Kinetics Activation Energy and Catalysts Collision Model n




- Slides: 18

Kinetics Activation Energy and Catalysts

Collision Model n n We have discussed that molecules must collide to react. These collisions require both sufficient Kinetic Energy as well as correct orientation in order for the reaction to proceed.

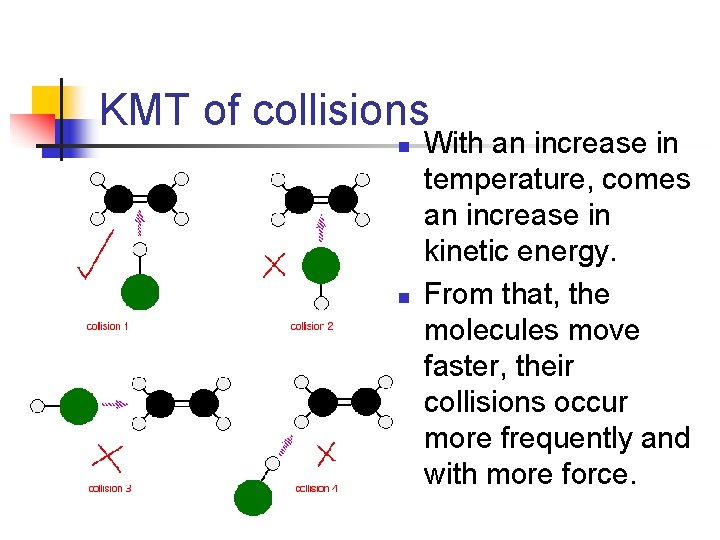
KMT of collisions n n With an increase in temperature, comes an increase in kinetic energy. From that, the molecules move faster, their collisions occur more frequently and with more force.

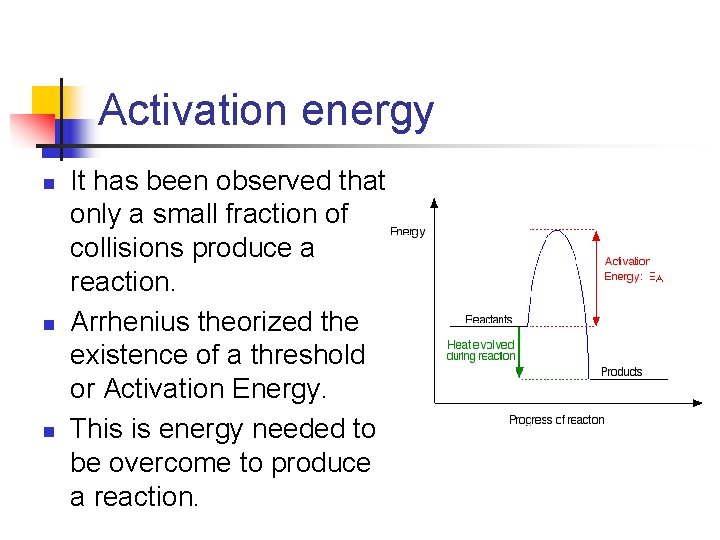
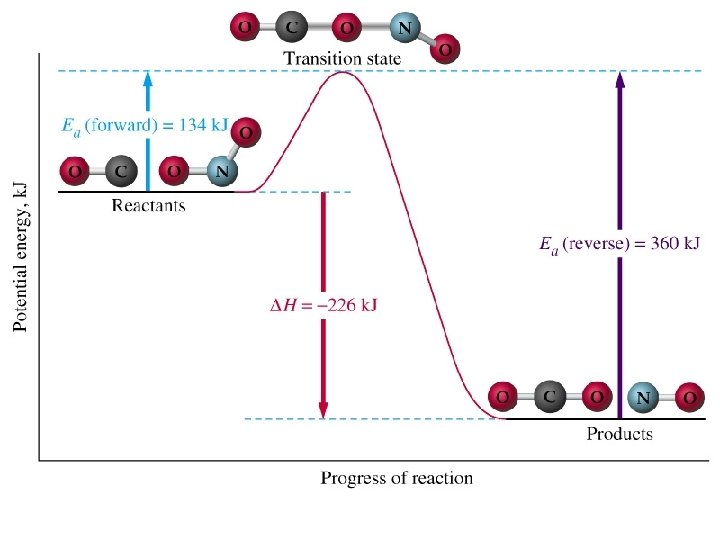
Activation energy n n n It has been observed that only a small fraction of collisions produce a reaction. Arrhenius theorized the existence of a threshold or Activation Energy. This is energy needed to be overcome to produce a reaction.

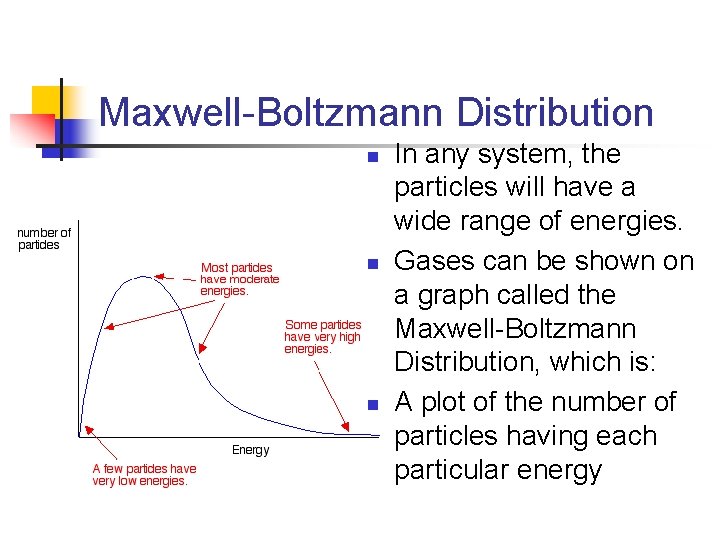
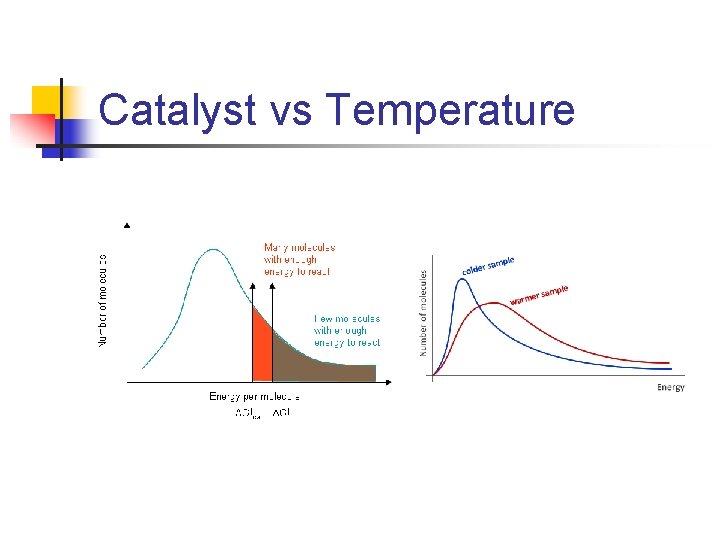
Maxwell-Boltzmann Distribution n In any system, the particles will have a wide range of energies. Gases can be shown on a graph called the Maxwell-Boltzmann Distribution, which is: A plot of the number of particles having each particular energy

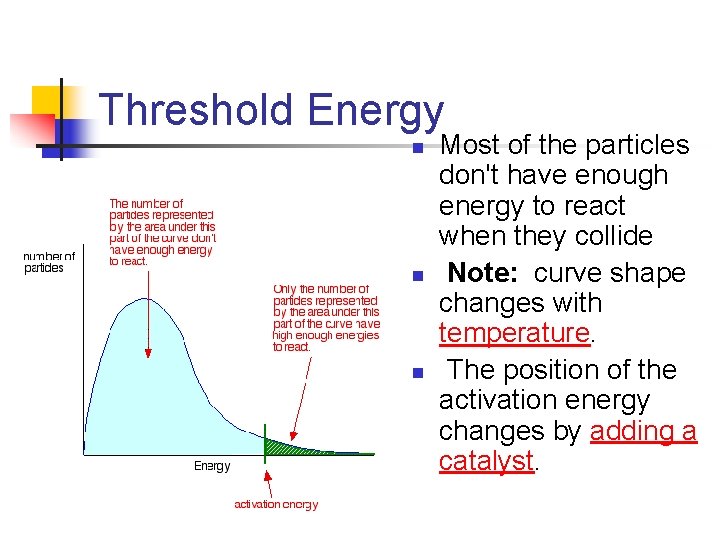
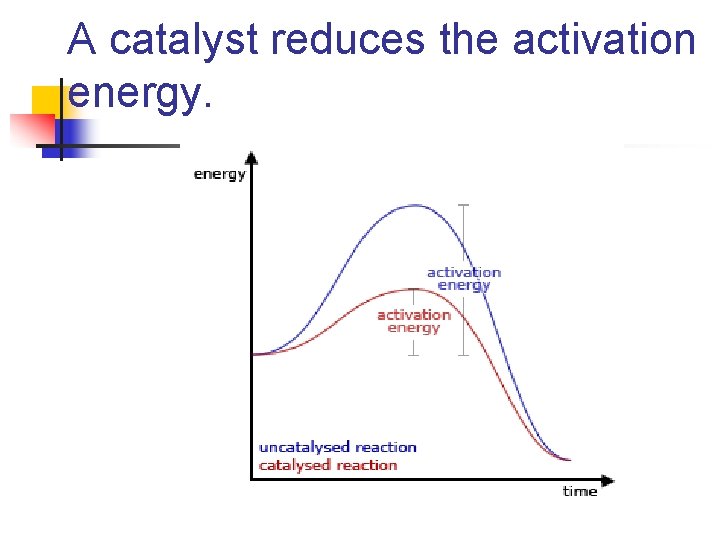
Threshold Energy n n n Most of the particles don't have enough energy to react when they collide Note: curve shape changes with temperature. The position of the activation energy changes by adding a catalyst.

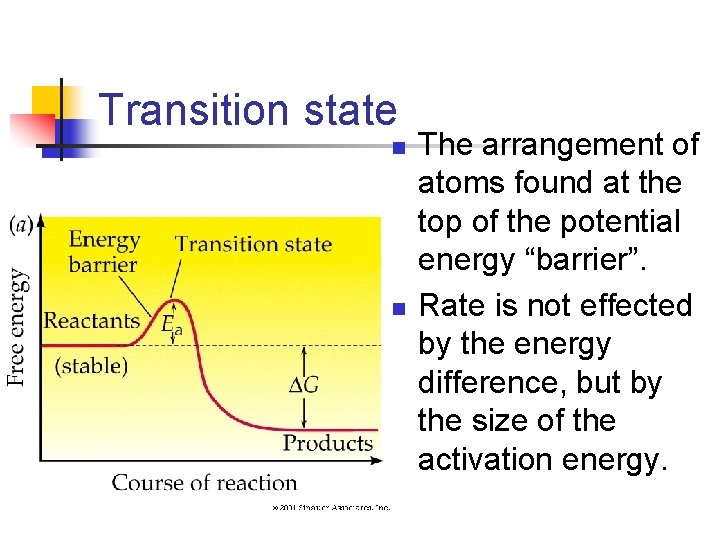
Transition state n n The arrangement of atoms found at the top of the potential energy “barrier”. Rate is not effected by the energy difference, but by the size of the activation energy.

Proposed mechanism

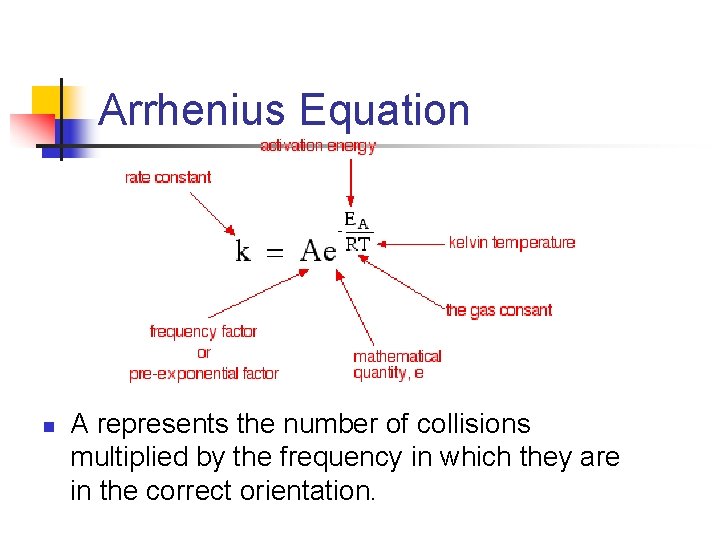
Arrhenius Equation n A represents the number of collisions multiplied by the frequency in which they are in the correct orientation.

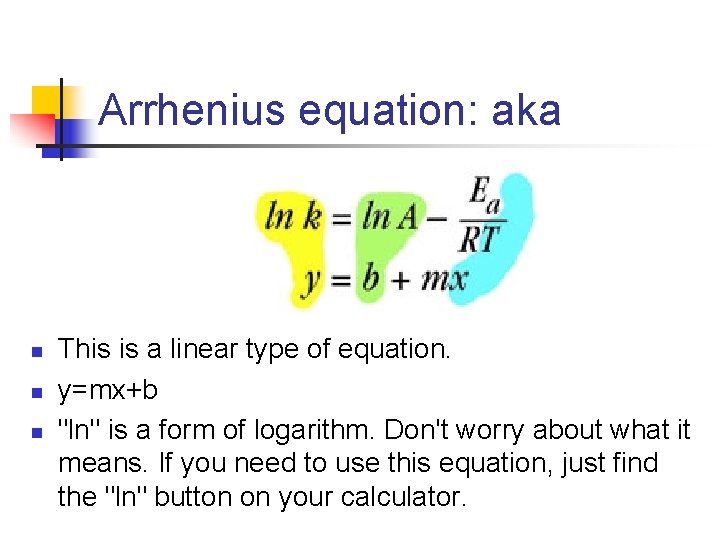
Arrhenius equation: aka n n n This is a linear type of equation. y=mx+b "ln" is a form of logarithm. Don't worry about what it means. If you need to use this equation, just find the "ln" button on your calculator.

Example n n n CH 4(g) + 2 S 2(g) CS 2(g) + 2 H 2 S(g) At 550ºC the rate constant is 1. 1 M-1 sec-1 and at 625ºC the rate constant is 6. 4 M-1 sec-1. Find Ea for this reaction.

Solution n n Substitute values into ln(k 2/k 1)=Ea/8. 3145 J/K • mol(1/T 1 -1/T 2) ln(6. 4/1. 1)=Ea/8. 3145 J/K • mol(1/823 K 1/898 K) Ea=1. 4 x 105 J/mol

Catalyst n n A catalyst is a substance that speeds up a reaction without being consumed by it. Examples include biological enzymes, sometimes acids or even metal surfaces.

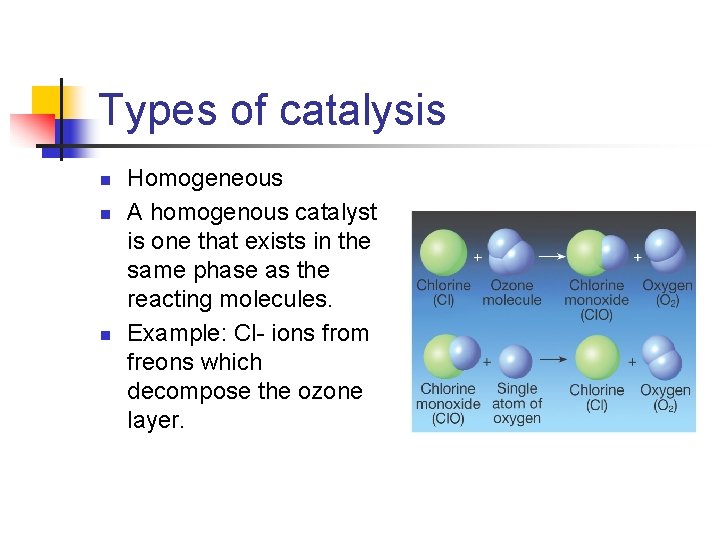
Types of catalysis n n n Homogeneous A homogenous catalyst is one that exists in the same phase as the reacting molecules. Example: Cl- ions from freons which decompose the ozone layer.

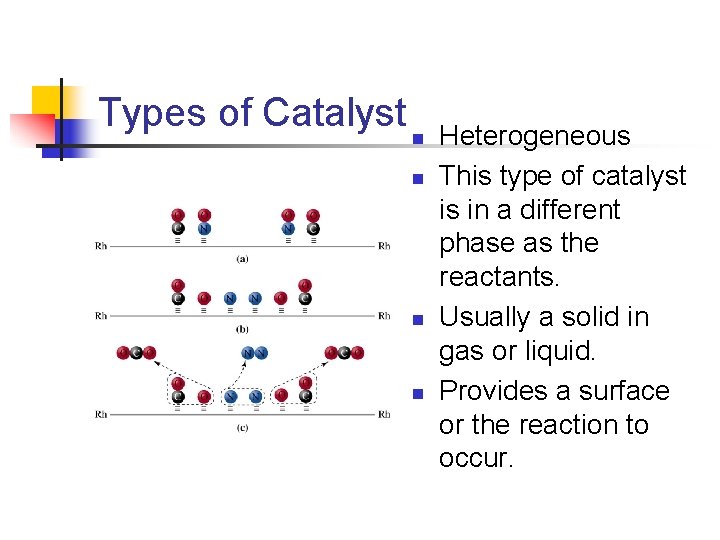
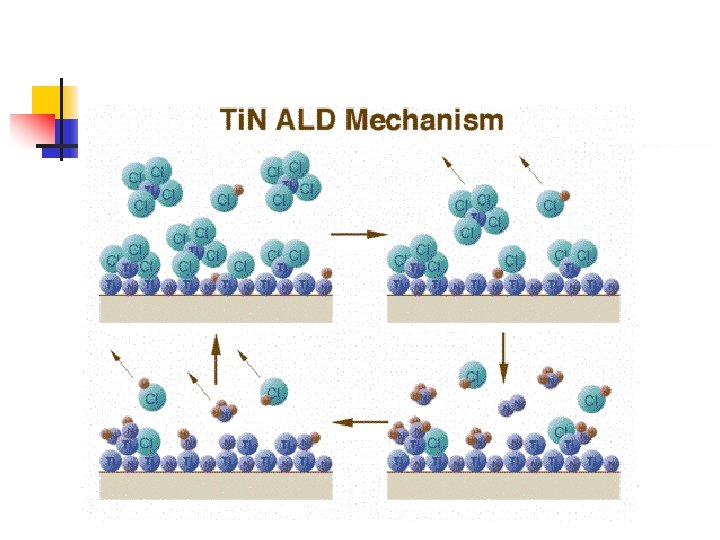
Types of Catalyst n n Heterogeneous This type of catalyst is in a different phase as the reactants. Usually a solid in gas or liquid. Provides a surface or the reaction to occur.

Catalyst

A catalyst reduces the activation energy.

Catalyst vs Temperature