ISMS Univ of Illinois June 2015 Highresolution Laser


- Slides: 28

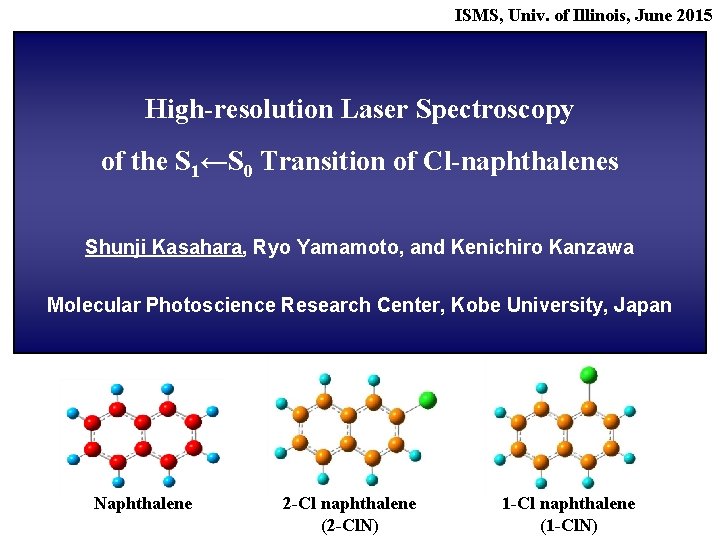
ISMS, Univ. of Illinois, June 2015 High-resolution Laser Spectroscopy of the S 1←S 0 Transition of Cl-naphthalenes Shunji Kasahara, Ryo Yamamoto, and Kenichiro Kanzawa Molecular Photoscience Research Center, Kobe University, Japan Naphthalene 2 -Cl naphthalene (2 -Cl. N) 1 -Cl naphthalene (1 -Cl. N)

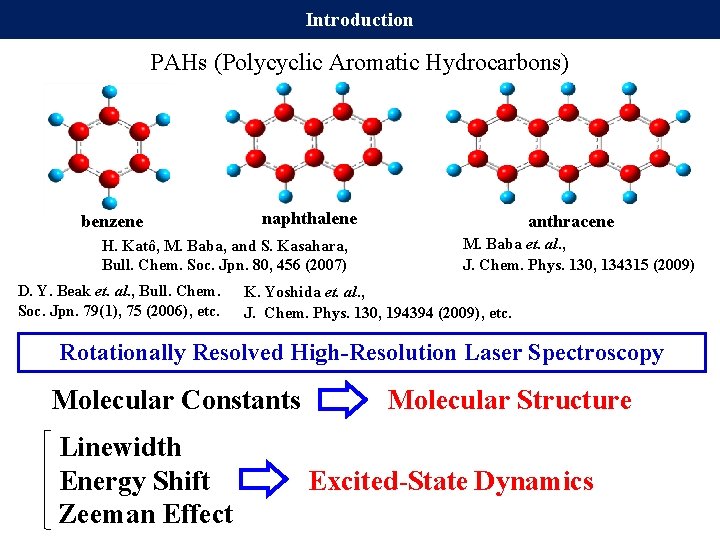
Introduction PAHs (Polycyclic Aromatic Hydrocarbons) benzene naphthalene H. Katô, M. Baba, and S. Kasahara, Bull. Chem. Soc. Jpn. 80, 456 (2007) D. Y. Beak et. al. , Bull. Chem. Soc. Jpn. 79(1), 75 (2006), etc. anthracene M. Baba et. al. , J. Chem. Phys. 130, 134315 (2009) K. Yoshida et. al. , J. Chem. Phys. 130, 194394 (2009), etc. Rotationally Resolved High-Resolution Laser Spectroscopy Molecular Constants Molecular Structure Linewidth Energy Shift Excited-State Dynamics Zeeman Effect

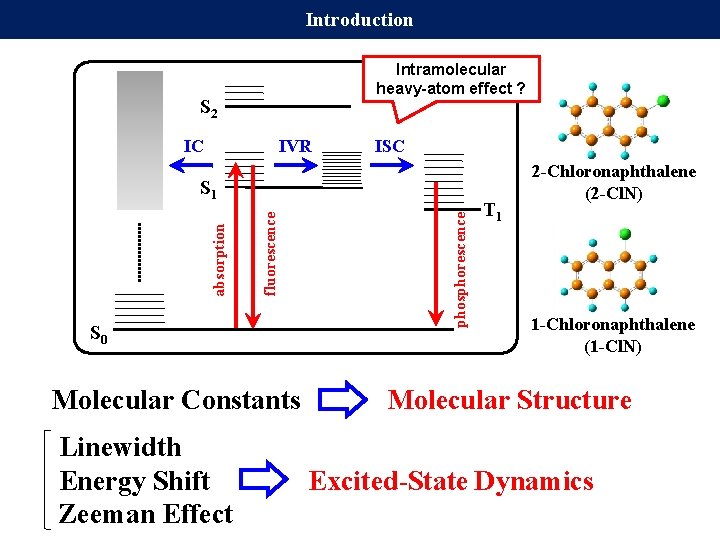
Introduction Intramolecular heavy-atom effect ? S 2 IC IVR ISC S 0 Molecular Constants phosphorescence fluorescence absorption S 1 T 1 2 -Chloronaphthalene (2 -Cl. N) 1 -Chloronaphthalene (1 -Cl. N) Molecular Structure Linewidth Energy Shift Excited-State Dynamics Zeeman Effect

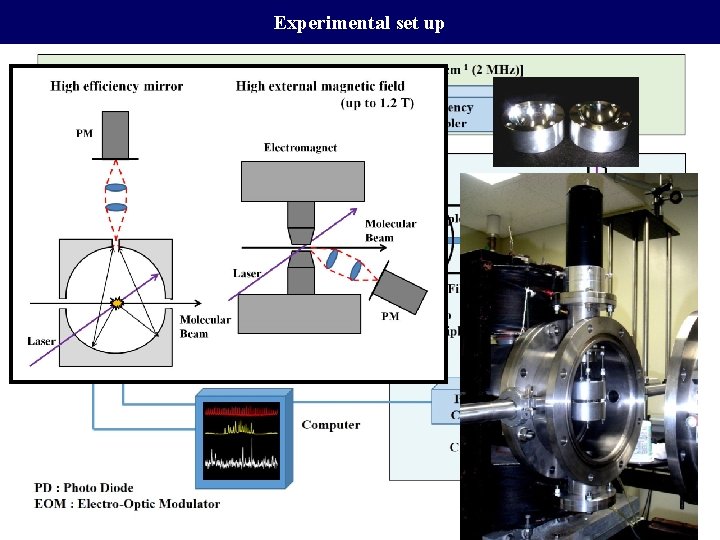
Experimental set up

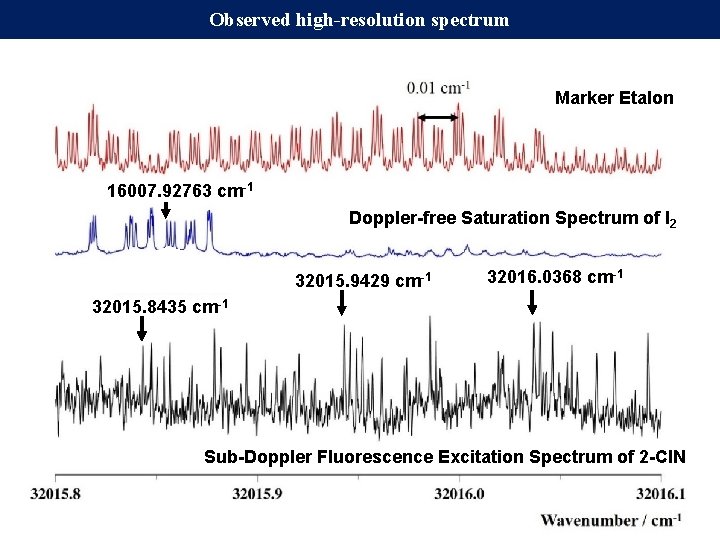
Observed high-resolution spectrum Marker Etalon 16007. 92763 cm-1 Doppler-free Saturation Spectrum of I 2 32015. 9429 cm-1 32016. 0368 cm-1 32015. 8435 cm-1 Sub-Doppler Fluorescence Excitation Spectrum of 2 -Cl. N

2 -Cl naphthalene (2 -Cl. N)

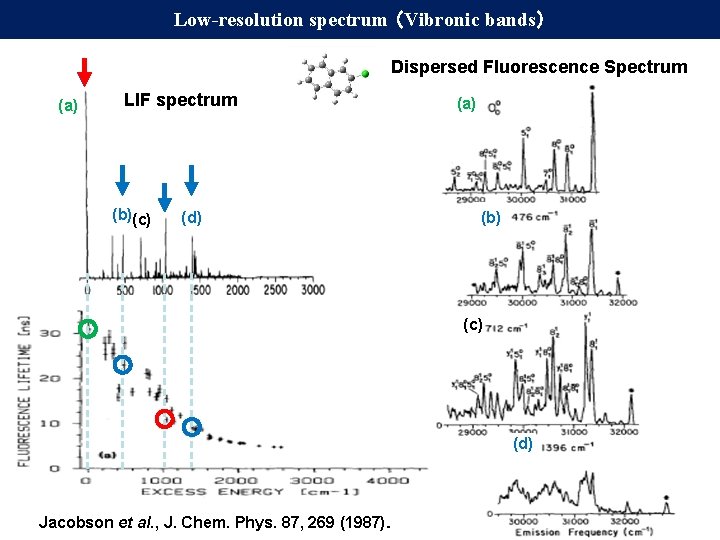
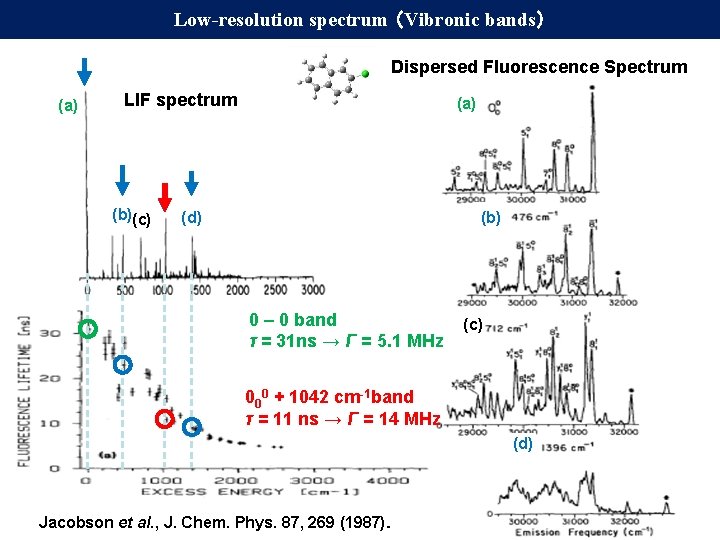
Low-resolution spectrum (Vibronic bands) Dispersed Fluorescence Spectrum (a) LIF spectrum (b)(c) (d) (a) (b) (c) (d) Jacobson et al. , J. Chem. Phys. 87, 269 (1987).

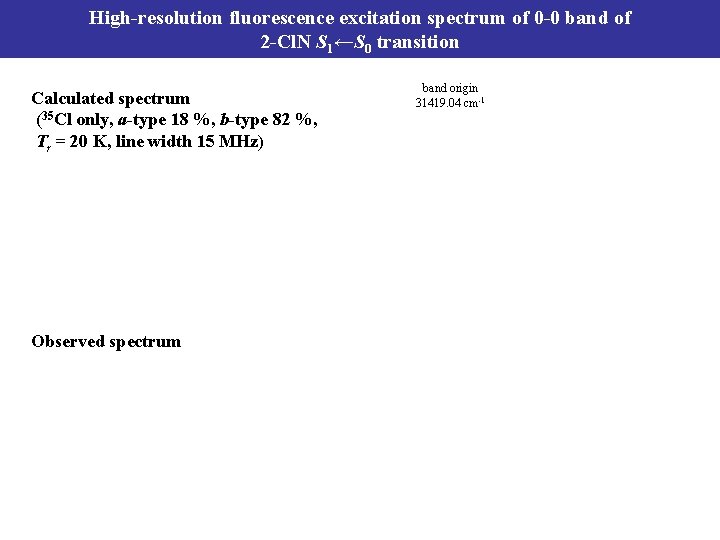
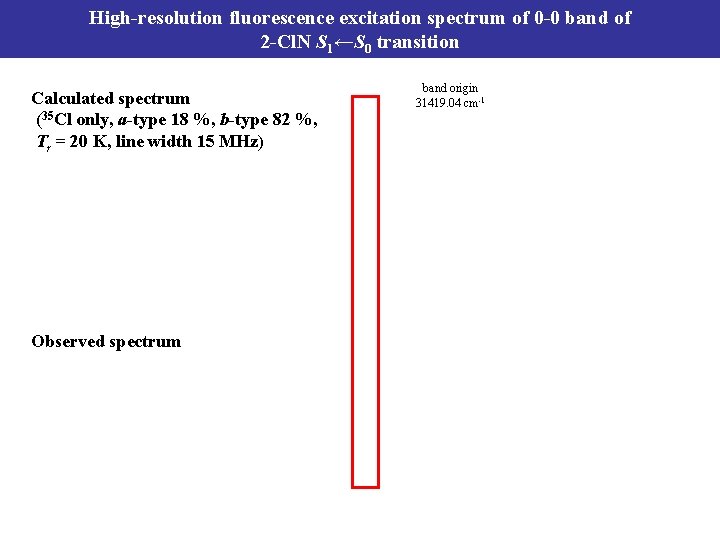
High-resolution fluorescence excitation spectrum of 0 -0 band of 2 -Cl. N S 1←S 0 transition Calculated spectrum (35 Cl only, a-type 18 %, b-type 82 %, Tr = 20 K, line width 15 MHz) Observed spectrum band origin 31419. 04 cm-1

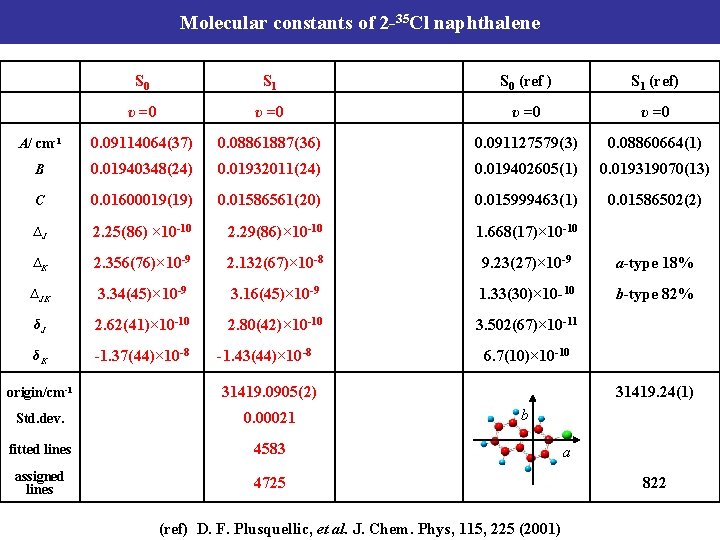
Molecular constants of 2 -35 Cl naphthalene S 0 S 1 S 0 (ref ) S 1 (ref) υ =0 A/ cm-1 0. 09114064(37) 0. 08861887(36) 0. 091127579(3) 0. 08860664(1) B 0. 01940348(24) 0. 01932011(24) 0. 019402605(1) 0. 019319070(13) C 0. 01600019(19) 0. 01586561(20) 0. 015999463(1) 0. 01586502(2) ∆J 2. 25(86) × 10 -10 2. 29(86)× 10 -10 1. 668(17)× 10 -10 ∆K 2. 356(76)× 10 -9 2. 132(67)× 10 -8 9. 23(27)× 10 -9 a-type 18% ∆JK 3. 34(45)× 10 -9 3. 16(45)× 10 -9 1. 33(30)× 10 -10 b-type 82% δJ 2. 62(41)× 10 -10 2. 80(42)× 10 -10 3. 502(67)× 10 -11 δK -1. 37(44)× 10 -8 -1. 43(44)× 10 -8 6. 7(10)× 10 -10 origin/cm-1 31419. 0905(2) 31419. 24(1) Std. dev. 0. 00021 b fitted lines 4583 assigned lines 4725 (ref) D. F. Plusquellic, et al. J. Chem. Phys, 115, 225 (2001) a 822

High-resolution fluorescence excitation spectrum of 0 -0 band of 2 -Cl. N S 1←S 0 transition Calculated spectrum (35 Cl only, a-type 18 %, b-type 82 %, Tr = 20 K, line width 15 MHz) Observed spectrum band origin 31419. 04 cm-1

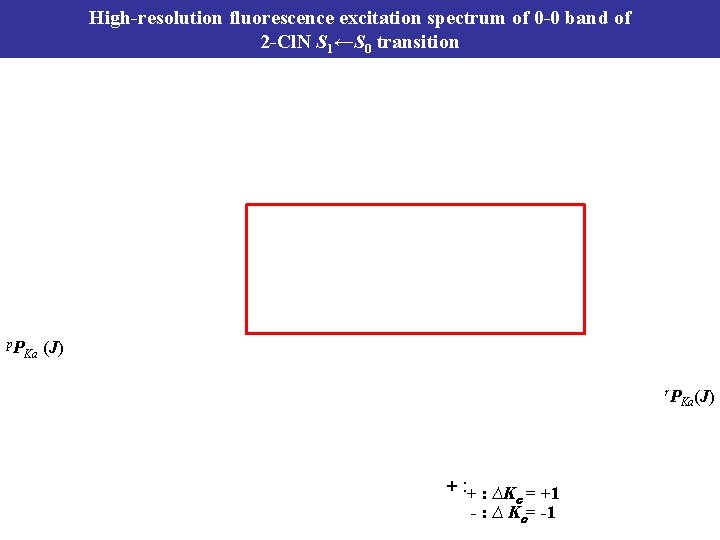
High-resolution fluorescence excitation spectrum of 0 -0 band of 2 -Cl. N S 1←S 0 transition p. P Ka (J) r. P + : ∆K = +1 c - : ∆ Kc= -1 Ka(J)

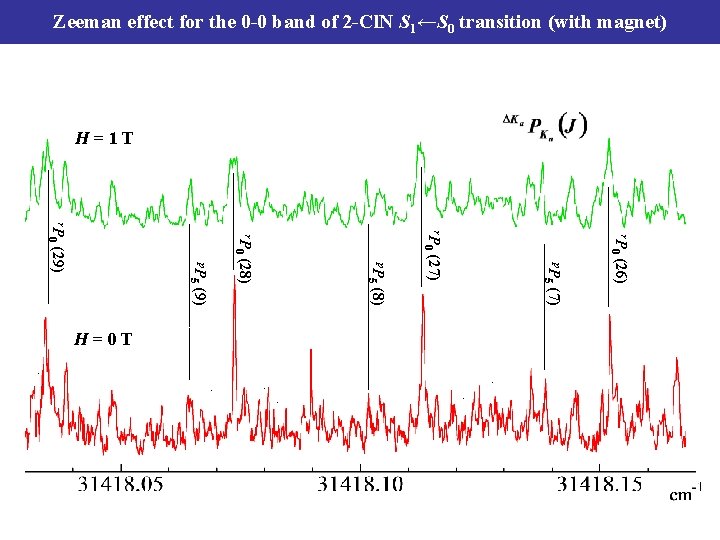
Zeeman effect for the 0 -0 band of 2 -Cl. N S 1←S 0 transition (with magnet) H=1 T 0 0 0 5 5 (26) p. P 5 (28) p. P (27) (29) r. P 0 r. P (7) (8) (9) H=0 T

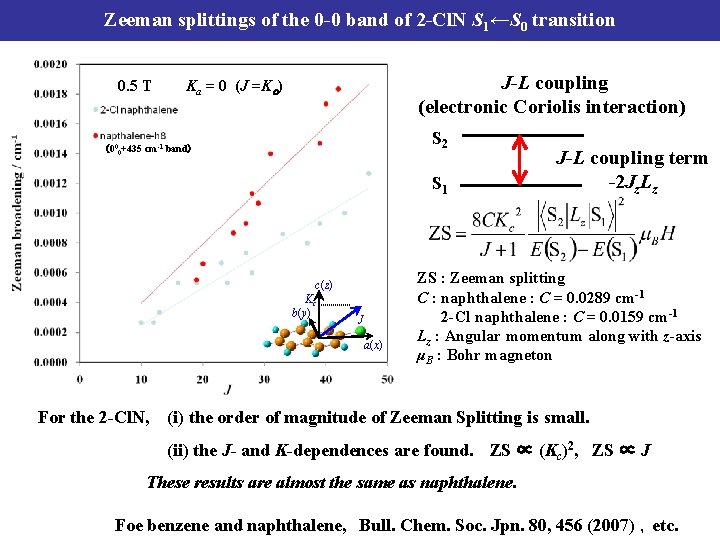
Zeeman splittings of the 0 -0 band of 2 -Cl. N S 1←S 0 transition 0. 5 T J-L coupling (electronic Coriolis interaction) Ka = 0 (J =Kc) S 2 (000+435 cm-1 band) S 1 c(z) Kc b(y) J a(x) For the 2 -Cl. N, J-L coupling term -2 Jz. Lz ZS : Zeeman splitting C : naphthalene : C = 0. 0289 cm-1 2 -Cl naphthalene : C = 0. 0159 cm-1 Lz : Angular momentum along with z-axis μB : Bohr magneton (i) the order of magnitude of Zeeman Splitting is small. (ii) the J- and K-dependences are found. ZS ∝ (Kc)2, ZS ∝ J These results are almost the same as naphthalene. Foe benzene and naphthalene, Bull. Chem. Soc. Jpn. 80, 456 (2007) ,etc.

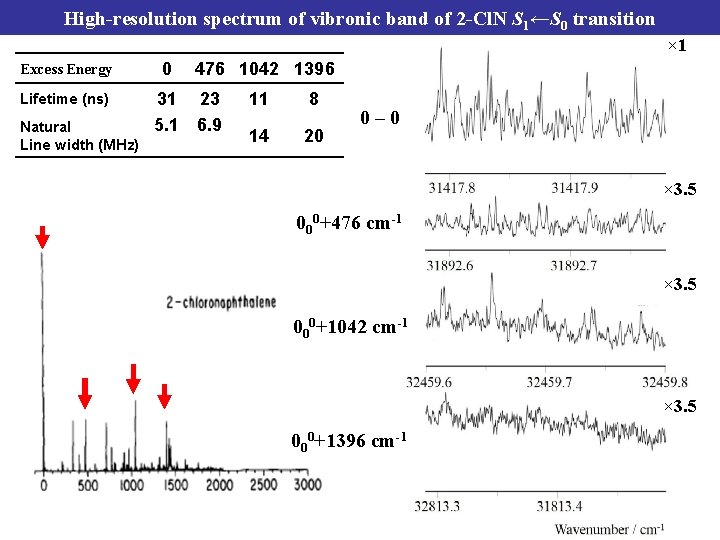
Low-resolution spectrum (Vibronic bands) Dispersed Fluorescence Spectrum (a) LIF spectrum (b)(c) (a) (b) (d) 0 – 0 band τ = 31 ns → Γ = 5. 1 MHz (c) 000 + 1042 cm-1 band τ = 11 ns → Γ = 14 MHz (d) Jacobson et al. , J. Chem. Phys. 87, 269 (1987).

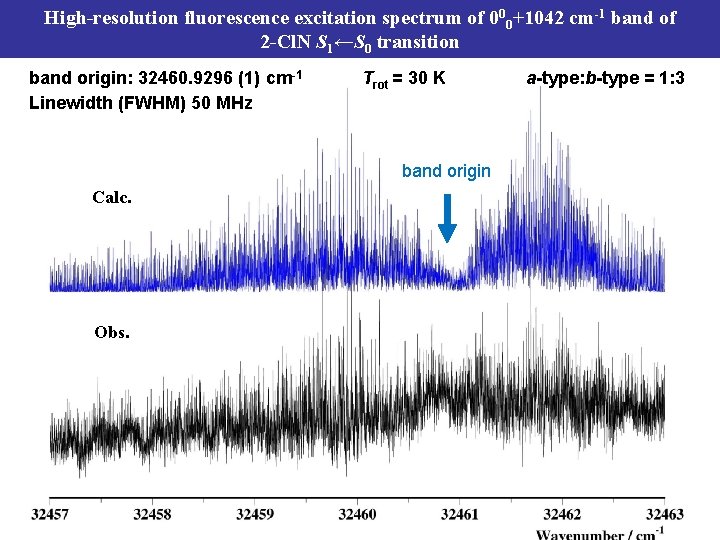
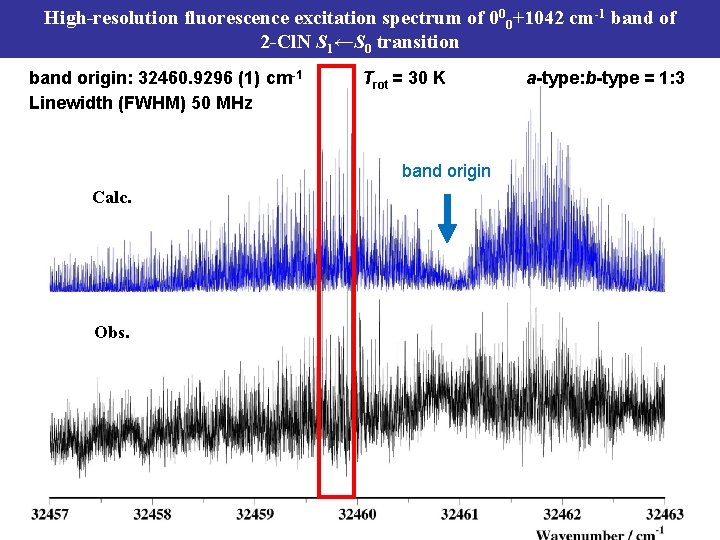
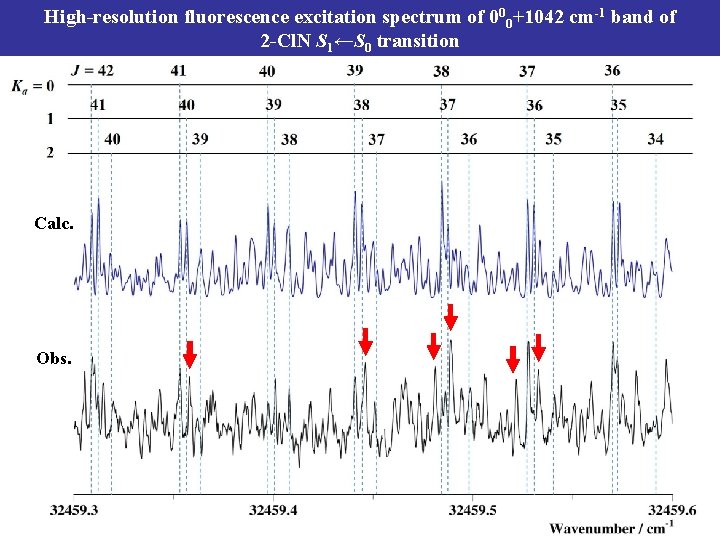
High-resolution fluorescence excitation spectrum of 000+1042 cm-1 band of 2 -Cl. N S 1←S 0 transition band origin: 32460. 9296 (1) cm-1 Linewidth (FWHM) 50 MHz Trot = 30 K band origin Calc. Obs. a-type: b-type = 1: 3

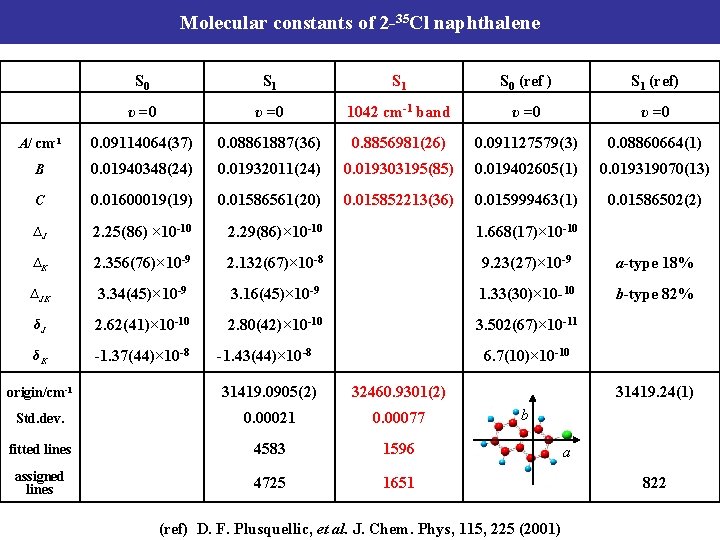
Molecular constants of 2 -35 Cl naphthalene S 0 S 1 S 0 (ref ) S 1 (ref) υ =0 1042 cm-1 band υ =0 A/ cm-1 0. 09114064(37) 0. 08861887(36) 0. 8856981(26) 0. 091127579(3) 0. 08860664(1) B 0. 01940348(24) 0. 01932011(24) 0. 019303195(85) 0. 019402605(1) 0. 019319070(13) C 0. 01600019(19) 0. 01586561(20) 0. 015852213(36) 0. 015999463(1) 0. 01586502(2) ∆J 2. 25(86) × 10 -10 2. 29(86)× 10 -10 1. 668(17)× 10 -10 ∆K 2. 356(76)× 10 -9 2. 132(67)× 10 -8 9. 23(27)× 10 -9 a-type 18% ∆JK 3. 34(45)× 10 -9 3. 16(45)× 10 -9 1. 33(30)× 10 -10 b-type 82% δJ 2. 62(41)× 10 -10 2. 80(42)× 10 -10 3. 502(67)× 10 -11 δK -1. 37(44)× 10 -8 -1. 43(44)× 10 -8 6. 7(10)× 10 -10 origin/cm-1 31419. 0905(2) 32460. 9301(2) 31419. 24(1) Std. dev. 0. 00021 0. 00077 b fitted lines 4583 1596 assigned lines 4725 1651 (ref) D. F. Plusquellic, et al. J. Chem. Phys, 115, 225 (2001) a 822

High-resolution fluorescence excitation spectrum of 000+1042 cm-1 band of 2 -Cl. N S 1←S 0 transition band origin: 32460. 9296 (1) cm-1 Linewidth (FWHM) 50 MHz Trot = 30 K band origin Calc. Obs. a-type: b-type = 1: 3

High-resolution fluorescence excitation spectrum of 000+1042 cm-1 band of 2 -Cl. N S 1←S 0 transition Calc. Obs.

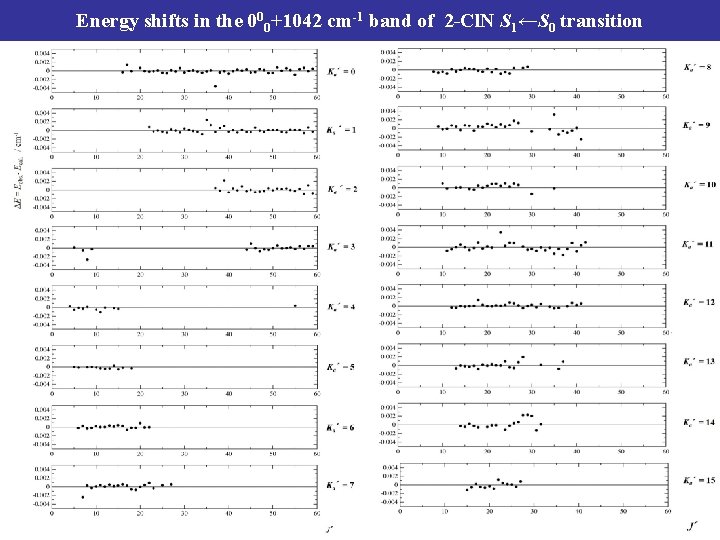
Energy shifts in the 000+1042 cm-1 band of 2 -Cl. N S 1←S 0 transition

High-resolution spectrum of vibronic band of 2 -Cl. N S 1←S 0 transition × 1 Excess Energy 0 31 5. 1 Natural Line width (MHz) Lifetime (ns) 476 1042 1396 23 6. 9 11 8 14 20 0– 0 × 3. 5 000+476 cm-1 × 3. 5 000+1042 cm-1 × 3. 5 000+1396 cm-1

1 -Cl naphthalene (2 -Cl. N)

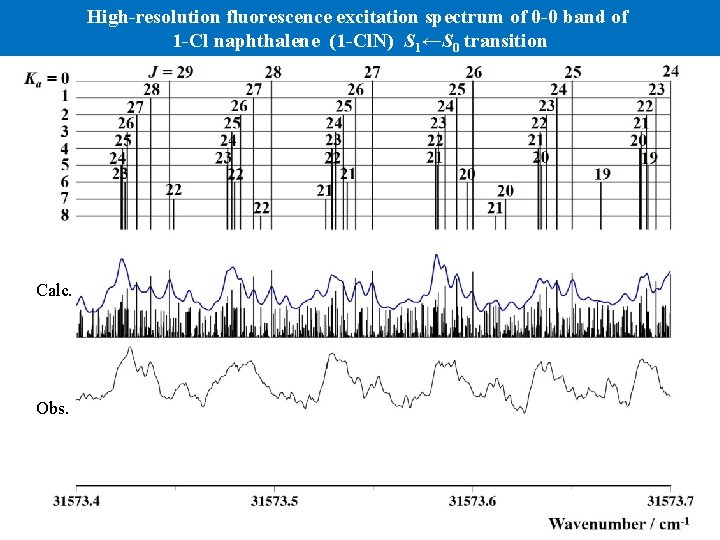
High-resolution fluorescence excitation spectrum of 0 -0 band of 1 -Cl naphthalene (1 -Cl. N) S 1←S 0 transition S 0: A = 0. 050572(30), B = 0. 030095(84), C = 0. 0191249(35) [cm-1] S 1: A = 0. 049720(31), B = 0. 029615(84), C = 0. 0188965(35) [cm-1] band origin 31574. 7730(3) cm-1 Trot = 60 K a-type: b-type = 1: 2 Linewidth (FWHM) 120 MHz band origin Calc. Obs. Wavenumber / cm-1

High-resolution fluorescence excitation spectrum of 0 -0 band of 1 -Cl naphthalene (1 -Cl. N) S 1←S 0 transition Calc. Obs.

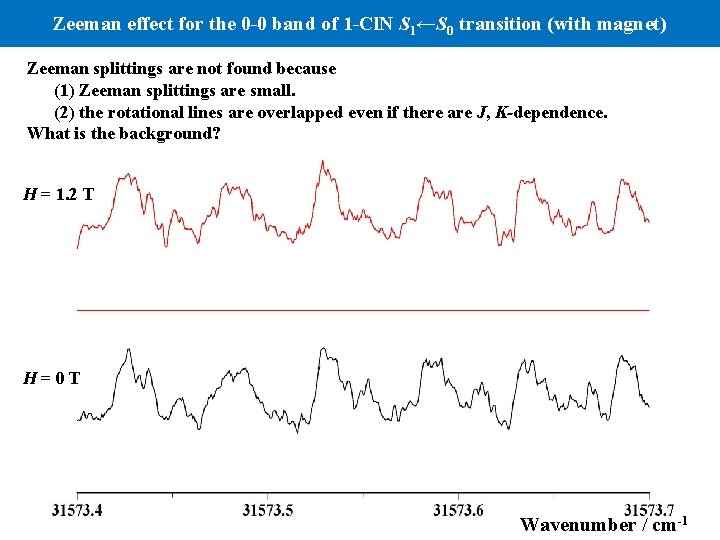
Zeeman effect for the 0 -0 band of 1 -Cl. N S 1←S 0 transition (with magnet) Zeeman splittings are not found because (1) Zeeman splittings are small. (2) the rotational lines are overlapped even if there are J, K-dependence. What is the background? H = 1. 2 T H=0 T Wavenumber / cm-1

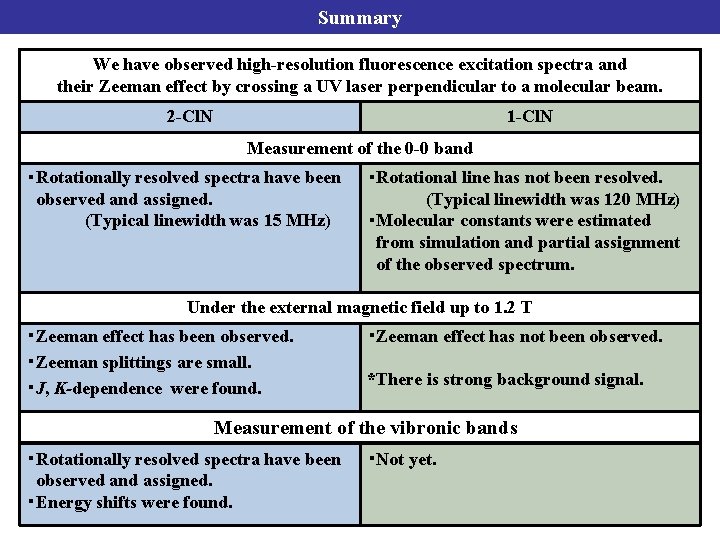
Summary We have observed high-resolution fluorescence excitation spectra and their Zeeman effect by crossing a UV laser perpendicular to a molecular beam. 2 -Cl. N 1 -Cl. N Measurement of the 0 -0 band ・Rotationally resolved spectra have been observed and assigned. (Typical linewidth was 15 MHz) ・Rotational line has not been resolved. (Typical linewidth was 120 MHz) ・Molecular constants were estimated from simulation and partial assignment of the observed spectrum. Under the external magnetic field up to 1. 2 T ・Zeeman effect has been observed. ・Zeeman splittings are small. ・J, K-dependence were found. ・Zeeman effect has not been observed. *There is strong background signal. Measurement of the vibronic bands ・Rotationally resolved spectra have been observed and assigned. ・Energy shifts were found. ・Not yet.

Thank you for your attension !

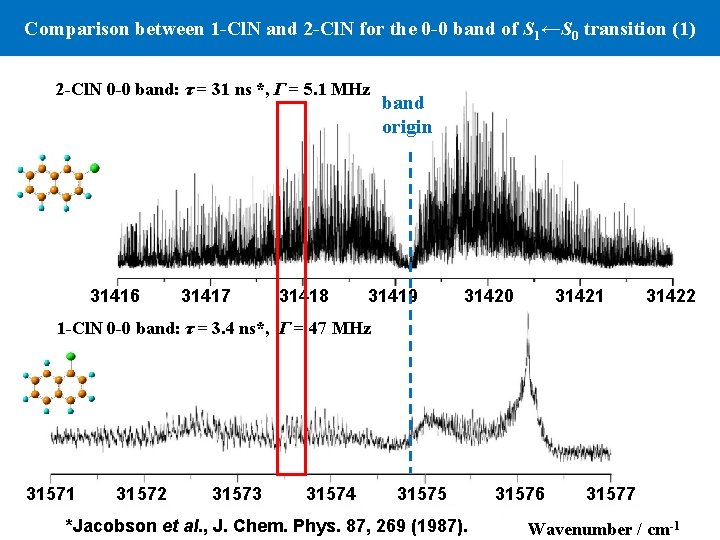
Comparison between 1 -Cl. N and 2 -Cl. N for the 0 -0 band of S 1←S 0 transition (1) 2 -Cl. N 0 -0 band: τ = 31 ns *, Γ = 5. 1 MHz band origin 31416 31417 31418 31419 31420 31421 31422 1 -Cl. N 0 -0 band: τ = 3. 4 ns*, Γ = 47 MHz 31571 31572 31573 31574 31575 31576 31577 *Jacobson et al. , J. Chem. Phys. 87, 269 (1987). Wavenumber / cm-1

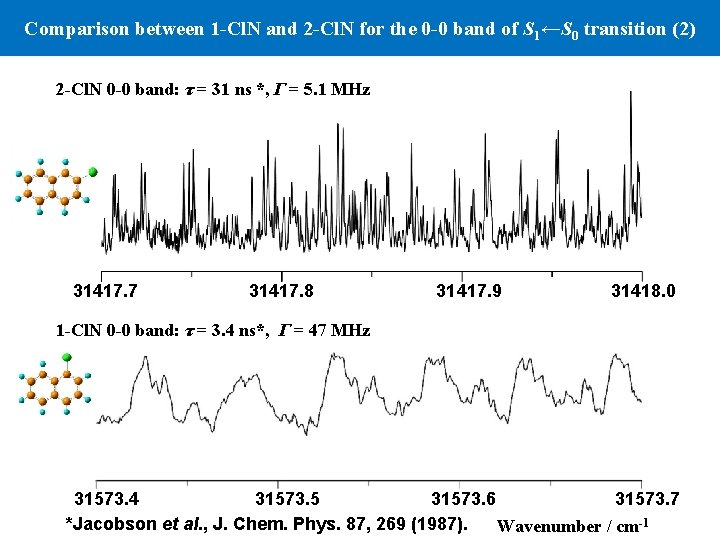
Comparison between 1 -Cl. N and 2 -Cl. N for the 0 -0 band of S 1←S 0 transition (2) 2 -Cl. N 0 -0 band: τ = 31 ns *, Γ = 5. 1 MHz 31417. 7 31417. 8 31417. 9 31418. 0 1 -Cl. N 0 -0 band: τ = 3. 4 ns*, Γ = 47 MHz 31573. 4 31573. 5 31573. 6 31573. 7 *Jacobson et al. , J. Chem. Phys. 87, 269 (1987). Wavenumber / cm-1
 Flacs speaking tasks
Flacs speaking tasks Isms
Isms Hvad er iso 27001
Hvad er iso 27001 Pims
Pims Conservatism
Conservatism Iso 27000 series
Iso 27000 series Isms
Isms Project plan for iso 27001 implementation
Project plan for iso 27001 implementation Isms
Isms Isms 1042
Isms 1042 Isms org
Isms org Isms in literature
Isms in literature Isms spectroscopy
Isms spectroscopy Isms keller
Isms keller Isms roadmap
Isms roadmap The 5 isms
The 5 isms Isms guiding principles
Isms guiding principles Ent université tours
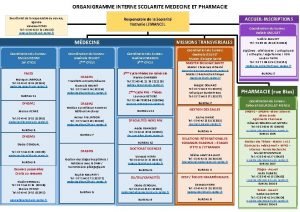
Ent université tours Scolarité pharmacie nantes
Scolarité pharmacie nantes Http:fsi-st univ-boumerdes-dz
Http:fsi-st univ-boumerdes-dz (univ. caxias do sul) escolha a alternativa que completa
(univ. caxias do sul) escolha a alternativa que completa Fs univ umbb
Fs univ umbb Peroxydation
Peroxydation Centre universitaire nour el bachir el bayadh
Centre universitaire nour el bachir el bayadh Moodle ustv
Moodle ustv Sug grant
Sug grant Lon capa ohio university
Lon capa ohio university Dysopyramide
Dysopyramide Ut arlington demographics
Ut arlington demographics