KS 4 Useful Products from Organic Sources ORGANIC











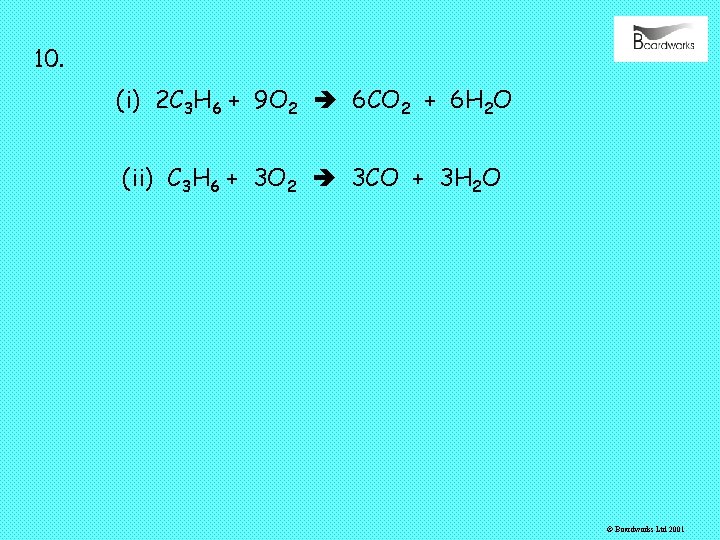
- Slides: 47

KS 4: Useful Products from Organic Sources ORGANIC CHEMISTRY © Boardworks Ltd 2001

Crude Oil Crude oil is a mixture; it contains hundreds of different compounds, some small and some extremely large. Nearly all these compounds contain carbon and hydrogen only and are called hydrocarbons. Hydrocarbons are molecules that contain carbon and hydrogen only. The compounds present in crude oil are separated, processed and purified in an oil refinery. These processes are covered in the following slides. © Boardworks Ltd 2001

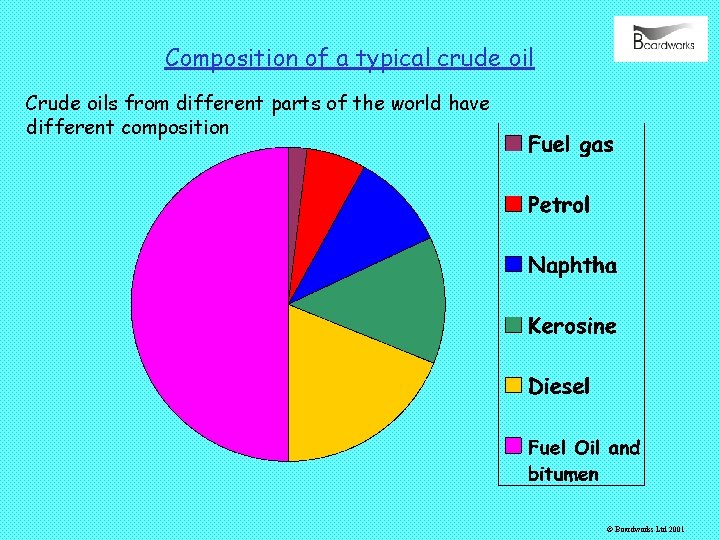
Composition of a typical crude oil Crude oils from different parts of the world have different composition © Boardworks Ltd 2001

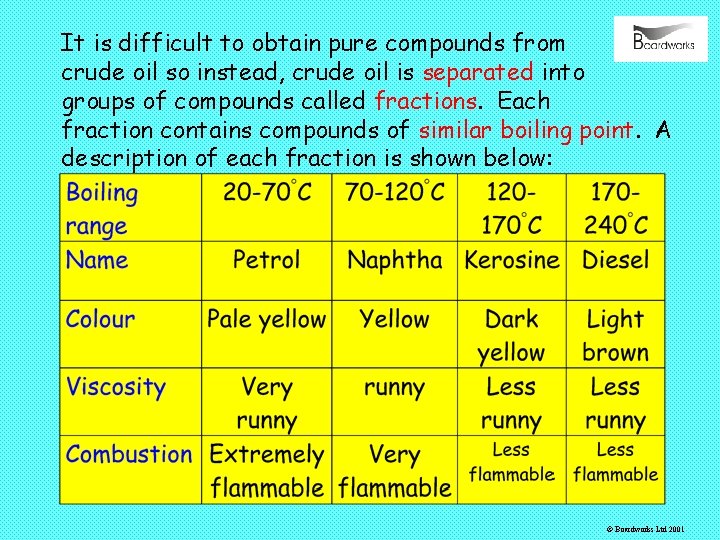
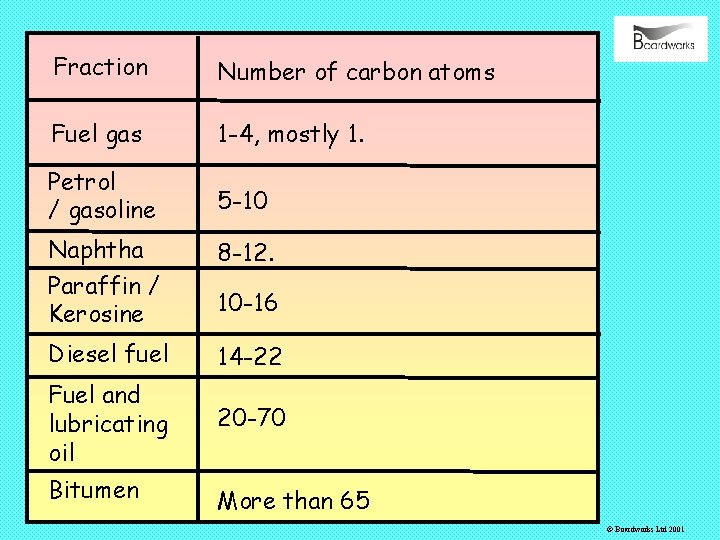
It is difficult to obtain pure compounds from crude oil so instead, crude oil is separated into groups of compounds called fractions. Each fraction contains compounds of similar boiling point. A description of each fraction is shown below: © Boardworks Ltd 2001

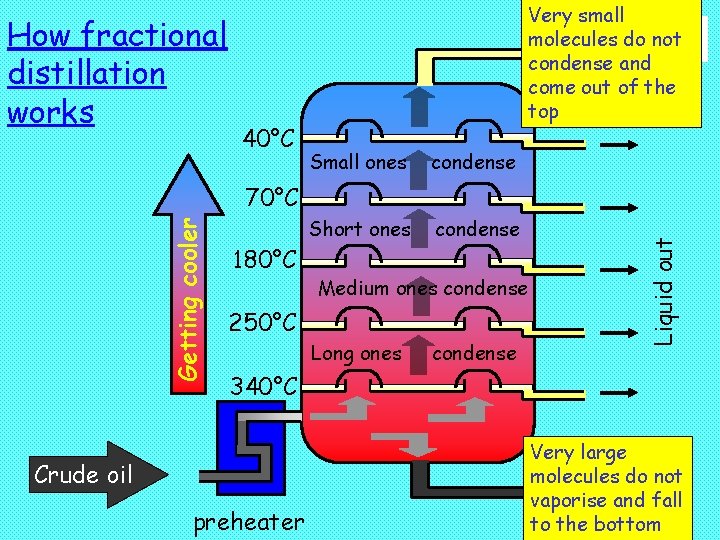
Fractional distillation Crude oil is split into fractions using fractional distillation. Here crude oil is heated and pumped into the bottom of a tall tower containing trays. Compounds with low boiling points rise towards the top of the tower where they condense. Compounds with higher boiling point condense lower in the column. Thus each tray contains liquids (fractions) of different boiling point. The liquid on each tray is continuously pumped away. A fractional distillation tower works continuously, unlike distillation in the laboratory. © Boardworks Ltd 2001

How fractional distillation works 40°C Very small molecules do not condense and come out of the top Small ones condense Short ones condense 180°C Medium ones condense 250°C Long ones condense Liquid out Getting cooler 70°C 340°C Crude oil preheater Very large molecules do not vaporise and fall to the bottom © Boardworks Ltd 2001

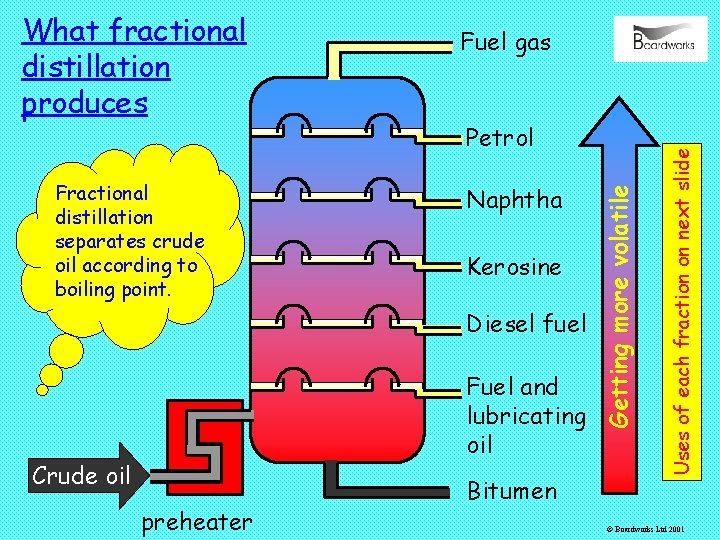
Petrol Naphtha Kerosine Diesel fuel Fuel and lubricating oil Crude oil preheater Bitumen Uses of each fraction on next slide Fractional distillation separates crude oil according to boiling point. Fuel gas Getting more volatile What fractional distillation produces © Boardworks Ltd 2001

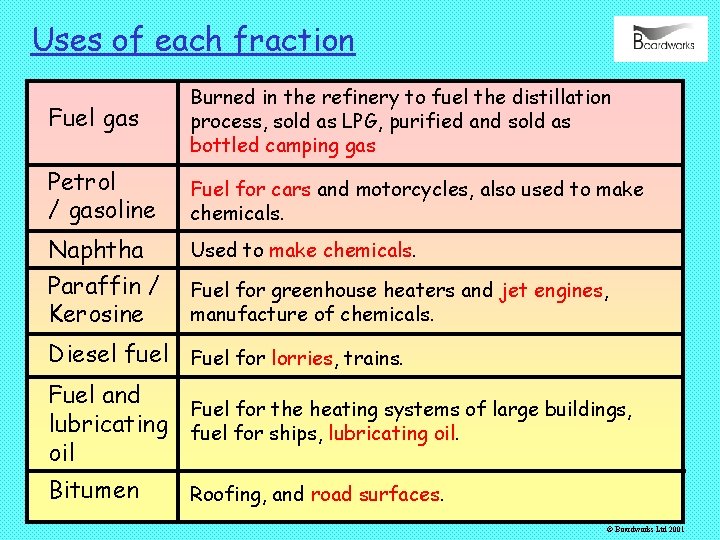
Uses of each fraction Fuel gas Burned in the refinery to fuel the distillation process, sold as LPG, purified and sold as bottled camping gas Petrol / gasoline Fuel for cars and motorcycles, also used to make chemicals. Naphtha Paraffin / Kerosine Used to make chemicals. Fuel for greenhouse heaters and jet engines, manufacture of chemicals. Diesel fuel Fuel for lorries, trains. Fuel and Fuel for the heating systems of large buildings, lubricating fuel for ships, lubricating oil Bitumen Roofing, and road surfaces. © Boardworks Ltd 2001

Fraction Number of carbon atoms Fuel gas 1 -4, mostly 1. Petrol / gasoline 5 -10 Naphtha Paraffin / Kerosine 8 -12. Diesel fuel 14 -22 Fuel and lubricating oil 20 -70 Bitumen More than 65 10 -16 © Boardworks Ltd 2001

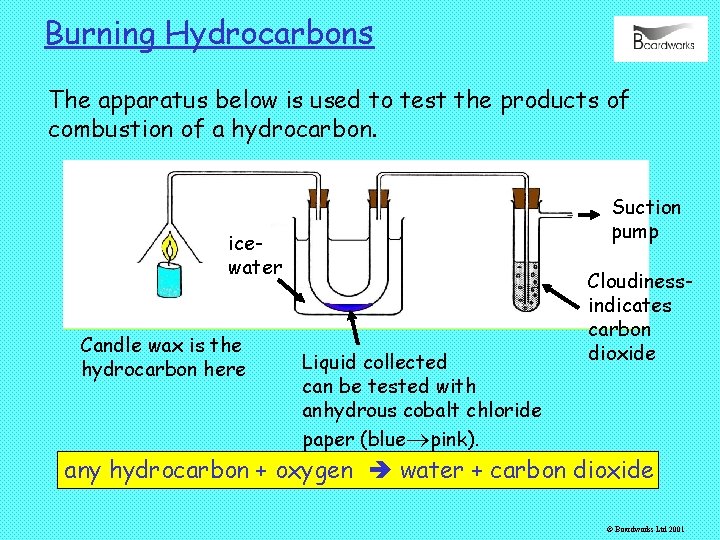
Burning Hydrocarbons The apparatus below is used to test the products of combustion of a hydrocarbon. Suction pump icewater Candle wax is the hydrocarbon here Liquid collected can be tested with anhydrous cobalt chloride paper (blue pink). Cloudinessindicates carbon dioxide any hydrocarbon + oxygen water + carbon dioxide © Boardworks Ltd 2001

Alkanes Carbon atoms are unusual in that they are able to form strong covalent bonds to each other. Therefore, carbon is able to form chains of atoms of different length and so make molecules of different size. There are many thousands of molecules containing C-C bonds. In this course you learn about two groups of these compounds, alkanes and alkenes. Alkanes are hydrocarbons (molecules containing carbon and hydrogen only) in which each carbon atom is covalently bonded to four other atoms via 4 single covalent bonds. The other atoms can be either carbon or hydrogen. © Boardworks Ltd 2001

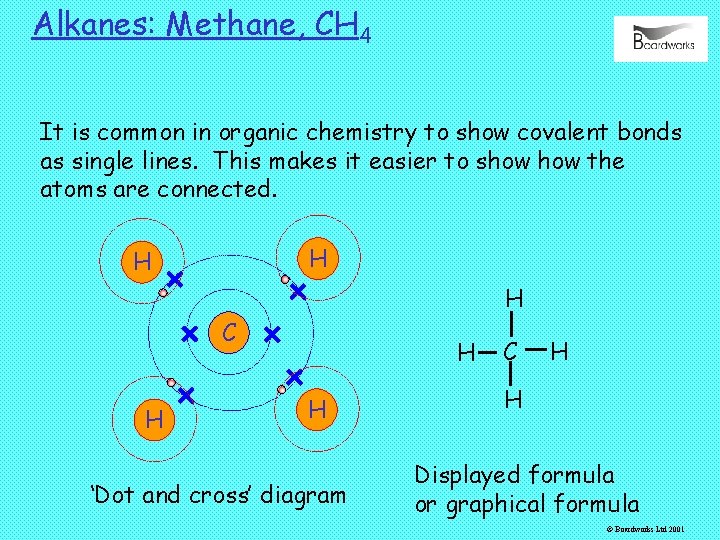
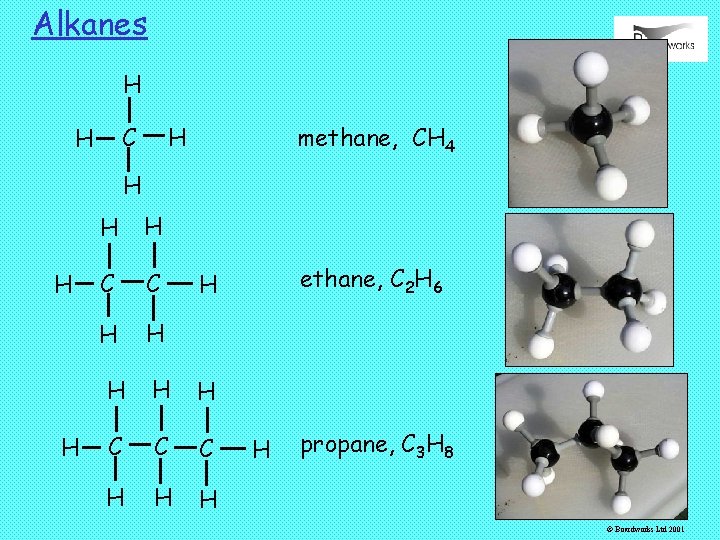
Alkanes: Methane, CH 4 It is common in organic chemistry to show covalent bonds as single lines. This makes it easier to show the atoms are connected. H H H C H H H ‘Dot and cross’ diagram C H H Displayed formula or graphical formula © Boardworks Ltd 2001

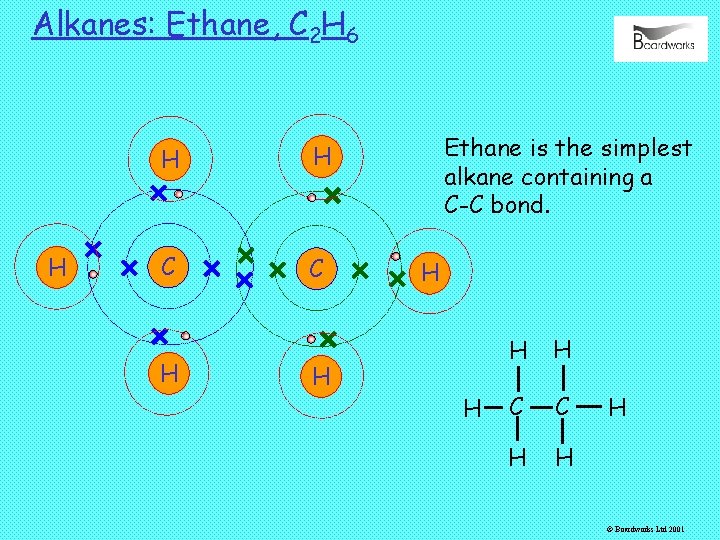
Alkanes: Ethane, C 2 H 6 H H H C C H H Ethane is the simplest alkane containing a C-C bond. H H C C H H H © Boardworks Ltd 2001

Alkanes H H C H methane, CH 4 H H H C C H H ethane, C 2 H 6 H H C C C H H propane, C 3 H 8 © Boardworks Ltd 2001

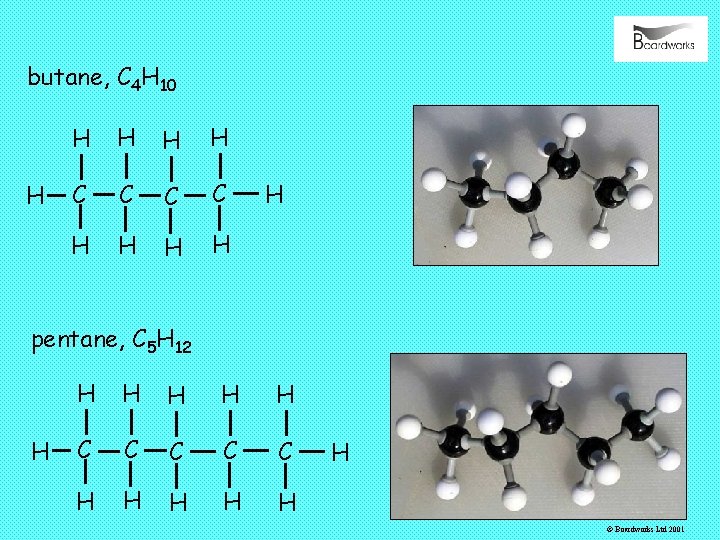
butane, C 4 H 10 H H H C C H H H pentane, C 5 H 12 H H H C C C H H H © Boardworks Ltd 2001

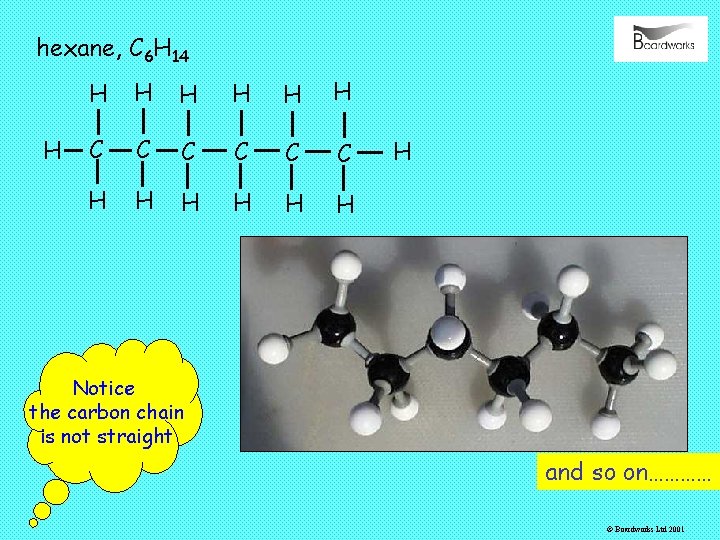
hexane, C 6 H 14 H H H H C C C H H H H Notice the carbon chain is not straight and so on………… © Boardworks Ltd 2001

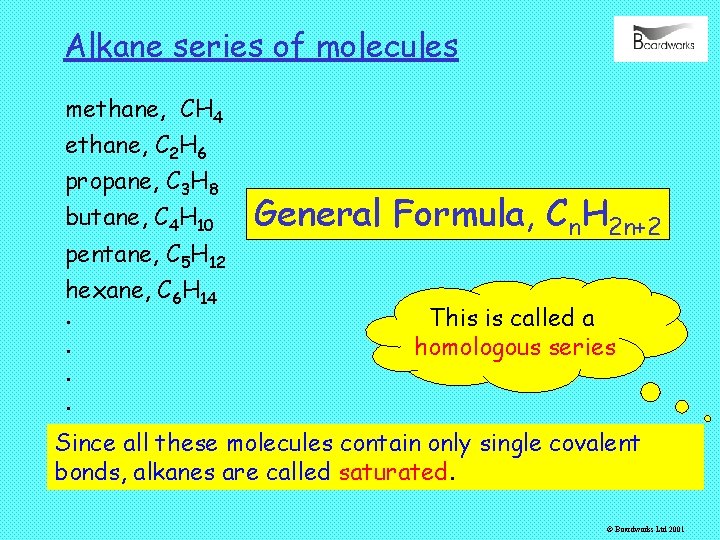
Alkane series of molecules methane, CH 4 ethane, C 2 H 6 propane, C 3 H 8 butane, C 4 H 10 pentane, C 5 H 12 hexane, C 6 H 14. . General Formula, Cn. H 2 n+2 This is called a homologous series Since all these molecules contain only single covalent bonds, alkanes are called saturated. © Boardworks Ltd 2001

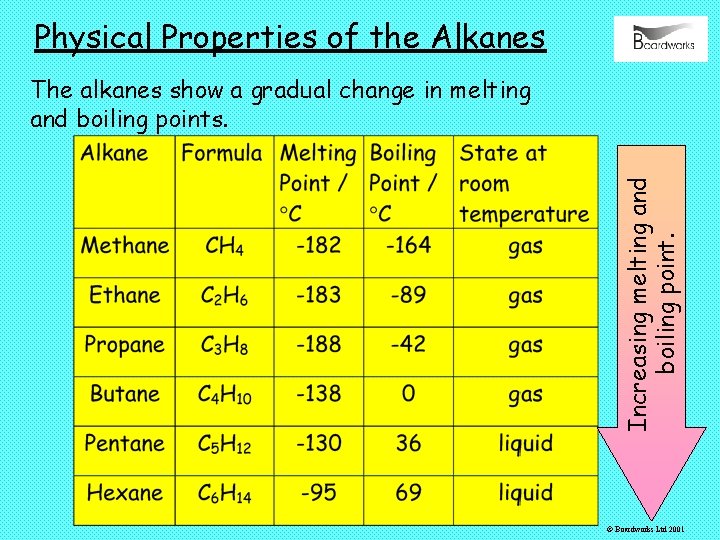
Physical Properties of the Alkanes Increasing melting and boiling point. The alkanes show a gradual change in melting and boiling points. © Boardworks Ltd 2001

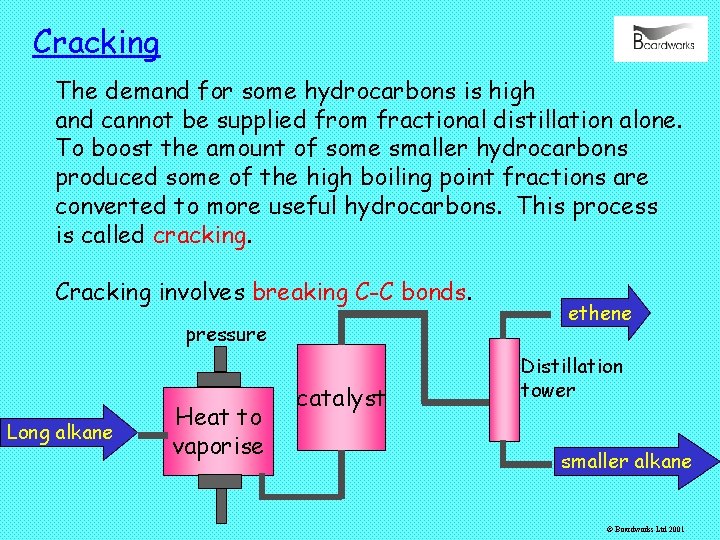
Cracking The demand for some hydrocarbons is high and cannot be supplied from fractional distillation alone. To boost the amount of some smaller hydrocarbons produced some of the high boiling point fractions are converted to more useful hydrocarbons. This process is called cracking. Cracking involves breaking C-C bonds. pressure Long alkane Heat to vaporise catalyst ethene Distillation tower smaller alkane © Boardworks Ltd 2001

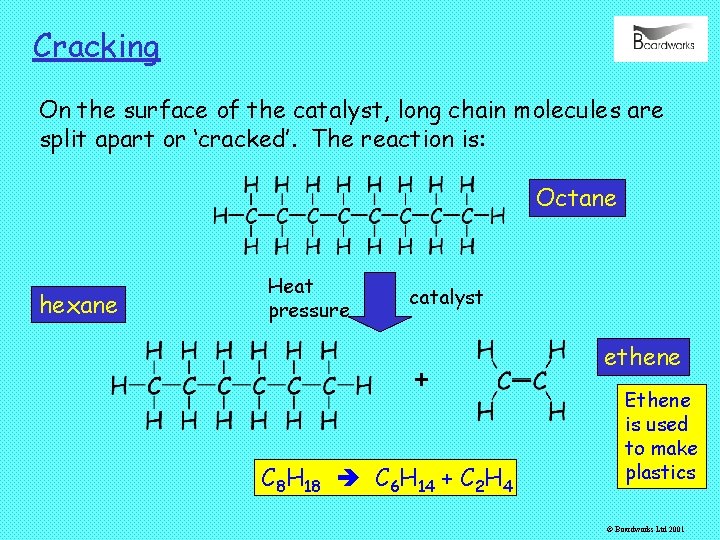
Cracking On the surface of the catalyst, long chain molecules are split apart or ‘cracked’. The reaction is: Octane hexane Heat pressure catalyst + C 8 H 18 C 6 H 14 + C 2 H 4 ethene Ethene is used to make plastics © Boardworks Ltd 2001

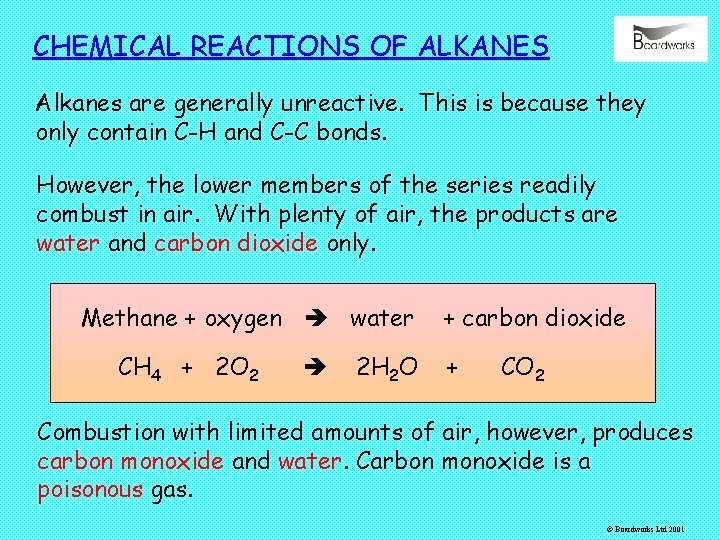
CHEMICAL REACTIONS OF ALKANES Alkanes are generally unreactive. This is because they only contain C-H and C-C bonds. However, the lower members of the series readily combust in air. With plenty of air, the products are water and carbon dioxide only. Methane + oxygen water CH 4 + 2 O 2 2 H 2 O + carbon dioxide + CO 2 Combustion with limited amounts of air, however, produces carbon monoxide and water. Carbon monoxide is a poisonous gas. © Boardworks Ltd 2001

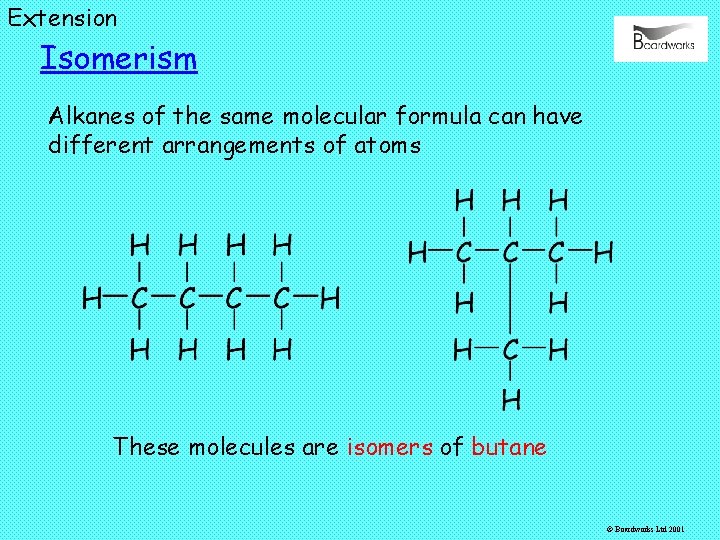
Extension Isomerism Alkanes of the same molecular formula can have different arrangements of atoms These molecules are isomers of butane © Boardworks Ltd 2001

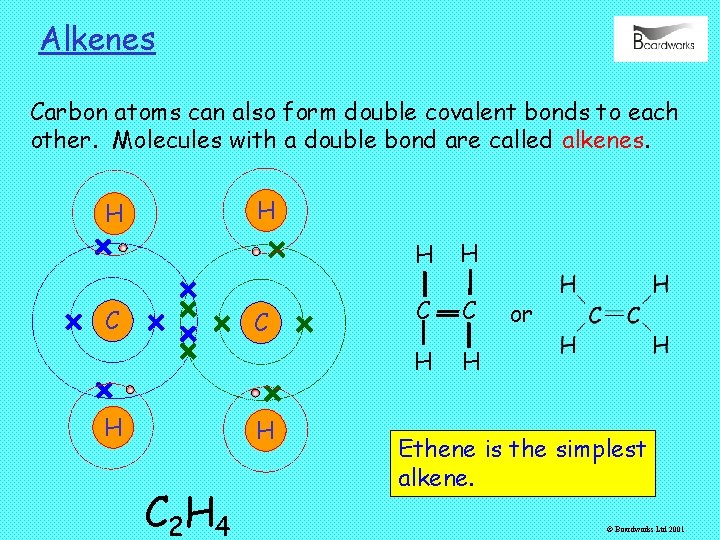
Alkenes Carbon atoms can also form double covalent bonds to each other. Molecules with a double bond are called alkenes. H H C C H H C 2 H 4 H H C C H H or Ethene is the simplest alkene. © Boardworks Ltd 2001

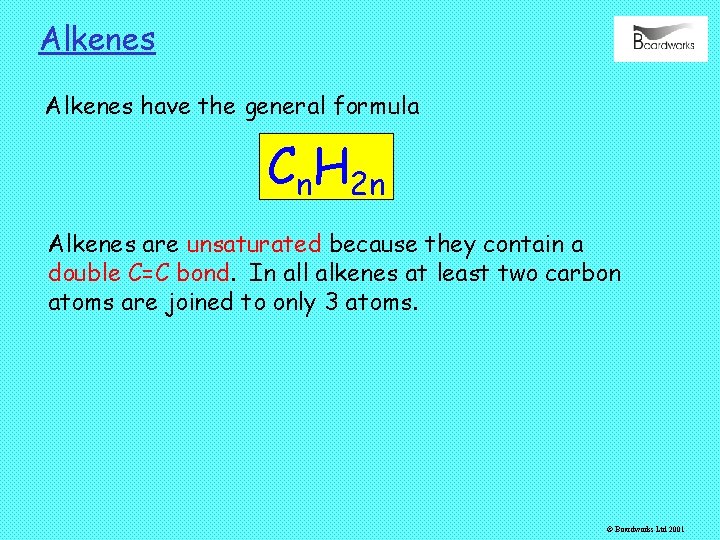
Alkenes have the general formula Cn. H 2 n Alkenes are unsaturated because they contain a double C=C bond. In all alkenes at least two carbon atoms are joined to only 3 atoms. © Boardworks Ltd 2001

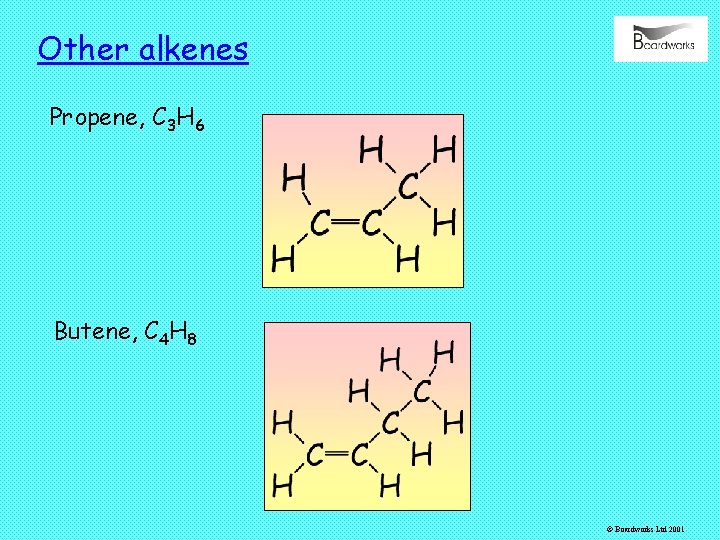
Other alkenes Propene, C 3 H 6 Butene, C 4 H 8 © Boardworks Ltd 2001

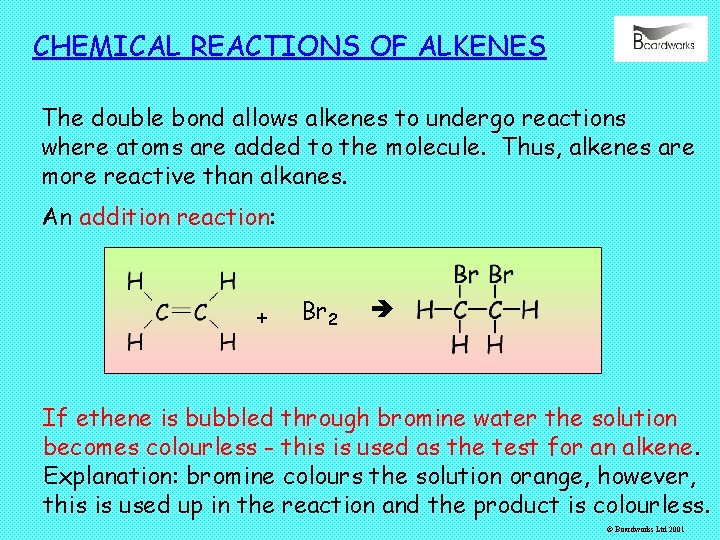
CHEMICAL REACTIONS OF ALKENES The double bond allows alkenes to undergo reactions where atoms are added to the molecule. Thus, alkenes are more reactive than alkanes. An addition reaction: + Br 2 If ethene is bubbled through bromine water the solution becomes colourless - this is used as the test for an alkene. Explanation: bromine colours the solution orange, however, this is used up in the reaction and the product is colourless. © Boardworks Ltd 2001

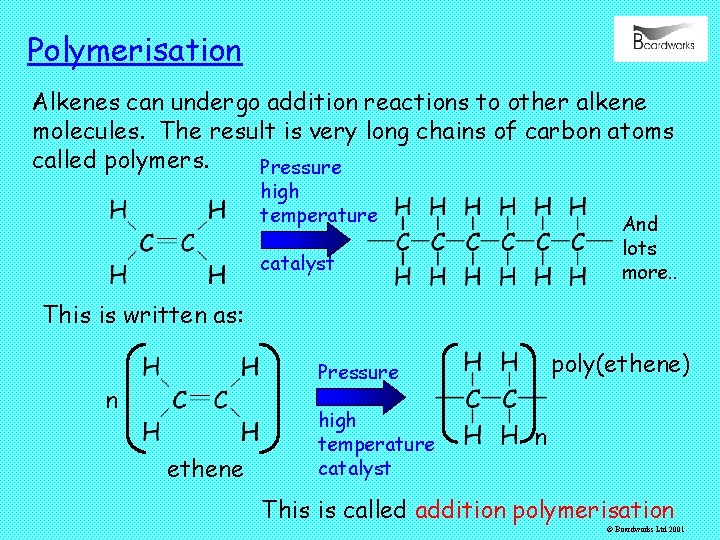
Polymerisation Alkenes can undergo addition reactions to other alkene molecules. The result is very long chains of carbon atoms called polymers. Pressure high temperature And lots more. . catalyst This is written as: poly(ethene) Pressure n ethene high temperature catalyst n This is called addition polymerisation © Boardworks Ltd 2001

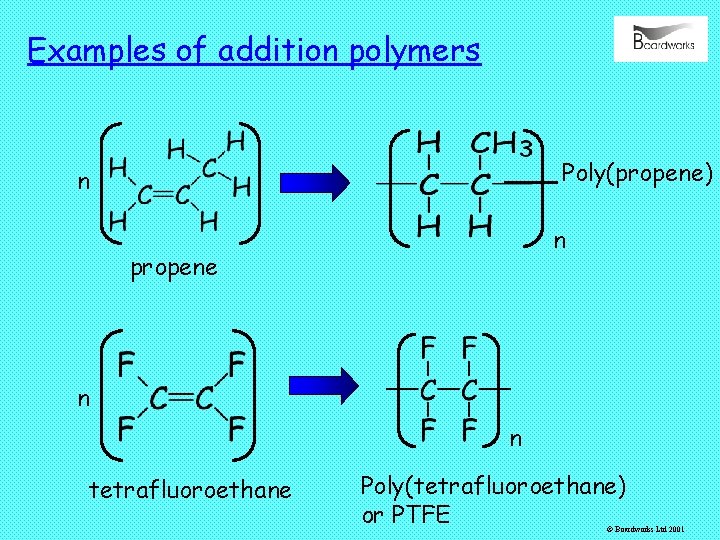
Examples of addition polymers Poly(propene) n n propene n n tetrafluoroethane Poly(tetrafluoroethane) or PTFE © Boardworks Ltd 2001

Three very useful plastics © Boardworks Ltd 2001

Poly(ethene) Bottles (HDPE) Poly(propene) boxes (PP) © Boardworks Ltd 2001

REVIEW QUESTIONS © Boardworks Ltd 2001

1. i) How is crude oil split into fractions ii) Name the seven fractions produced by fractional distillation of crude oil. iii) Give three differences between fractions. 2. i) What is meant by cracking? ii) Complete the formula below: C 8 H 18 + C 2 H 4 iii) The starting hydrocarbon is octane, name the two products of this reaction. iv) Describe how you could test for the presence of ethene. © Boardworks Ltd 2001

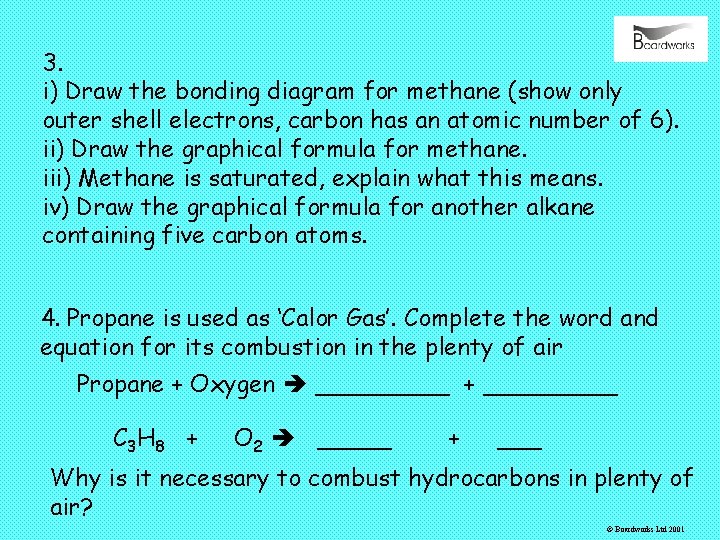
3. i) Draw the bonding diagram for methane (show only outer shell electrons, carbon has an atomic number of 6). ii) Draw the graphical formula for methane. iii) Methane is saturated, explain what this means. iv) Draw the graphical formula for another alkane containing five carbon atoms. 4. Propane is used as ‘Calor Gas’. Complete the word and equation for its combustion in the plenty of air Propane + Oxygen _____ + _____ C 3 H 8 + O 2 _____ + ___ Why is it necessary to combust hydrocarbons in plenty of air? © Boardworks Ltd 2001

5. i) In a homologous series the physical properties change gradually in the same direction. Plot a graph of boiling point against number of carbons for the alkanes. Use this graph to predict the boiling point of decane, C 10 H 22. 6. i) Write down the graphical formula for propene. ii) Write down the equation for the reaction of propene with bromine. iii) Predict the reaction of propene with hydrogen and write down the equation. Iv) What type of reaction is this? © Boardworks Ltd 2001

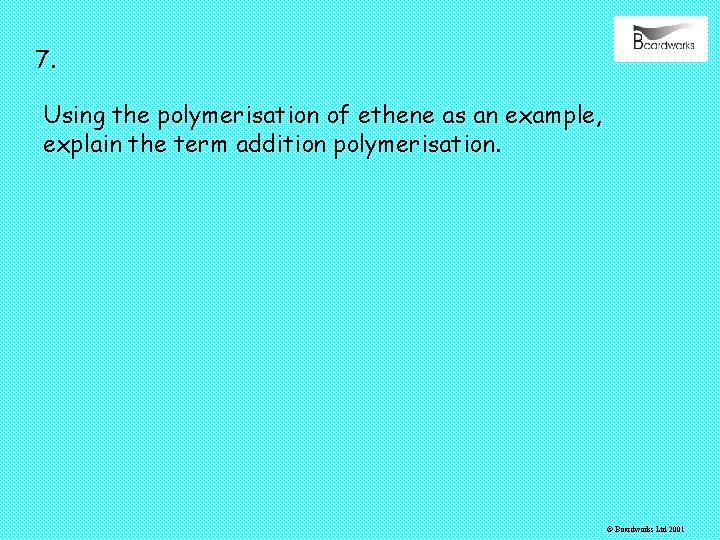
7. Using the polymerisation of ethene as an example, explain the term addition polymerisation. © Boardworks Ltd 2001

Harder Questions 8. Draw graphical formula for the three isomers of pentane. 9. Draw graphical formula for three isomers of butene. 10. Write down the equation for the combustion of propene in plenty of air and with a restricted amount of air. © Boardworks Ltd 2001

ANSWERS TO REVIEW QUESTIONS © Boardworks Ltd 2001

1. i) Crude oil is split into ‘fractions’ of different boiling point by fractional distillation. Here crude oil is vaporised and passed into a fractionating tower. Towards the top of the tower, where it is cooler, compounds of low boiling point condense and are collected on trays from which they are removed. Lower in the tower, higher boiling point fractions are collected. ii) Refinery gas / Petrol or gasoline / Naphtha / Kerosine or paraffin / Diesel / Oil / Bitumen iii) boiling point / viscosity / colour / size of hydrocarbon molecule © Boardworks Ltd 2001

2. i) Cracking is the process used to convert long hydrocarbon molecules into smaller ones. The hydrocarbon liquid is vaporised and passed over a catalyst. On the surface of the catalyst the carbon chain is split to produce ethene and a smaller alkane. ii) C 8 H 18 C 6 H 14 + C 2 H 4 iii) C 6 H 14 is hexane, C 2 H 4 is ethene iv) You could pass the gas through bromine water. The bromine water, which is orange, will become colourless. © Boardworks Ltd 2001

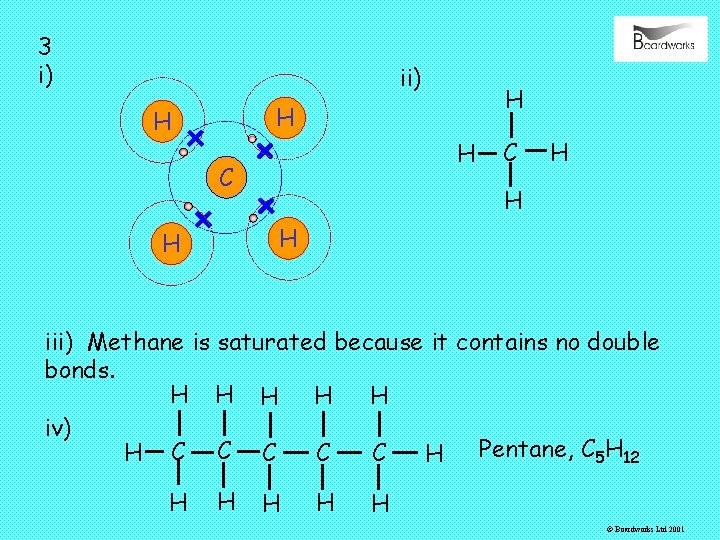
3 i) ii) H H C C H H iii) Methane is saturated because it contains no double bonds. H H H iv) Pentane, C 5 H 12 C H C C H H H © Boardworks Ltd 2001

4. Propane + Oxygen carbon dioxide + water C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O In insufficient air/oxygen, carbon monoxide and water are produced. Carbon monoxide is a poisonous gas. © Boardworks Ltd 2001

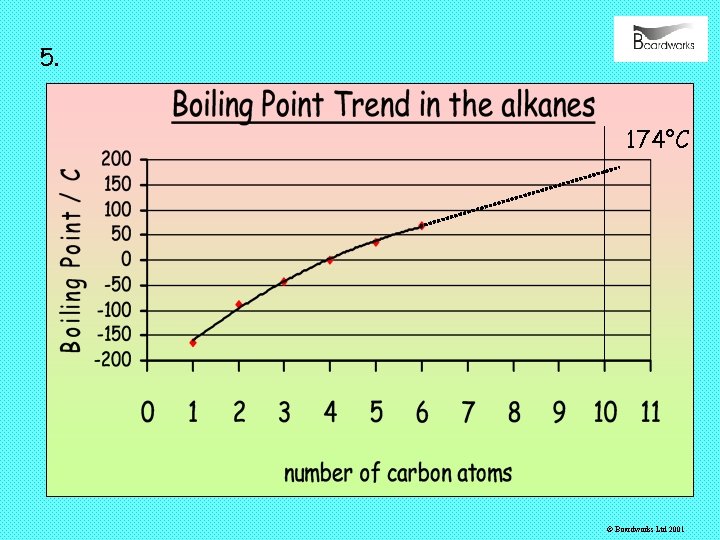
5. 174°C © Boardworks Ltd 2001

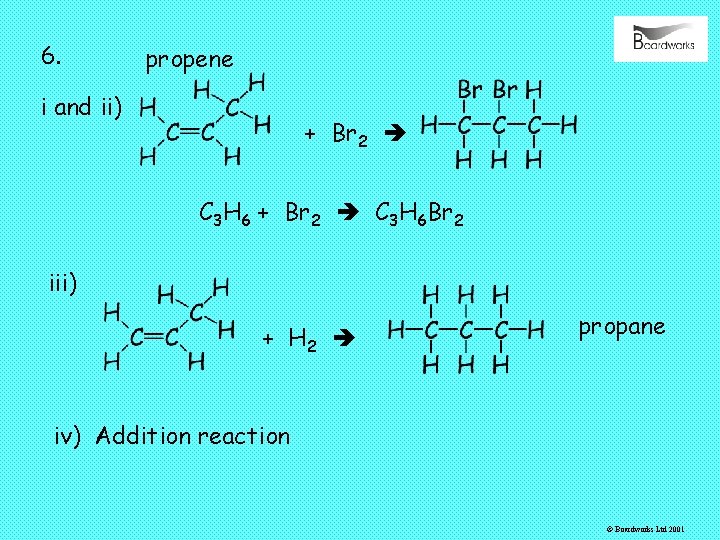
6. propene i and ii) + Br 2 C 3 H 6 Br 2 iii) + H 2 propane iv) Addition reaction © Boardworks Ltd 2001

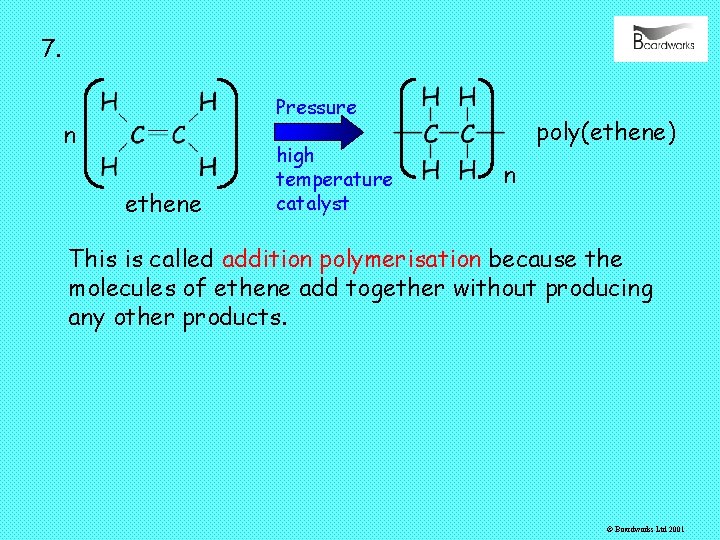
7. Pressure n ethene high temperature catalyst poly(ethene) n This is called addition polymerisation because the molecules of ethene add together without producing any other products. © Boardworks Ltd 2001

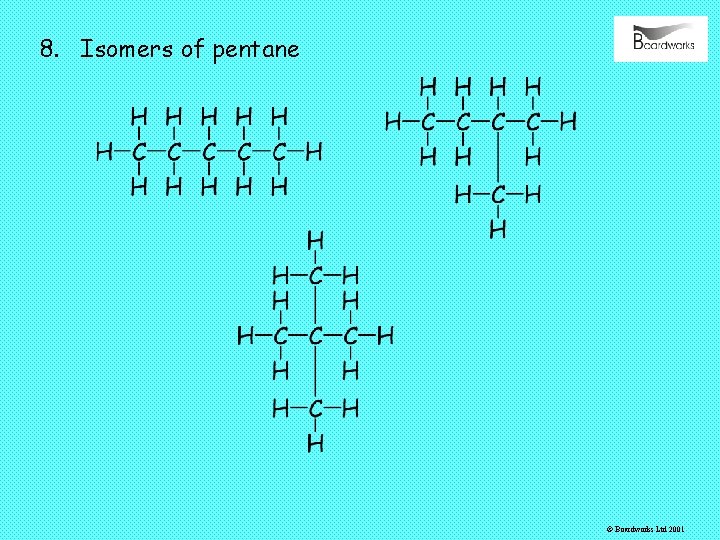
8. Isomers of pentane © Boardworks Ltd 2001

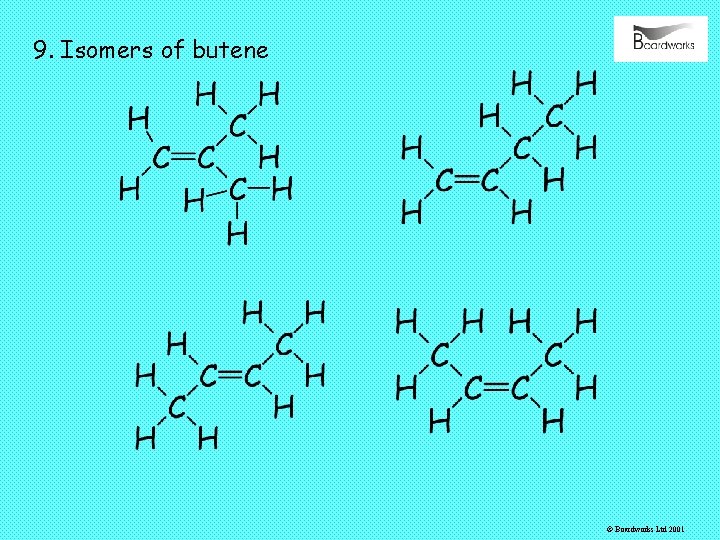
9. Isomers of butene © Boardworks Ltd 2001
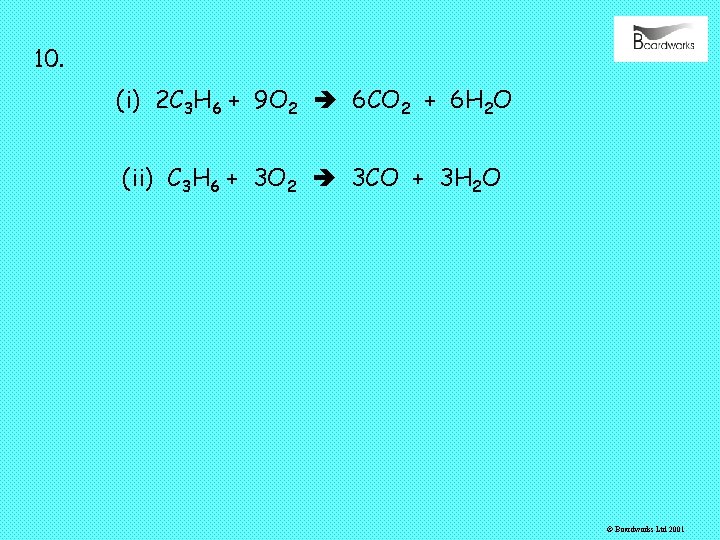
10. (i) 2 C 3 H 6 + 9 O 2 6 CO 2 + 6 H 2 O (ii) C 3 H 6 + 3 O 2 3 CO + 3 H 2 O © Boardworks Ltd 2001
 Water resources important
Water resources important Print and web sources
Print and web sources How to increase nitrogen in soil
How to increase nitrogen in soil Swot analysis of organic products
Swot analysis of organic products Walmart capstone presentation
Walmart capstone presentation Swo analysis
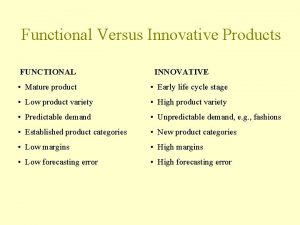
Swo analysis Functional vs innovative supply chain
Functional vs innovative supply chain Pepsi products vs coke products
Pepsi products vs coke products The place of useful learning
The place of useful learning Tool support for testing
Tool support for testing Useful microbes adalah
Useful microbes adalah Making data useful
Making data useful In drilling machine mcq
In drilling machine mcq Useful life of property plant and equipment
Useful life of property plant and equipment A material is useful when
A material is useful when Linearly independent vectors
Linearly independent vectors How do database applications make databases more useful?
How do database applications make databases more useful? Ratio of useful energy to total input energy
Ratio of useful energy to total input energy What is data converted into a meaningful and useful context
What is data converted into a meaningful and useful context The tree philip larkin
The tree philip larkin Why is mass more useful than weight for measuring matter
Why is mass more useful than weight for measuring matter Introduction for informal email
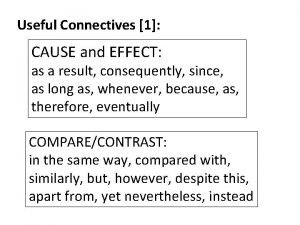
Introduction for informal email Connectives of cause and effect
Connectives of cause and effect Aqa gcse history 12 mark questions
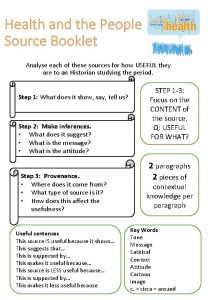
Aqa gcse history 12 mark questions How useful is this source
How useful is this source The cost to manufacture an unfinished unit is $120
The cost to manufacture an unfinished unit is $120 Useful block
Useful block How are atomic and molecular orbitals related?
How are atomic and molecular orbitals related? The rea data model
The rea data model Useful adb commands
Useful adb commands Mole background
Mole background Engineering and managerial economics
Engineering and managerial economics Usable vs useful
Usable vs useful A healthful eating plan can include sensible snacks
A healthful eating plan can include sensible snacks Thank you and have a great day email
Thank you and have a great day email Ppe structure
Ppe structure What is the most useful property of the semimetals
What is the most useful property of the semimetals Why is product analysis useful to a designer
Why is product analysis useful to a designer Thermal energy
Thermal energy How to calculate mode from mean and median
How to calculate mode from mean and median Importance of central tendency
Importance of central tendency The srs document is useful in various contexts
The srs document is useful in various contexts The most important thing in life essay
The most important thing in life essay How useful is this source
How useful is this source Business english dialogues
Business english dialogues Useful expressions for speaking
Useful expressions for speaking Thank you ladies and gentlemen
Thank you ladies and gentlemen Triangular shaped population pyramid
Triangular shaped population pyramid