtration Soluble Iron Corrosion control Assess Hazard

Remediation of Legionella Contaminated Tower Water Absence of Disease 1. 2. 3. Close bleed valve. Dose the cooling tower sump with a minimum of 180 ppm of C-992 and allow the product to circulate for 6 hours. Restart the bleed of the cooling tower.

Remediation of Legionella Contaminated Tower Water “Wisconsin Protocol” Cooling Tower Associated with Disease 1. 2. 3. * 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Power down cooling tower fan Close bleed valve. Close air intakes within 100 ft of the cooling tower. Use a 3 M™ Disc Filter 2125 P 2 respirator or equivalent Discontinue regular chemical treatment. Adjust p. H to 7. 5 -8. 0. Add chlorine to initially establish 50 ppm free chlorine. Add a dispersant or low foaming surfactant. Maintain 10 ppm of free chlorine for 24 hours. Confirm the residual at least every 2 hours. Drain, refill and repeat steps 5 -9 until tower is visually clean Mechanically clean all cooling tower surfaces and components *Refill and bring free chlorine to 10 ppm for 1 hour. Test and confirm absence of Legionella. If still present, repeat procedure.

Risk Management Plan Summary § § § Make your Risk Management Plan relevant and simple. There are few standards; guidelines are the norm. All guidelines are steered by validation. Risk Management is an iterative process of continuing improvement. So long as the process of assessment, mitigation and validation is followed, presence of Legionella is simply an opportunity for improvement. Risk Management can be your friend.

Questions?

Bibliography 1. Guidelines for the Surveillance, Investigation, and Control of Legionnaires’ Disease in Florida. www. floridahealth. gov/healthyenvironments/indoor. . . /Legionella. pdf 2. Legionnaires’ disease. The control of Legionella bacteria in water systems. Approved Code of Practice and guidance. ww. hseni. gov. uk/l 8_legionnaires__disease_the_control_of_legionella_bacteria_i n_water_systems. pdf 3. Guidelines for Preventing Health-Care--Associated Pneumonia, 2003 www. cdc. gov/mmwr/preview/mmwrhtml/rr 5303 a 1. htm 4. Texas Department of State Health Services Report of the Texas Legionnaires’ Disease Task Force. www. dshs. state. tx. us/idcu/disease/legionnaires/taskforce/ 5. ASHRAE Legionellosis Position Statement www. ashrae. org/File%20 Library/doc. Lib/About%20 Us/Position. Documents/ASHRA E_PD_Legionellosis_2012. pdf 6. OSHA Technical Manual https: //www. osha. gov/dts/osta/otm_iii/otm_iii_7. html#app_iii: 7_3
 Market risk assessment
Market risk assessment Third party risk management conference 2019 new york
Third party risk management conference 2019 new york Cboe risk management conference
Cboe risk management conference Financial risk management conference 2018
Financial risk management conference 2018 Key risk indicators for vendor management
Key risk indicators for vendor management Risk map
Risk map Business continuity indiana
Business continuity indiana Risk information sheet in software engineering
Risk information sheet in software engineering Oaep fall conference
Oaep fall conference Tgfoa
Tgfoa Wasbo fall conference
Wasbo fall conference Wasbo fall conference
Wasbo fall conference Njdv
Njdv Hester davis fall
Hester davis fall Fall risk form
Fall risk form National youth at risk conference
National youth at risk conference Risk minds conference amsterdam
Risk minds conference amsterdam Hendrick 2 fall risk model
Hendrick 2 fall risk model Fall risk assessment tool
Fall risk assessment tool Risk fall
Risk fall Incite jtac
Incite jtac Workforce planning conference 2018
Workforce planning conference 2018 Main planning conference
Main planning conference Workforce planning conference
Workforce planning conference Financial management chapter 8 risk and return
Financial management chapter 8 risk and return Chapter 34 risk management
Chapter 34 risk management Chapter 33 the basics of risk management
Chapter 33 the basics of risk management Pure risk examples
Pure risk examples Chapter 21 introduction to risk management
Chapter 21 introduction to risk management Chapter 14 risk management
Chapter 14 risk management Risk management and worker protection
Risk management and worker protection Chapter 14 risk management
Chapter 14 risk management Risk projection attempts to rate each risk in two ways
Risk projection attempts to rate each risk in two ways Risk mitigation avoidance
Risk mitigation avoidance How to calculate relative risk
How to calculate relative risk Residual risk and secondary risk pmp
Residual risk and secondary risk pmp Inherent risks examples
Inherent risks examples Absolute risk vs relative risk
Absolute risk vs relative risk Activity sheet 2: stock market calculations answer key
Activity sheet 2: stock market calculations answer key Medium risk examples
Medium risk examples Risk financing retention adalah
Risk financing retention adalah The biggest risk is not taking any risks
The biggest risk is not taking any risks Business risk vs audit risk
Business risk vs audit risk Business risk vs financial risk capital structure
Business risk vs financial risk capital structure Relative risk and attributable risk
Relative risk and attributable risk Population attributable risk formula
Population attributable risk formula Management chapter 5 planning and decision making
Management chapter 5 planning and decision making Strategic management chapter 3
Strategic management chapter 3 Chapter 16 money management and financial planning
Chapter 16 money management and financial planning What are some characteristics of a wise money manager
What are some characteristics of a wise money manager Management chapter 5 planning and decision making
Management chapter 5 planning and decision making Strategic planning vs tactical planning
Strategic planning vs tactical planning Planning balance sheet in urban planning
Planning balance sheet in urban planning Role segmentation workforce planning
Role segmentation workforce planning Proactive planning and reactive planning
Proactive planning and reactive planning Perencanaan agregat
Perencanaan agregat Long medium and short term planning in primary schools
Long medium and short term planning in primary schools Stages of language planning slideshare
Stages of language planning slideshare Aggregate planning is capacity planning for
Aggregate planning is capacity planning for Capacity aggregation example
Capacity aggregation example Post fall management
Post fall management Time management conference
Time management conference Enterprise content management rfp
Enterprise content management rfp Performance management conference
Performance management conference Customer experience management conference 2010
Customer experience management conference 2010 Local government asset management software
Local government asset management software Conference management middlesbrough
Conference management middlesbrough Relevance lost the rise and fall of management accounting
Relevance lost the rise and fall of management accounting Things fall apart 22
Things fall apart 22 Things fall apart chapters 20-25
Things fall apart chapters 20-25 Examples of okonkwo’s heroic behavior
Examples of okonkwo’s heroic behavior