ILUMIEN III OPTIMIZE PCI A Randomized Controlled Trial


- Slides: 19

ILUMIEN III: OPTIMIZE PCI A Randomized Controlled Trial Comparing OCT-Guided, IVUS-Guided and Angiography-Guided PCI Lancet 2016; 388: 2618 -28 Gregg W. Stone MD Columbia University Medical Center New-York Presybyterian Hospital Cardiovascular Research Foundation

Disclosures • Consultant to St. Jude

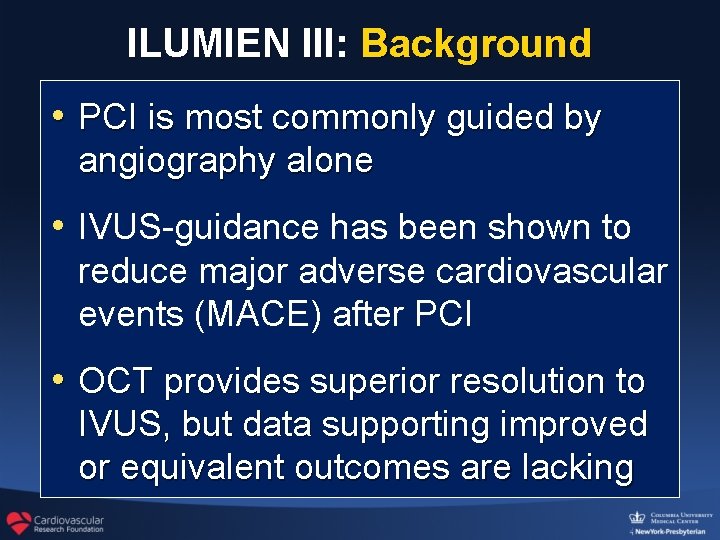
ILUMIEN III: Background • PCI is most commonly guided by angiography alone • IVUS-guidance has been shown to reduce major adverse cardiovascular events (MACE) after PCI • OCT provides superior resolution to IVUS, but data supporting improved or equivalent outcomes are lacking

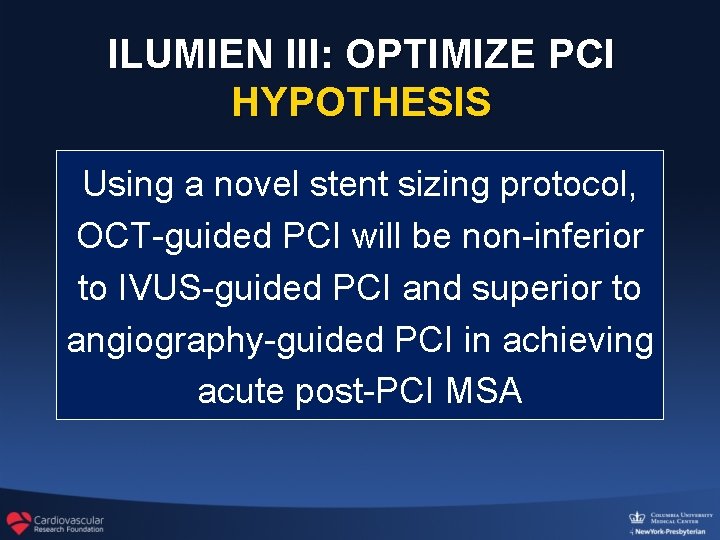
ILUMIEN III: OPTIMIZE PCI HYPOTHESIS Using a novel stent sizing protocol, OCT-guided PCI will be non-inferior to IVUS-guided PCI and superior to angiography-guided PCI in achieving acute post-PCI MSA

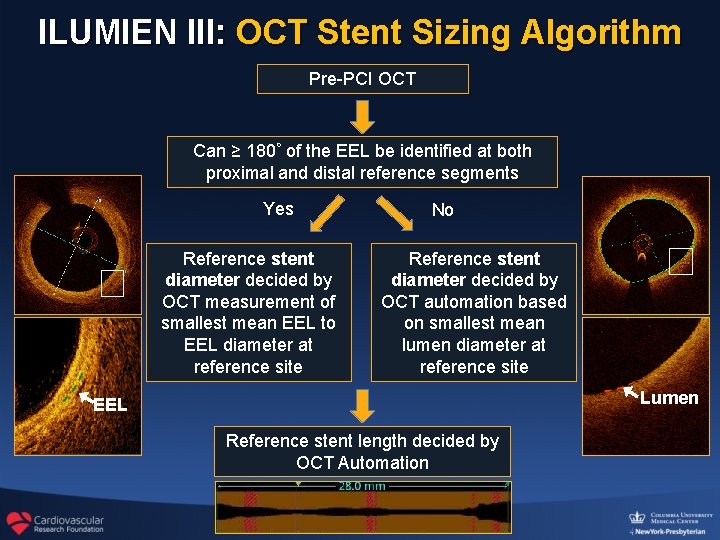
ILUMIEN III: OCT Stent Sizing Algorithm Pre-PCI OCT Can ≥ 180◦ of the EEL be identified at both proximal and distal reference segments Yes Reference stent diameter decided by OCT measurement of smallest mean EEL to EEL diameter at reference site No Reference stent diameter decided by OCT automation based on smallest mean lumen diameter at reference site Lumen EEL Reference stent length decided by OCT Automation

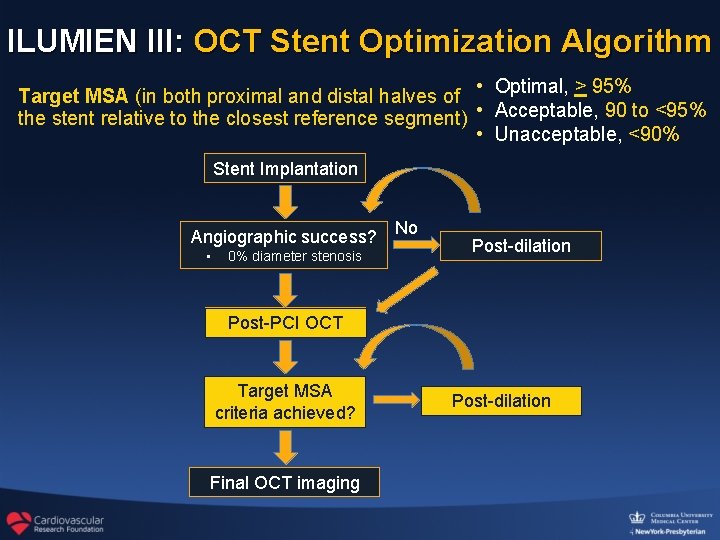
ILUMIEN III: OCT Stent Optimization Algorithm Target MSA (in both proximal and distal halves of • Optimal, > 95% the stent relative to the closest reference segment) • Acceptable, 90 to <95% • Unacceptable, <90% Stent Implantation Angiographic success? • 0% diameter stenosis No Post-dilation Post-PCI OCT Target MSA criteria achieved? Final OCT imaging Post-dilation

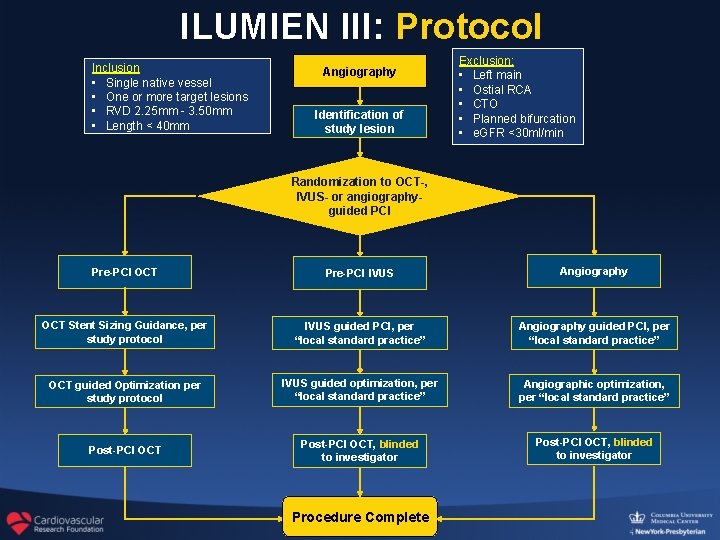
ILUMIEN III: Protocol Inclusion • Single native vessel • One or more target lesions • RVD 2. 25 mm - 3. 50 mm • Length < 40 mm Angiography Identification of study lesion Exclusion: • Left main • Ostial RCA • CTO • Planned bifurcation • e. GFR <30 ml/min Randomization to OCT-, IVUS- or angiographyguided PCI Pre-PCI OCT Pre-PCI IVUS Angiography OCT Stent Sizing Guidance, per study protocol IVUS guided PCI, per “local standard practice” Angiography guided PCI, per “local standard practice” OCT guided Optimization per study protocol IVUS guided optimization, per “local standard practice” Angiographic optimization, per “local standard practice” Post-PCI OCT, blinded to investigator Procedure Complete

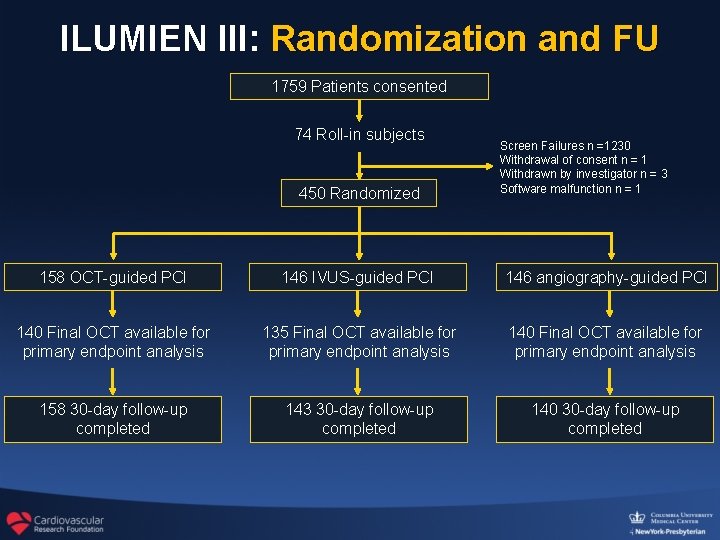
ILUMIEN III: Randomization and FU 1759 Patients consented 74 Roll-in subjects 450 Randomized Screen Failures n =1230 Withdrawal of consent n = 1 Withdrawn by investigator n = 3 Software malfunction n = 1 158 OCT-guided PCI 146 IVUS-guided PCI 146 angiography-guided PCI 140 Final OCT available for primary endpoint analysis 135 Final OCT available for primary endpoint analysis 140 Final OCT available for primary endpoint analysis 158 30 -day follow-up completed 143 30 -day follow-up completed 140 30 -day follow-up completed

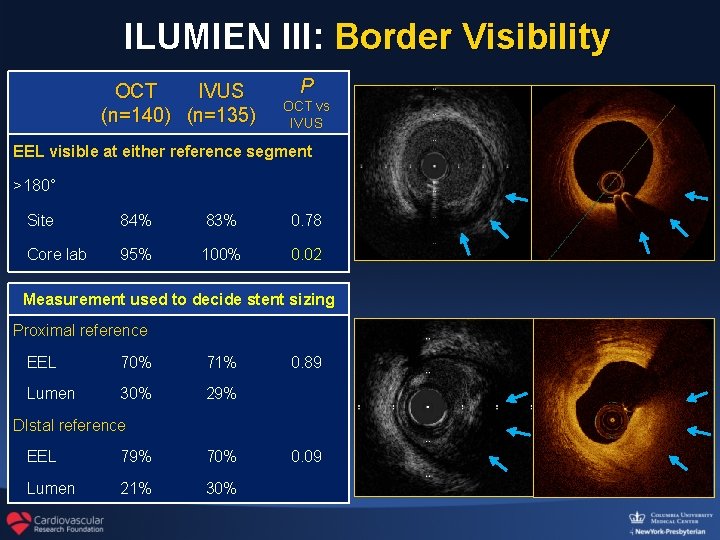
ILUMIEN III: Border Visibility OCT IVUS (n=140) (n=135) P OCT vs IVUS EEL visible at either reference segment >180° Site 84% 83% 0. 78 Core lab 95% 100% 0. 02 Measurement used to decide stent sizing Proximal reference EEL 70% 71% Lumen 30% 29% 0. 89 DIstal reference EEL 79% 70% Lumen 21% 30% 0. 09

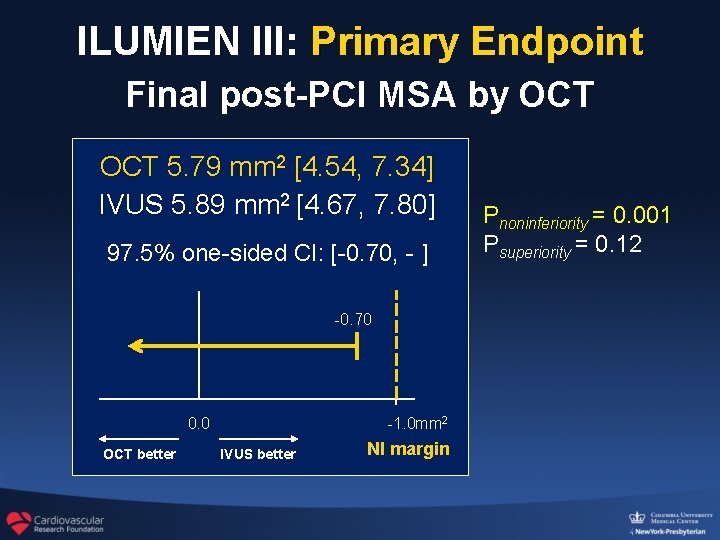
ILUMIEN III: Primary Endpoint Final post-PCI MSA by OCT 5. 79 mm 2 [4. 54, 7. 34] IVUS 5. 89 mm 2 [4. 67, 7. 80] 97. 5% one-sided CI: [-0. 70, - ] -0. 70 0. 0 OCT better -1. 0 mm 2 IVUS better NI margin Pnoninferiority = 0. 001 Psuperiority = 0. 12

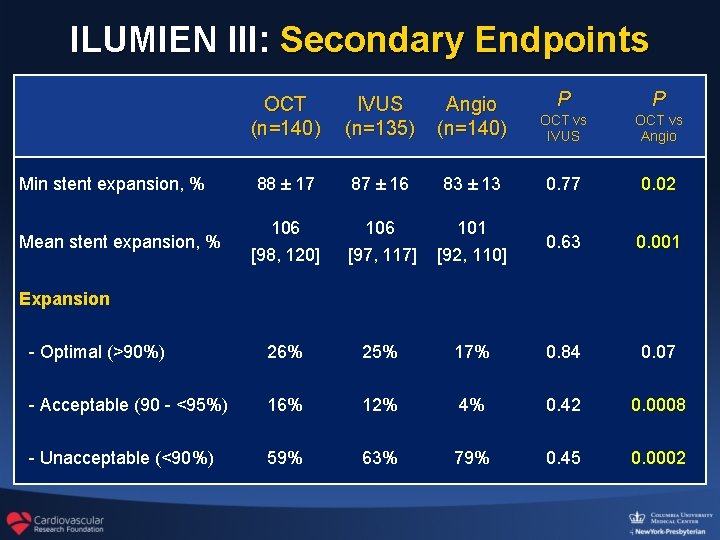
ILUMIEN III: Secondary Endpoints OCT (n=140) IVUS (n=135) Angio (n=140) P P OCT vs IVUS OCT vs Angio 88 ± 17 87 ± 16 83 ± 13 0. 77 0. 02 106 [98, 120] 106 [97, 117] 101 [92, 110] 0. 63 0. 001 - Optimal (>90%) 26% 25% 17% 0. 84 0. 07 - Acceptable (90 - <95%) 16% 12% 4% 0. 42 0. 0008 - Unacceptable (<90%) 59% 63% 79% 0. 45 0. 0002 Min stent expansion, % Mean stent expansion, % Expansion

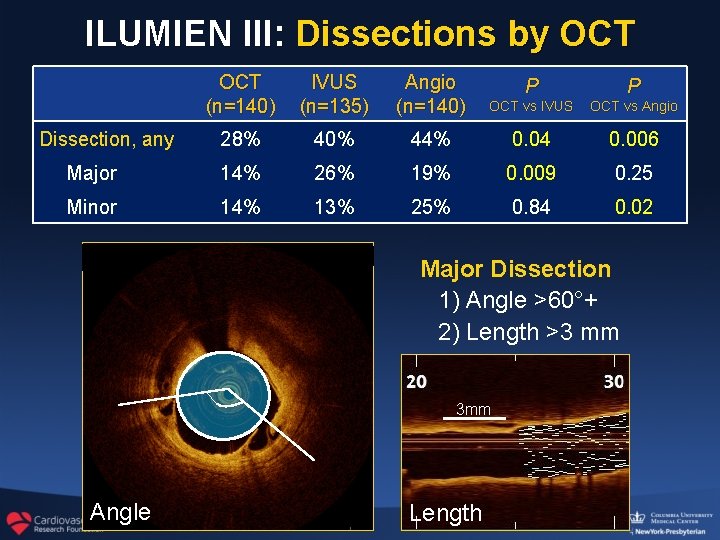
ILUMIEN III: Dissections by OCT (n=140) IVUS (n=135) Angio (n=140) P P OCT vs IVUS OCT vs Angio 28% 40% 44% 0. 04 0. 006 Major 14% 26% 19% 0. 009 0. 25 Minor 14% 13% 25% 0. 84 0. 02 Dissection, any Major Dissection 1) Angle >60°+ 2) Length >3 mm 3 mm Angle Length

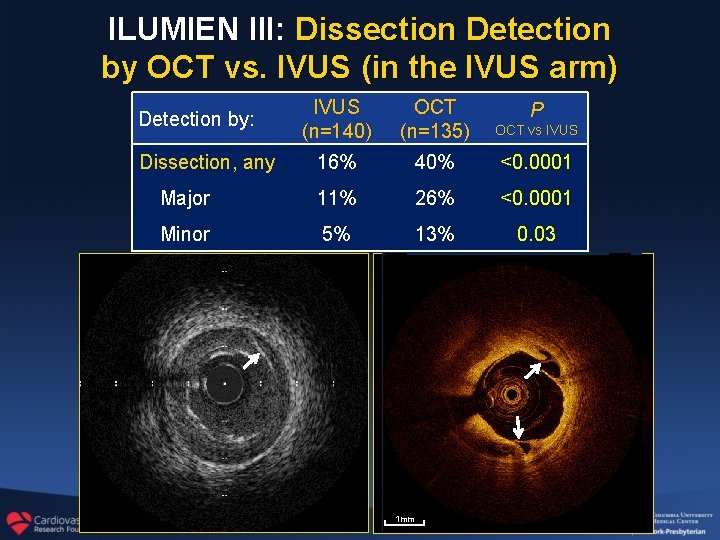
ILUMIEN III: Dissection Detection by OCT vs. IVUS (in the IVUS arm) IVUS (n=140) OCT (n=135) OCT vs IVUS 16% 40% <0. 0001 Major 11% 26% <0. 0001 Minor 5% 13% 0. 03 Detection by: Dissection, any 1 mm P

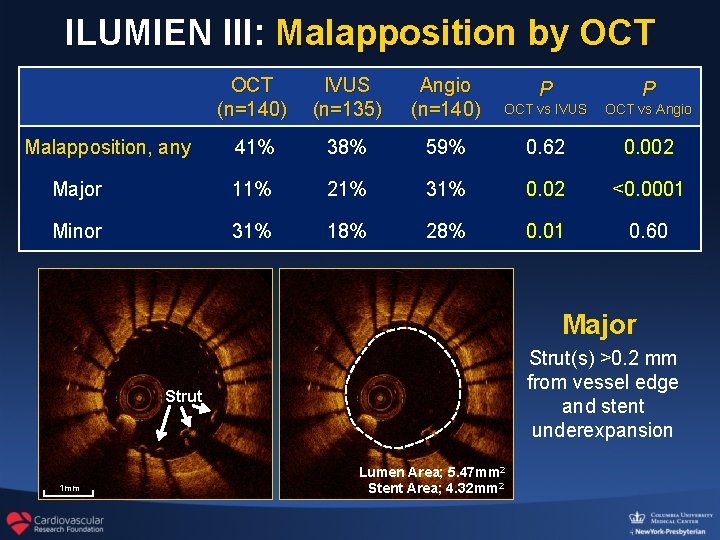
ILUMIEN III: Malapposition by OCT (n=140) IVUS (n=135) Angio (n=140) P P OCT vs IVUS OCT vs Angio 41% 38% 59% 0. 62 0. 002 Major 11% 21% 31% 0. 02 <0. 0001 Minor 31% 18% 28% 0. 01 0. 60 Malapposition, any Major Strut(s) >0. 2 mm from vessel edge and stent underexpansion Strut 1 mm Lumen Area; 5. 47 mm 2 Stent Area; 4. 32 mm 2

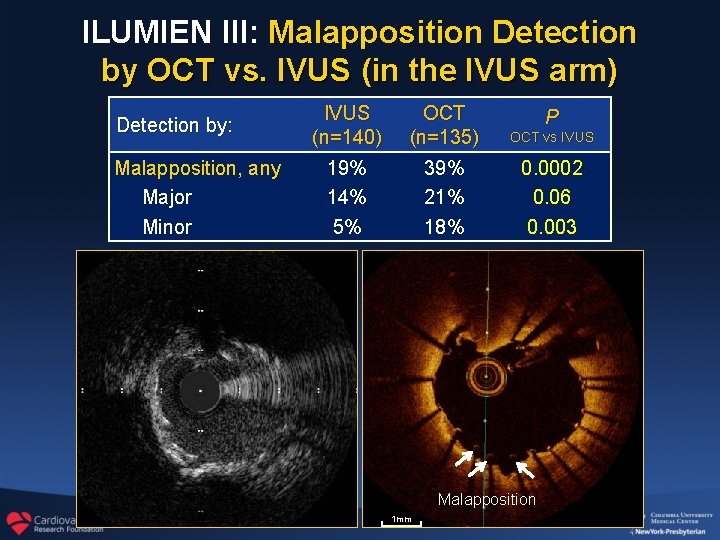
ILUMIEN III: Malapposition Detection by OCT vs. IVUS (in the IVUS arm) Detection by: Malapposition, any Major Minor IVUS (n=140) 19% 14% 5% OCT (n=135) 39% 21% 18% P OCT vs IVUS 0. 0002 0. 06 0. 003 Malapposition 1 mm

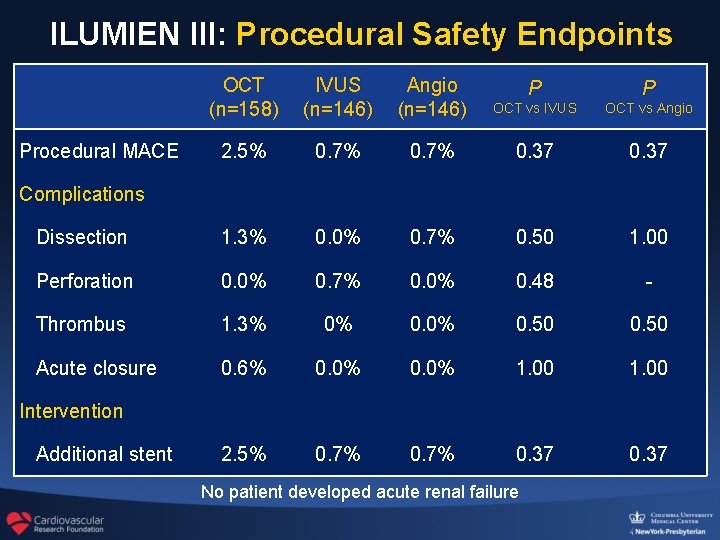
ILUMIEN III: Procedural Safety Endpoints OCT (n=158) IVUS (n=146) Angio (n=146) P P OCT vs IVUS OCT vs Angio 2. 5% 0. 7% 0. 37 Dissection 1. 3% 0. 0% 0. 7% 0. 50 1. 00 Perforation 0. 0% 0. 7% 0. 0% 0. 48 - Thrombus 1. 3% 0% 0. 50 Acute closure 0. 6% 0. 0% 1. 00 2. 5% 0. 7% 0. 37 Procedural MACE Complications Intervention Additional stent No patient developed acute renal failure

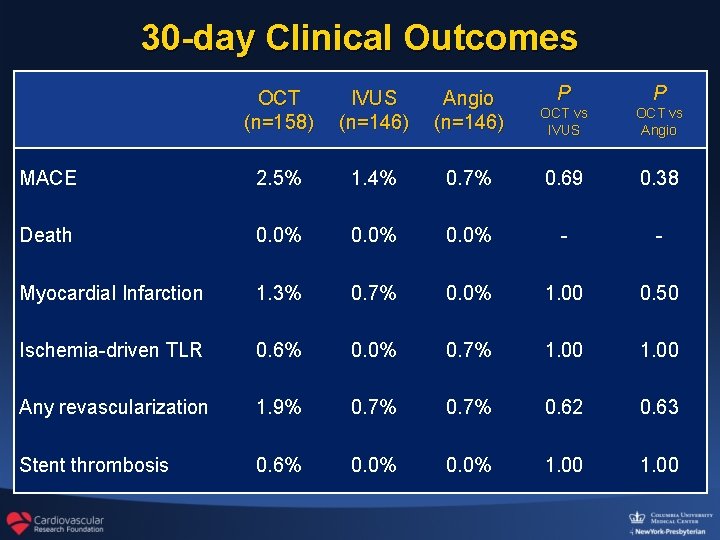
30 -day Clinical Outcomes OCT (n=158) IVUS (n=146) Angio (n=146) P P OCT vs IVUS OCT vs Angio MACE 2. 5% 1. 4% 0. 7% 0. 69 0. 38 Death 0. 0% - - Myocardial Infarction 1. 3% 0. 7% 0. 0% 1. 00 0. 50 Ischemia-driven TLR 0. 6% 0. 0% 0. 7% 1. 00 Any revascularization 1. 9% 0. 7% 0. 62 0. 63 Stent thrombosis 0. 6% 0. 0% 1. 00

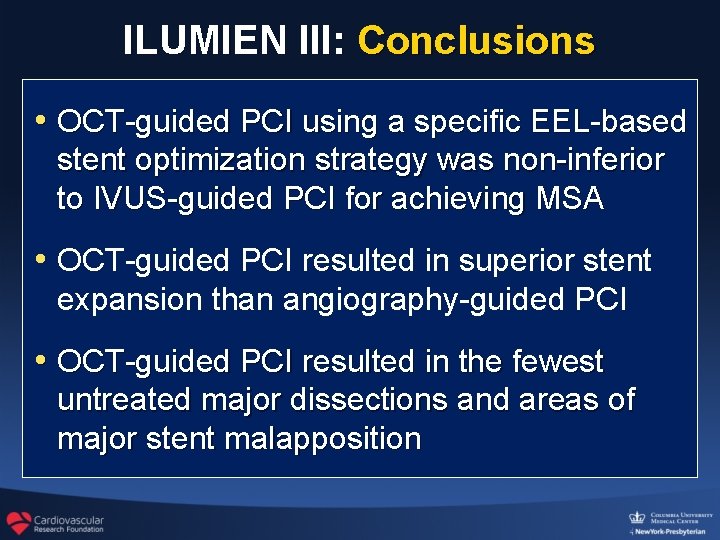
ILUMIEN III: Conclusions • OCT-guided PCI using a specific EEL-based stent optimization strategy was non-inferior to IVUS-guided PCI for achieving MSA • OCT-guided PCI resulted in superior stent expansion than angiography-guided PCI • OCT-guided PCI resulted in the fewest untreated major dissections and areas of major stent malapposition

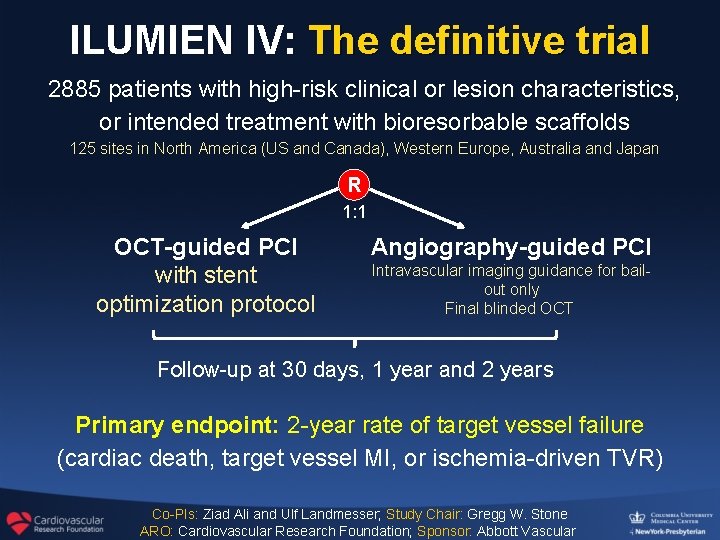
ILUMIEN IV: The definitive trial 2885 patients with high-risk clinical or lesion characteristics, or intended treatment with bioresorbable scaffolds 125 sites in North America (US and Canada), Western Europe, Australia and Japan R 1: 1 OCT-guided PCI with stent optimization protocol Angiography-guided PCI Intravascular imaging guidance for bailout only Final blinded OCT Follow-up at 30 days, 1 year and 2 years Primary endpoint: 2 -year rate of target vessel failure (cardiac death, target vessel MI, or ischemia-driven TVR) Co-PIs: Ziad Ali and Ulf Landmesser; Study Chair: Gregg W. Stone ARO: Cardiovascular Research Foundation; Sponsor: Abbott Vascular