ICCS eNewsletter CSI Winter 2013 Jacqueline Emmons MD


- Slides: 23

ICCS e-Newsletter CSI Winter 2013 Jacqueline Emmons, MD Department of Pathology University of Texas Southwestern Medical Center Dallas, Texas

History • 11 month old female with a history of fever and failure to thrive • On physical exam, the child was pale and lethargic.

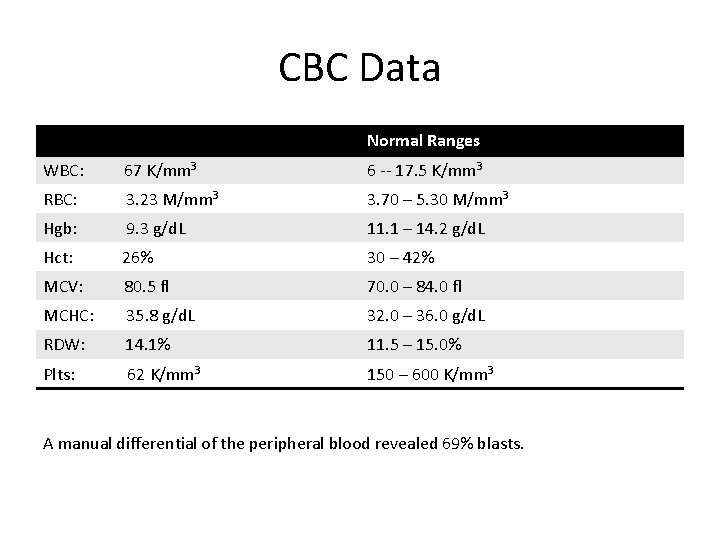
CBC Data Normal Ranges WBC: 67 K/mm 3 6 -- 17. 5 K/mm 3 RBC: 3. 23 M/mm 3 3. 70 – 5. 30 M/mm 3 Hgb: 9. 3 g/d. L 11. 1 – 14. 2 g/d. L Hct: 26% 30 – 42% MCV: 80. 5 fl 70. 0 – 84. 0 fl MCHC: 35. 8 g/d. L 32. 0 – 36. 0 g/d. L RDW: 14. 1% 11. 5 – 15. 0% Plts: 62 K/mm 3 150 – 600 K/mm 3 A manual differential of the peripheral blood revealed 69% blasts.

• A peripheral blood sample was received in the flow cytometry lab with the indication to “rule out acute leukemia. ” • Selected tubes from the diagnostic analysis are included in this case study for review.

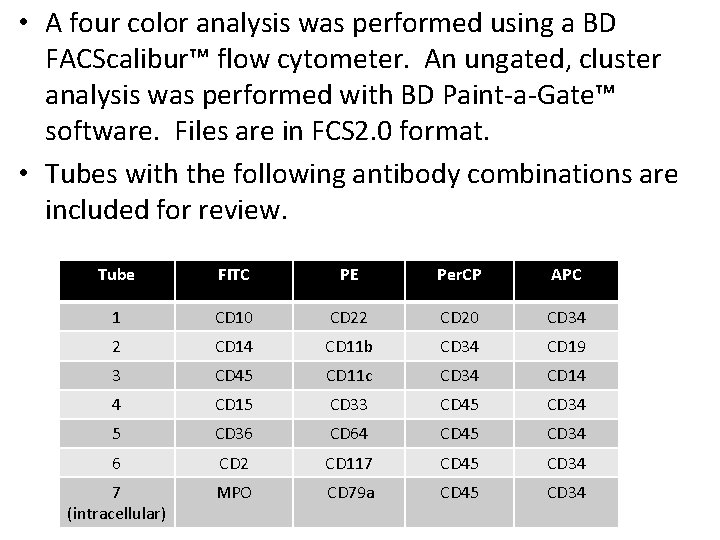
• A four color analysis was performed using a BD FACScalibur™ flow cytometer. An ungated, cluster analysis was performed with BD Paint-a-Gate™ software. Files are in FCS 2. 0 format. • Tubes with the following antibody combinations are included for review. Tube FITC PE Per. CP APC 1 CD 10 CD 22 CD 20 CD 34 2 CD 14 CD 11 b CD 34 CD 19 3 CD 45 CD 11 c CD 34 CD 14 4 CD 15 CD 33 CD 45 CD 34 5 CD 36 CD 64 CD 45 CD 34 6 CD 2 CD 117 CD 45 CD 34 7 (intracellular) MPO CD 79 a CD 45 CD 34

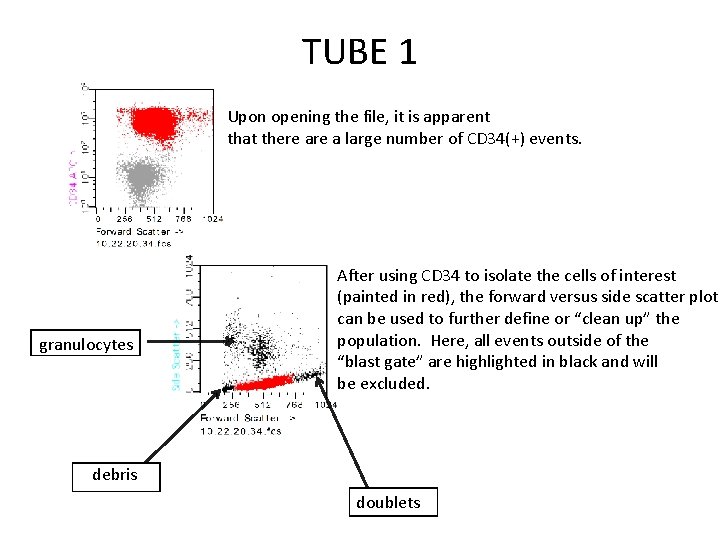
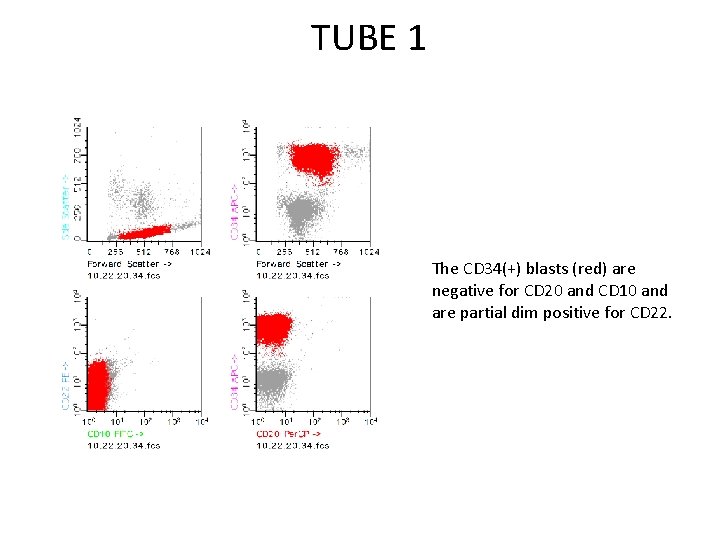
TUBE 1 Upon opening the file, it is apparent that there a large number of CD 34(+) events. granulocytes After using CD 34 to isolate the cells of interest (painted in red), the forward versus side scatter plot can be used to further define or “clean up” the population. Here, all events outside of the “blast gate” are highlighted in black and will be excluded. debris doublets

TUBE 1 The CD 34(+) blasts (red) are negative for CD 20 and CD 10 and are partial dim positive for CD 22.

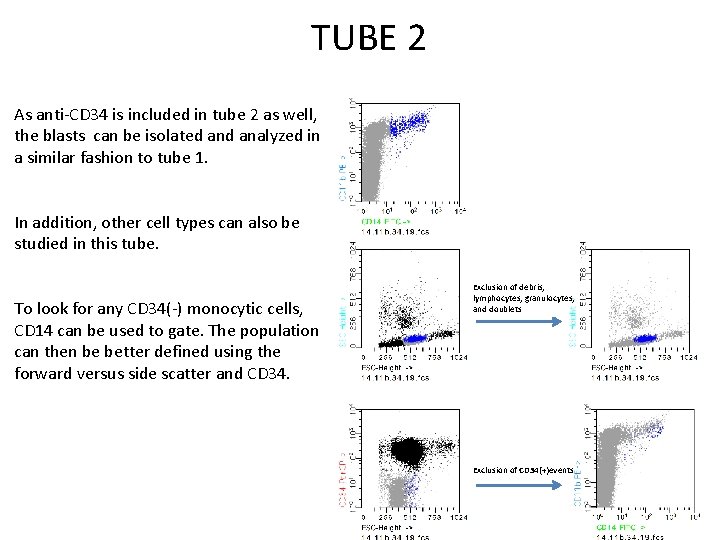
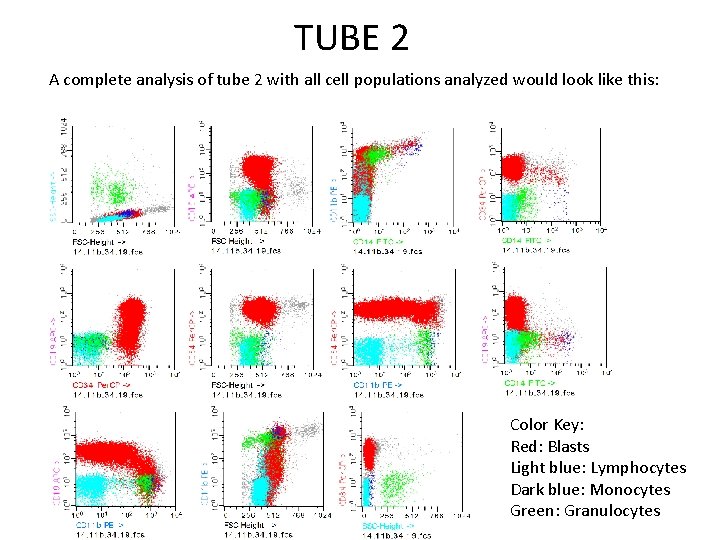
TUBE 2 As anti-CD 34 is included in tube 2 as well, the blasts can be isolated analyzed in a similar fashion to tube 1. In addition, other cell types can also be studied in this tube. To look for any CD 34(-) monocytic cells, CD 14 can be used to gate. The population can then be better defined using the forward versus side scatter and CD 34. Exclusion of debris, lymphocytes, granulocytes, and doublets Exclusion of CD 34(+)events

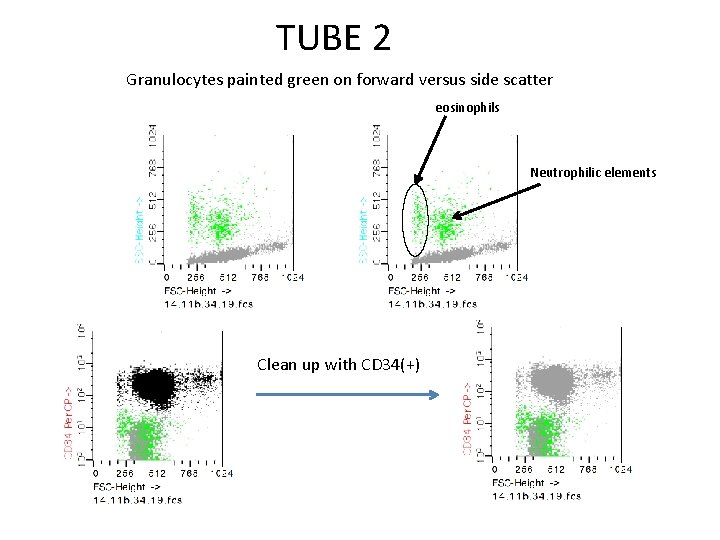
TUBE 2 Granulocytes painted green on forward versus side scatter eosinophils Neutrophilic elements Clean up with CD 34(+)

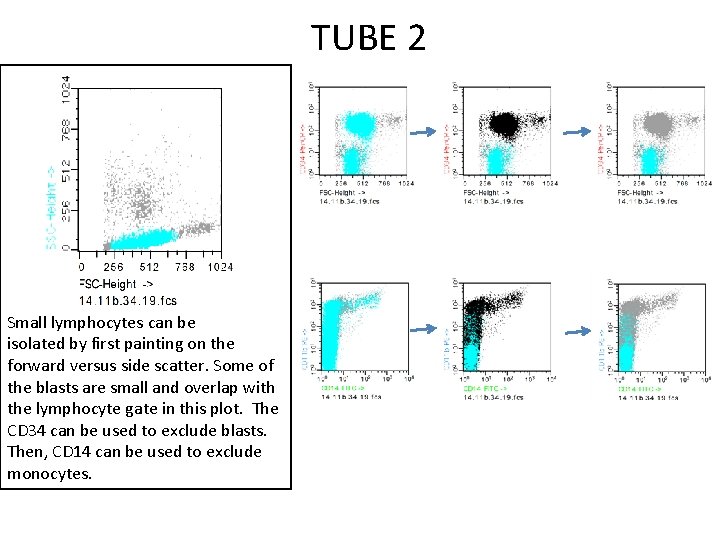
TUBE 2 Small lymphocytes can be isolated by first painting on the forward versus side scatter. Some of the blasts are small and overlap with the lymphocyte gate in this plot. The CD 34 can be used to exclude blasts. Then, CD 14 can be used to exclude monocytes.

TUBE 2 A complete analysis of tube 2 with all cell populations analyzed would look like this: Color Key: Red: Blasts Light blue: Lymphocytes Dark blue: Monocytes Green: Granulocytes

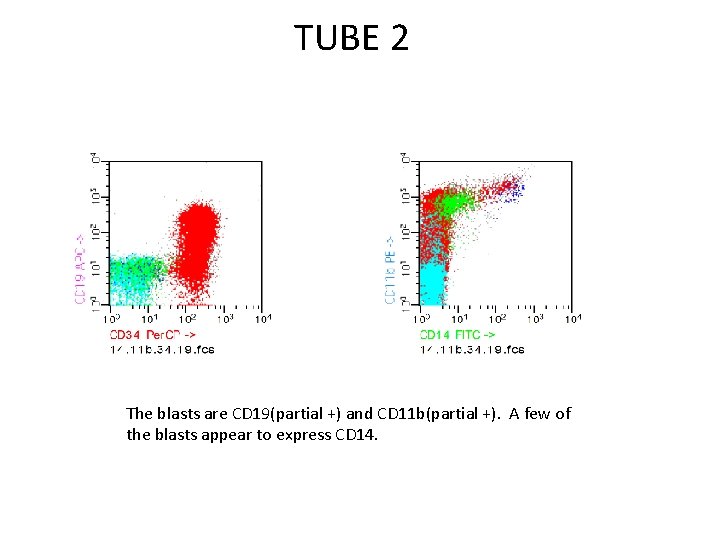
TUBE 2 The blasts are CD 19(partial +) and CD 11 b(partial +). A few of the blasts appear to express CD 14.

• Tubes 3 – 7 can all be analyzed in a similar manner to tubes 1 and 2. • The immunophenotype of the blasts is as follows: – – – – – CD 34(+) CD 2(-) CD 10(-) CD 11 b(partial +) CD 11 c(partial +) CD 14(few cells +) CD 15(partial +) CD 19(partial +) CD 20(-) CD 22(partial dim +) CD 33(variably +) CD 34(+) CD 45(moderately +) CD 64(partial +) Cytoplasmic CD 79 a(partial +) CD 117(few cells +) Cytoplasmic MPO(-)

• The blasts express markers of both myeloid and B-lymphoid differentiation. • In addition to nonspecific myeloid markers such as CD 15 and CD 33, the blasts express markers suggesting monocytic differentiation (CD 11 c, dual expression of CD 64 and CD 36, a few cells positive for CD 14) • B-lymphoid markers include partial strong expression of CD 19, partial expression of CD 22, and partial expression of CD 79 a

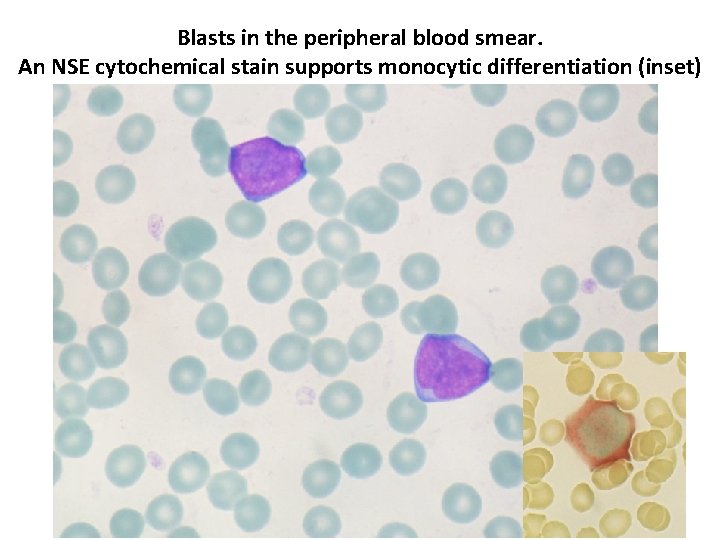
Blasts in the peripheral blood smear. An NSE cytochemical stain supports monocytic differentiation (inset)

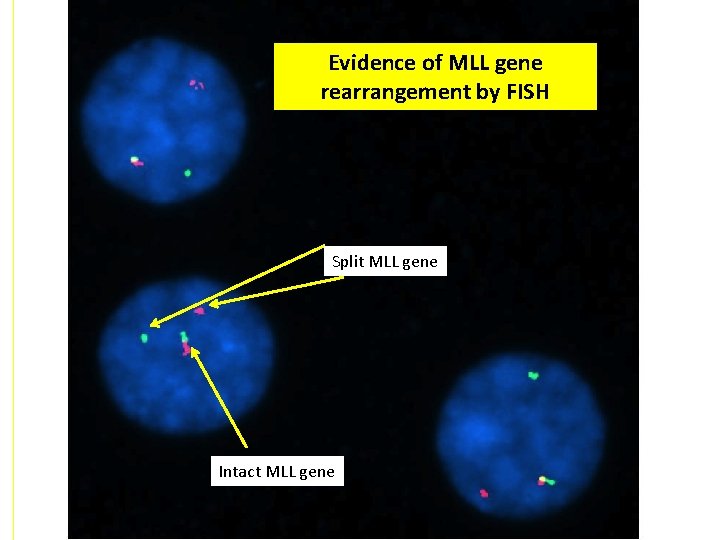
Evidence of MLL gene rearrangement by FISH Split MLL gene Intact MLL gene

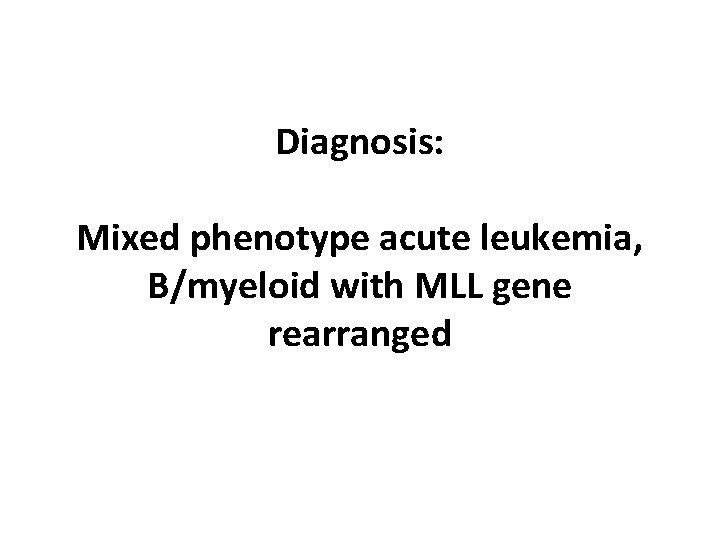
Diagnosis: Mixed phenotype acute leukemia, B/myeloid with MLL gene rearranged

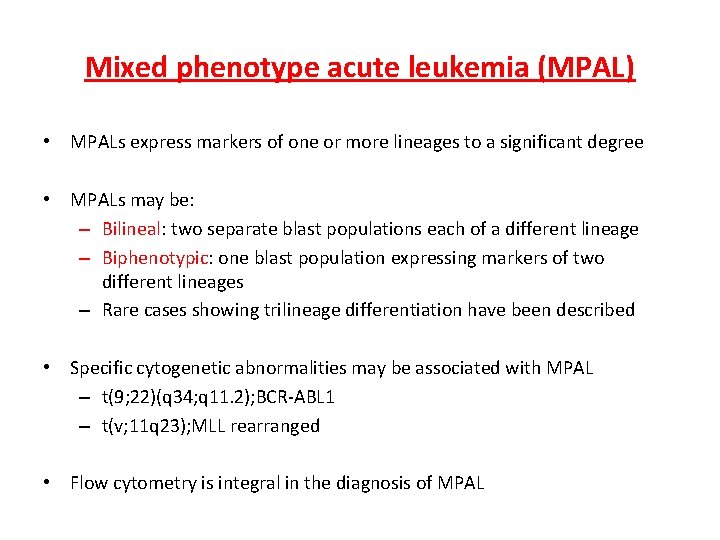
Mixed phenotype acute leukemia (MPAL) • MPALs express markers of one or more lineages to a significant degree • MPALs may be: – Bilineal: two separate blast populations each of a different lineage – Biphenotypic: one blast population expressing markers of two different lineages – Rare cases showing trilineage differentiation have been described • Specific cytogenetic abnormalities may be associated with MPAL – t(9; 22)(q 34; q 11. 2); BCR-ABL 1 – t(v; 11 q 23); MLL rearranged • Flow cytometry is integral in the diagnosis of MPAL

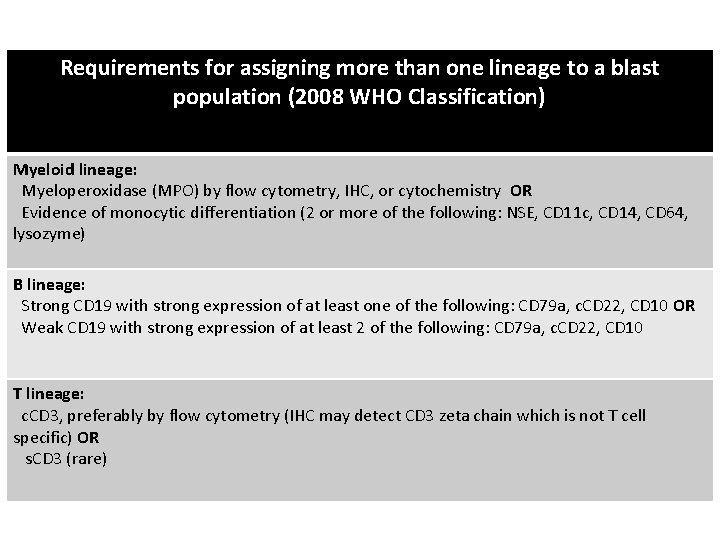
Requirements for assigning more than one lineage to a blast population (2008 WHO Classification) Myeloid lineage: Myeloperoxidase (MPO) by flow cytometry, IHC, or cytochemistry OR Evidence of monocytic differentiation (2 or more of the following: NSE, CD 11 c, CD 14, CD 64, lysozyme) B lineage: Strong CD 19 with strong expression of at least one of the following: CD 79 a, c. CD 22, CD 10 OR Weak CD 19 with strong expression of at least 2 of the following: CD 79 a, c. CD 22, CD 10 T lineage: c. CD 3, preferably by flow cytometry (IHC may detect CD 3 zeta chain which is not T cell specific) OR s. CD 3 (rare)

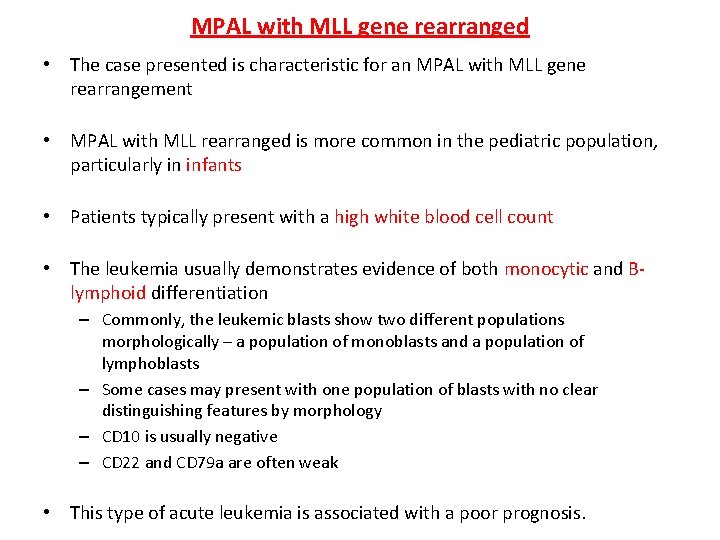
MPAL with MLL gene rearranged • The case presented is characteristic for an MPAL with MLL gene rearrangement • MPAL with MLL rearranged is more common in the pediatric population, particularly in infants • Patients typically present with a high white blood cell count • The leukemia usually demonstrates evidence of both monocytic and Blymphoid differentiation – Commonly, the leukemic blasts show two different populations morphologically – a population of monoblasts and a population of lymphoblasts – Some cases may present with one population of blasts with no clear distinguishing features by morphology – CD 10 is usually negative – CD 22 and CD 79 a are often weak • This type of acute leukemia is associated with a poor prognosis.

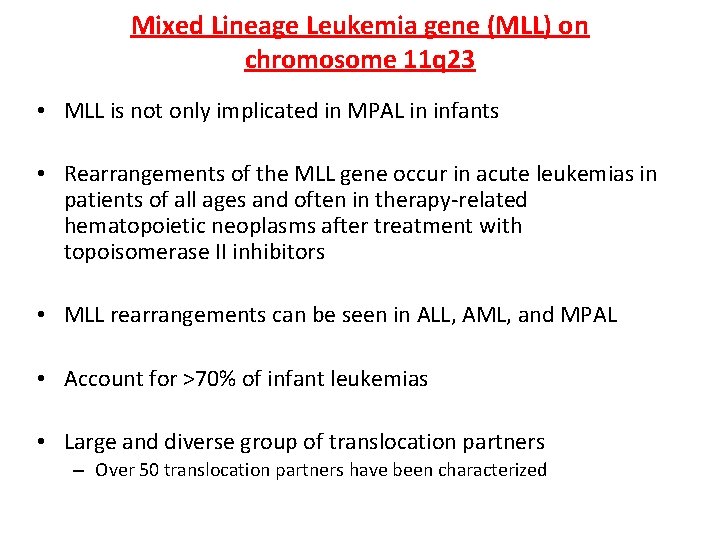
Mixed Lineage Leukemia gene (MLL) on chromosome 11 q 23 • MLL is not only implicated in MPAL in infants • Rearrangements of the MLL gene occur in acute leukemias in patients of all ages and often in therapy-related hematopoietic neoplasms after treatment with topoisomerase II inhibitors • MLL rearrangements can be seen in ALL, AML, and MPAL • Account for >70% of infant leukemias • Large and diverse group of translocation partners – Over 50 translocation partners have been characterized

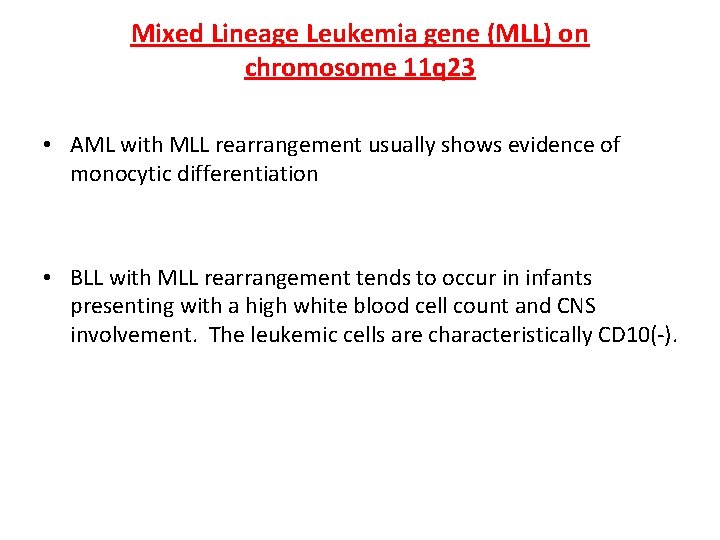
Mixed Lineage Leukemia gene (MLL) on chromosome 11 q 23 • AML with MLL rearrangement usually shows evidence of monocytic differentiation • BLL with MLL rearrangement tends to occur in infants presenting with a high white blood cell count and CNS involvement. The leukemic cells are characteristically CD 10(-).

References Swerdlow SH, Campo E, Harris NL, et al. , editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008. Muntean AG, Hess JL. The pathogenesis of mixed-lineage leukemia. Annu Rev Pathol. 2012; 7: 283 -301. Matutes E. , Pickl WF, et al. Mixed-phenotype acute leukemia: clinical and laboratory features and outcome in 100 patients defined according to the WHO 2008 classification. Blood. 2011 Mar 17; 117(11): 3163 -71. Sam TN, Kersey JH, Linabery AM, et al. MLL gene rearrangements in infant leukemia vary with age at diagnosis and selected demographic factors: a Children's Oncology Group (COG) study. Pediatr Blood Cancer. 2012 Jun; 58(6): 836 -9
 Jacqueline emmons
Jacqueline emmons Nathan emmons
Nathan emmons Delos emmons
Delos emmons Louisa emmons
Louisa emmons Iccs denver
Iccs denver Rbc normal range
Rbc normal range Iccs
Iccs Iata iccs
Iata iccs Iccs 2016 cytometry
Iccs 2016 cytometry Winter kommt flocken fallen nieder
Winter kommt flocken fallen nieder Es war eine mutter
Es war eine mutter Winter kommt winter kommt flocken fallen nieder
Winter kommt winter kommt flocken fallen nieder Jacqueline besser
Jacqueline besser Jacqueline saburido
Jacqueline saburido Jacqueline deurloo
Jacqueline deurloo Jupyter lab slides
Jupyter lab slides Jacqueline westcott
Jacqueline westcott Jacqui saburido
Jacqui saburido Jacqueline conard
Jacqueline conard Lucien gainsbourg caroline paulus
Lucien gainsbourg caroline paulus Dr house
Dr house Jacqueline chan md
Jacqueline chan md Teorema de chebyshev ejemplos
Teorema de chebyshev ejemplos Jacqueline bichsel
Jacqueline bichsel








































