Kinetics This is important determine rate laws units



![[reactants] & rate • rate law is a math relationship that shows how rate [reactants] & rate • rate law is a math relationship that shows how rate](https://slidetodoc.com/presentation_image_h2/068c2bcd81064d6436ffaa9ff7b7a5e7/image-5.jpg)

![Example Experiment [A] (M) [B] (M) Rate = -D[A]/Dt (M/s) 1 0. 10 0. Example Experiment [A] (M) [B] (M) Rate = -D[A]/Dt (M/s) 1 0. 10 0.](https://slidetodoc.com/presentation_image_h2/068c2bcd81064d6436ffaa9ff7b7a5e7/image-7.jpg)
![D [] with time • 1 st order rxn’s rate depends on the [] D [] with time • 1 st order rxn’s rate depends on the []](https://slidetodoc.com/presentation_image_h2/068c2bcd81064d6436ffaa9ff7b7a5e7/image-9.jpg)
- Slides: 16

Kinetics

This is important!!! • determine rate laws & units from experimental data • calculate rates & concentrations of reactants or products under given conditions • match mechanisms to rate laws • The next few chapters comprises over 50% of the test!!!

Rates • how quickly a reaction proceeds to the right (products) • units are D amount of reactants per D time (most commonly M/s) • as [reactants] & [products] change, so does the rate • rates are expressed as positive quantities, so negative sign is used on the slope to make it positive

Factors that affect rates • rates increase with more collisions or higher energy collisions, so… – [reactants] – higher [reactant] produce a faster rxn by increasing the frequency of collisions – temp – higher temp means higher energy collisions, collisions more often, and a higher percentage of successful collisions, thus higher rate – state of reactants – liquids & gases react faster because there are more particles available to collide. solids are limited to reacting particles on the surface. – catalyst – presence of catalyst increases rate by changing the mechanism of the rxn to one that has a higher success rate
![reactants rate rate law is a math relationship that shows how rate [reactants] & rate • rate law is a math relationship that shows how rate](https://slidetodoc.com/presentation_image_h2/068c2bcd81064d6436ffaa9ff7b7a5e7/image-5.jpg)
[reactants] & rate • rate law is a math relationship that shows how rate depends on [reactants] – where: a A + b B products, – the rate law is: Rate = k[A]m[B]n • • k = rate constant (determined experimentally) [A] = molar concentration of A [B] = molar concentration of B m & n = small, whole number that relate to # of m’cules that collide and determine the rxn order

m&n • are NOT the coefficients from the balanced rxn • can ONLY be found experimentally • if Rate = k[A]1[B]2, the rxn is said to be first order with respect to A, second order with respect to B, and third order overall
![Example Experiment A M B M Rate DADt Ms 1 0 10 0 Example Experiment [A] (M) [B] (M) Rate = -D[A]/Dt (M/s) 1 0. 10 0.](https://slidetodoc.com/presentation_image_h2/068c2bcd81064d6436ffaa9ff7b7a5e7/image-7.jpg)
Example Experiment [A] (M) [B] (M) Rate = -D[A]/Dt (M/s) 1 0. 10 0. 04 2 0. 10 0. 20 0. 08 3 0. 20 0. 32 Determine the rate order with respect to each of the reactants and the rate constant, k.

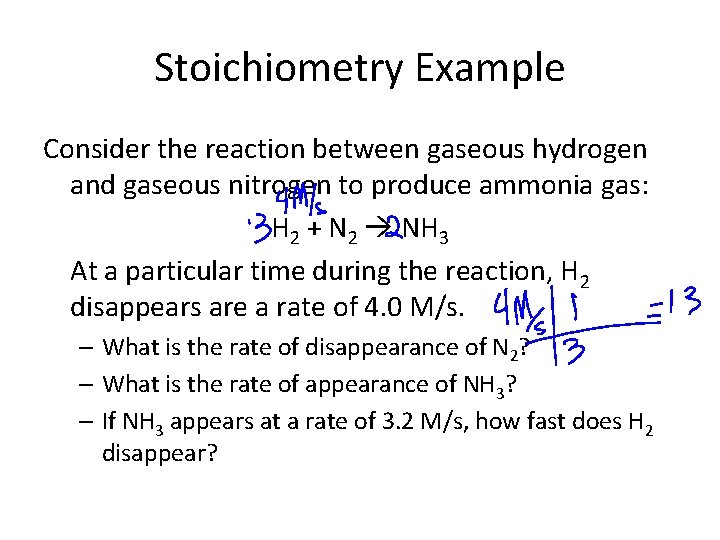
Stoichiometry Example Consider the reaction between gaseous hydrogen and gaseous nitrogen to produce ammonia gas: H 2 + N 2 NH 3 At a particular time during the reaction, H 2 disappears are a rate of 4. 0 M/s. – What is the rate of disappearance of N 2? – What is the rate of appearance of NH 3? – If NH 3 appears at a rate of 3. 2 M/s, how fast does H 2 disappear?
![D with time 1 st order rxns rate depends on the D [] with time • 1 st order rxn’s rate depends on the []](https://slidetodoc.com/presentation_image_h2/068c2bcd81064d6436ffaa9ff7b7a5e7/image-9.jpg)
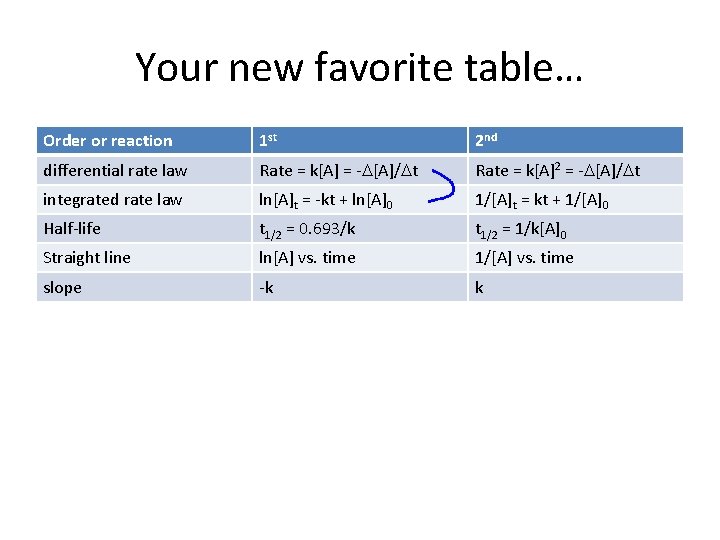
D [] with time • 1 st order rxn’s rate depends on the [] of a single reactant raised to the 1 st power. – to recognize 1 st order rxns, graph ln[A] vs. time and you will get a straight line whose slope = -k • 2 nd order rxn’s rate depends on the [] of a single reactant raised to the 2 nd power – to recognize 2 nd order rxns, graph 1/[A] vs. time and you will get a straight line whose slope = k

Your new favorite table… Order or reaction 1 st 2 nd differential rate law Rate = k[A] = -D[A]/Dt Rate = k[A]2 = -D[A]/Dt integrated rate law ln[A]t = -kt + ln[A]0 1/[A]t = kt + 1/[A]0 Half-life t 1/2 = 0. 693/k t 1/2 = 1/k[A]0 Straight line ln[A] vs. time 1/[A] vs. time slope -k k

Temp & rate • generally rate increases with temp • higher temp = higher energy = more collisions = more successful collisions = higher speed of m’cules • [] changes will not change k, but T will • know the vocab: – activation energy – transition state – catalyst

• know how to label Ea, Ereac, Eprod, DH, & transition state • know how to recognize endothermic vs. exothermic rxns

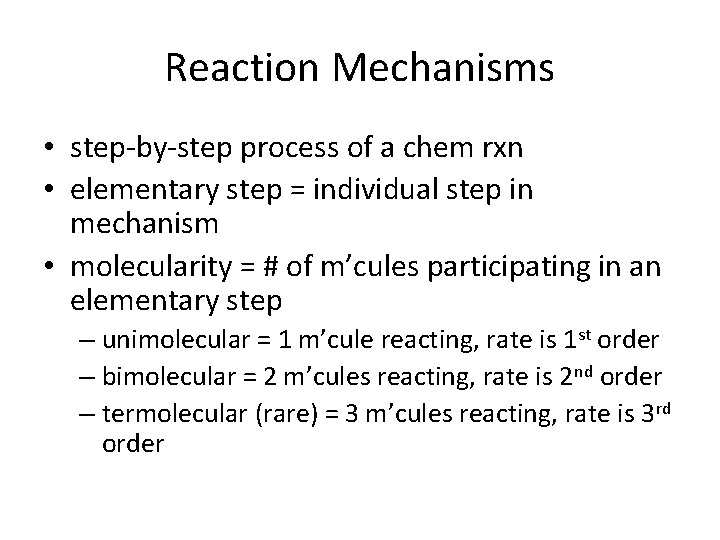
Reaction Mechanisms • step-by-step process of a chem rxn • elementary step = individual step in mechanism • molecularity = # of m’cules participating in an elementary step – unimolecular = 1 m’cule reacting, rate is 1 st order – bimolecular = 2 m’cules reacting, rate is 2 nd order – termolecular (rare) = 3 m’cules reacting, rate is 3 rd order

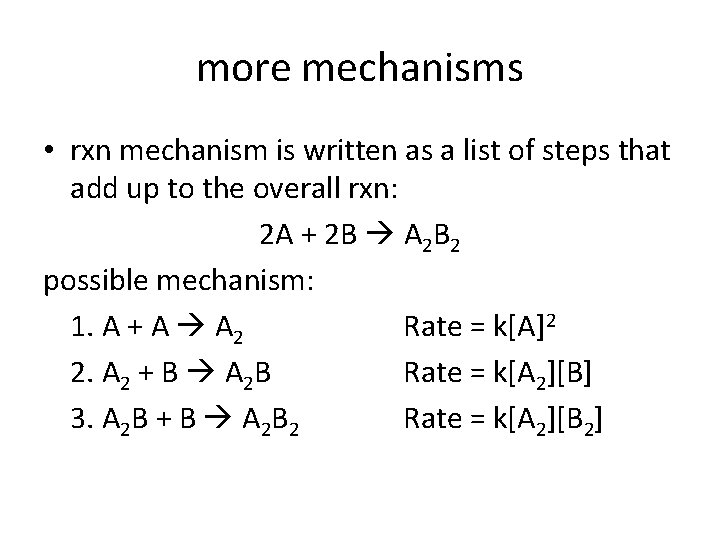
more mechanisms • rxn mechanism is written as a list of steps that add up to the overall rxn: 2 A + 2 B A 2 B 2 possible mechanism: 1. A + A A 2 Rate = k[A]2 2. A 2 + B A 2 B Rate = k[A 2][B] 3. A 2 B + B A 2 B 2 Rate = k[A 2][B 2]

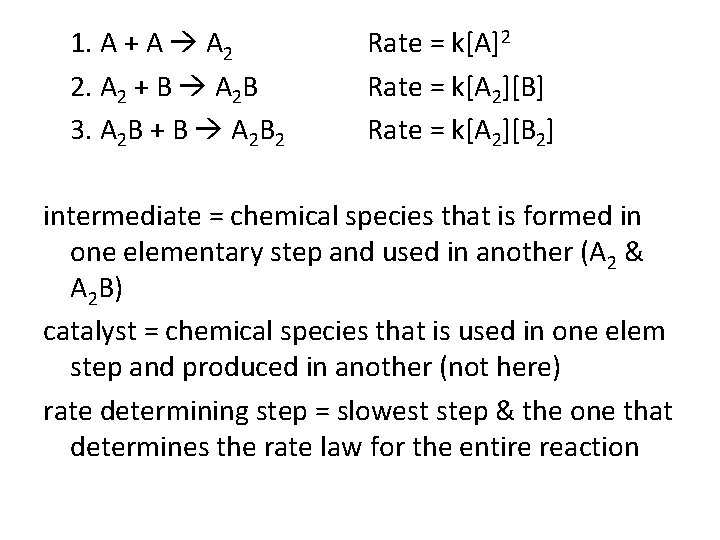
1. A + A A 2 2. A 2 + B A 2 B 3. A 2 B + B A 2 B 2 Rate = k[A]2 Rate = k[A 2][B] Rate = k[A 2][B 2] intermediate = chemical species that is formed in one elementary step and used in another (A 2 & A 2 B) catalyst = chemical species that is used in one elem step and produced in another (not here) rate determining step = slowest step & the one that determines the rate law for the entire reaction

Catalysts • catalyst = used to increase the rate of the rxn, but is not used up in the rxn itself, removes the slowest step in the rxn, lowers the Ea.