ICCS eNewsletter CSI Fall 2014 Uni Path Denver






- Slides: 25

ICCS e-Newsletter CSI Fall 2014 Uni. Path - Denver, CO Richard Quinones, MLS(ASCP) Hong Lin, Ph. D Cristina Mc. Laughlin, MD

Presentation Clinical History • 67 -year-old female with history of T-prolymphocytic leukemia (T-PLL). • Originally presented in 2010 with leukocytosis, anemia, thrombocytopenia, peripheral lymphadenopathy and massive splenomegaly. • Currently status post second allogeneic stem cell transplant, after multiple rounds of treatment with anti-CD 52 antibody (Campath; alemtuzumab). – First transplant with matched related donor in 2013 after first relapse. – Second transplant with matched unrelated donor in 2014 after 2 extramedullary relapses. Specimens Submitted for Analysis • Bone marrow for 60 -day evaluation post second transplant. – – Peripheral blood received in EDTA for morphology. Bone marrow aspirate received in EDTA for morphological and molecular testing. Bone marrow aspirate received in Na. Hep for flow cytometry and cytogenetics. Two trephine needle core biopsies of bone marrow (0. 6 cm and 0. 8 cm in length by 0. 4 cm each in diameter) received in B+ for fixation and decalcification. • Subsequent soft tissue biopsy of a right neck mass for flow cytometry. • Follow-up peripheral blood for morphology and flow cytometry. 2

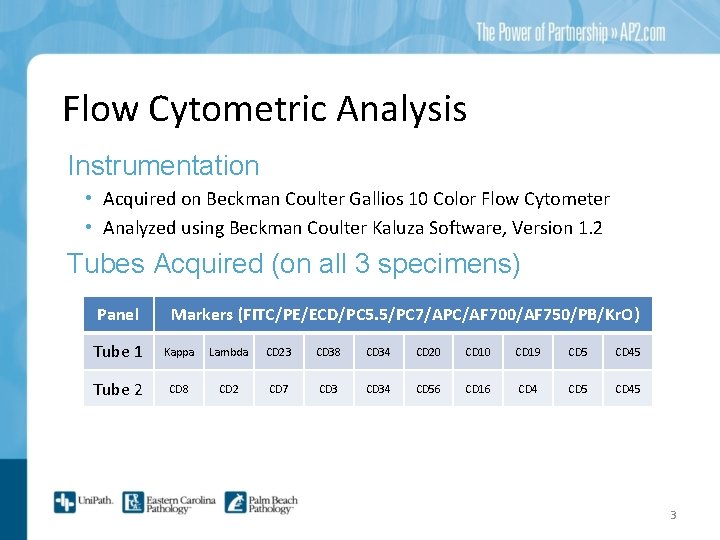
Flow Cytometric Analysis Instrumentation • Acquired on Beckman Coulter Gallios 10 Color Flow Cytometer • Analyzed using Beckman Coulter Kaluza Software, Version 1. 2 Tubes Acquired (on all 3 specimens) Panel Markers (FITC/PE/ECD/PC 5. 5/PC 7/APC/AF 700/AF 750/PB/Kr. O) Tube 1 Kappa Lambda CD 23 CD 38 CD 34 CD 20 CD 19 CD 5 CD 45 Tube 2 CD 8 CD 2 CD 7 CD 34 CD 56 CD 16 CD 4 CD 5 CD 45 3

60 days post second transplant BONE MARROW FINDINGS

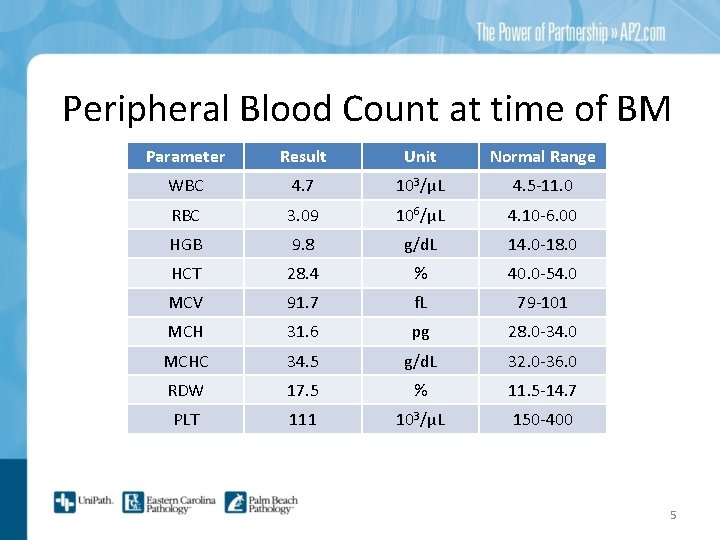
Peripheral Blood Count at time of BM Parameter Result Unit Normal Range WBC 4. 7 103/μL 4. 5 -11. 0 RBC 3. 09 106/μL 4. 10 -6. 00 HGB 9. 8 g/d. L 14. 0 -18. 0 HCT 28. 4 % 40. 0 -54. 0 MCV 91. 7 f. L 79 -101 MCH 31. 6 pg 28. 0 -34. 0 MCHC 34. 5 g/d. L 32. 0 -36. 0 RDW 17. 5 % 11. 5 -14. 7 PLT 111 103/μL 150 -400 5

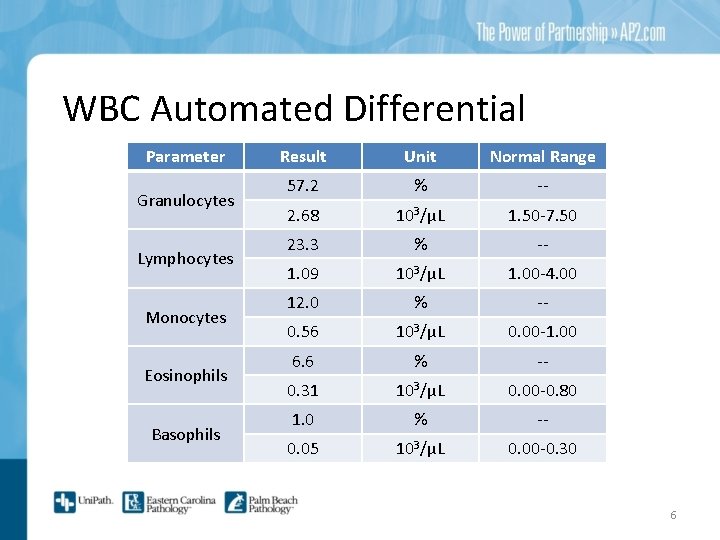
WBC Automated Differential Parameter Granulocytes Lymphocytes Monocytes Eosinophils Basophils Result Unit Normal Range 57. 2 % -- 2. 68 103/μL 1. 50 -7. 50 23. 3 % -- 1. 09 103/μL 1. 00 -4. 00 12. 0 % -- 0. 56 103/μL 0. 00 -1. 00 6. 6 % -- 0. 31 103/μL 0. 00 -0. 80 1. 0 % -- 0. 05 103/μL 0. 00 -0. 30 6

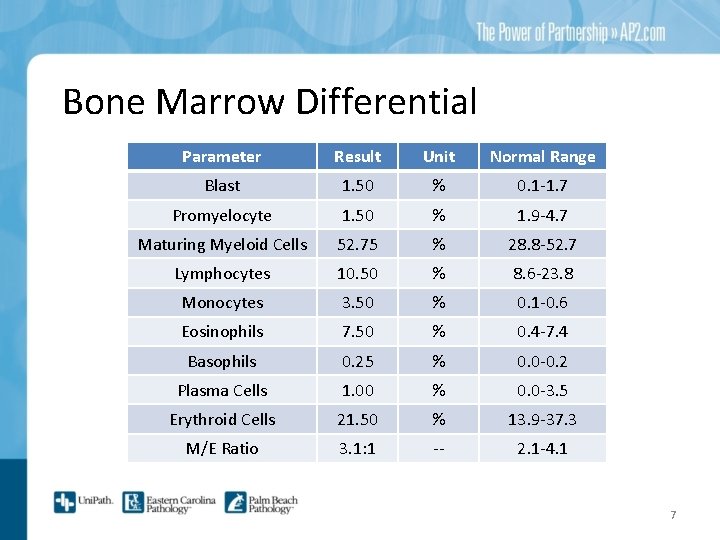
Bone Marrow Differential Parameter Result Unit Normal Range Blast 1. 50 % 0. 1 -1. 7 Promyelocyte 1. 50 % 1. 9 -4. 7 Maturing Myeloid Cells 52. 75 % 28. 8 -52. 7 Lymphocytes 10. 50 % 8. 6 -23. 8 Monocytes 3. 50 % 0. 1 -0. 6 Eosinophils 7. 50 % 0. 4 -7. 4 Basophils 0. 25 % 0. 0 -0. 2 Plasma Cells 1. 00 % 0. 0 -3. 5 Erythroid Cells 21. 50 % 13. 9 -37. 3 M/E Ratio 3. 1: 1 -- 2. 1 -4. 1 7

Morphology - Bone Marrow Aspirate • There is trilineage hematopoiesis with maturation. Myeloid and erythroid lineages show no evidence of dysplasia. No increase in blasts. No lymphoid or plasma cell aggregates. • Iron staining demonstrated markedly increased storage iron with decreased iron utilization. No ring sideroblasts were noted. Particle Prep • Rare interstitial and perivascular aggregates of small, mature lymphocytes which show slight/moderate nuclear irregularities, condensed chromatin, and scant cytoplasm. Core Biopsy • Patient exhibits normal cellularity of 30 -40%. Bone trabeculae are appropriate. 8

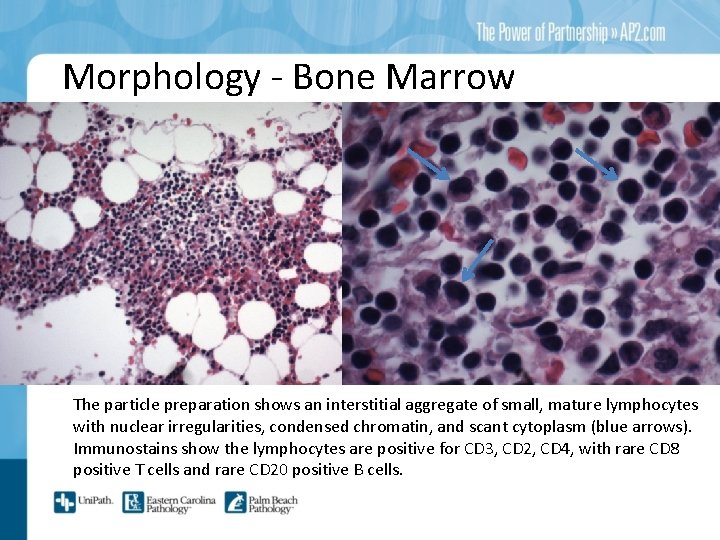
Morphology - Bone Marrow The particle preparation shows an interstitial aggregate of small, mature lymphocytes with nuclear irregularities, condensed chromatin, and scant cytoplasm (blue arrows). Immunostains show the lymphocytes are positive for CD 3, CD 2, CD 4, with rare CD 8 positive T cells and rare CD 20 positive B cells.

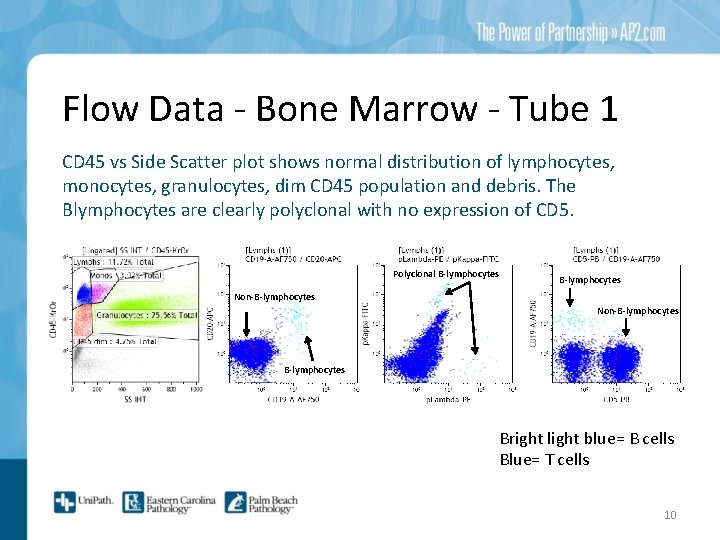
Flow Data - Bone Marrow - Tube 1 CD 45 vs Side Scatter plot shows normal distribution of lymphocytes, monocytes, granulocytes, dim CD 45 population and debris. The Blymphocytes are clearly polyclonal with no expression of CD 5. Polyclonal B-lymphocytes Non-B-lymphocytes Bright light blue= B cells Blue= T cells 10

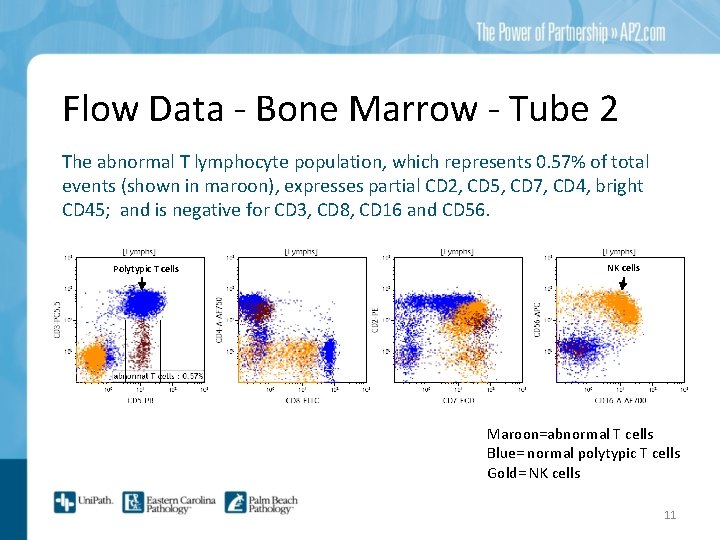
Flow Data - Bone Marrow - Tube 2 The abnormal T lymphocyte population, which represents 0. 57% of total events (shown in maroon), expresses partial CD 2, CD 5, CD 7, CD 4, bright CD 45; and is negative for CD 3, CD 8, CD 16 and CD 56. Polytypic T cells NK cells Maroon=abnormal T cells Blue= normal polytypic T cells Gold= NK cells 11

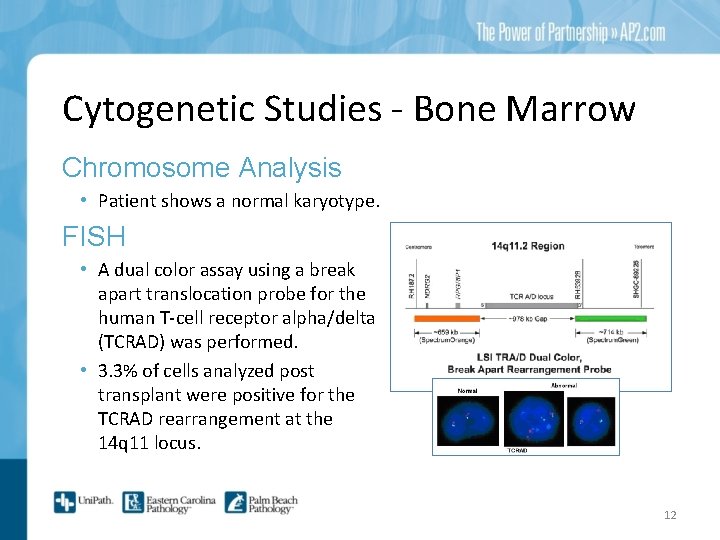
Cytogenetic Studies - Bone Marrow Chromosome Analysis • Patient shows a normal karyotype. FISH • A dual color assay using a break apart translocation probe for the human T-cell receptor alpha/delta (TCRAD) was performed. • 3. 3% of cells analyzed post transplant were positive for the TCRAD rearrangement at the 14 q 11 locus. 12

Patient developed an increasingly uncomfortable and rapidly growing right neck mass. Needle core biopsy with flow cytometry analysis was performed one week after the bone marrow biopsy. RIGHT NECK MASS

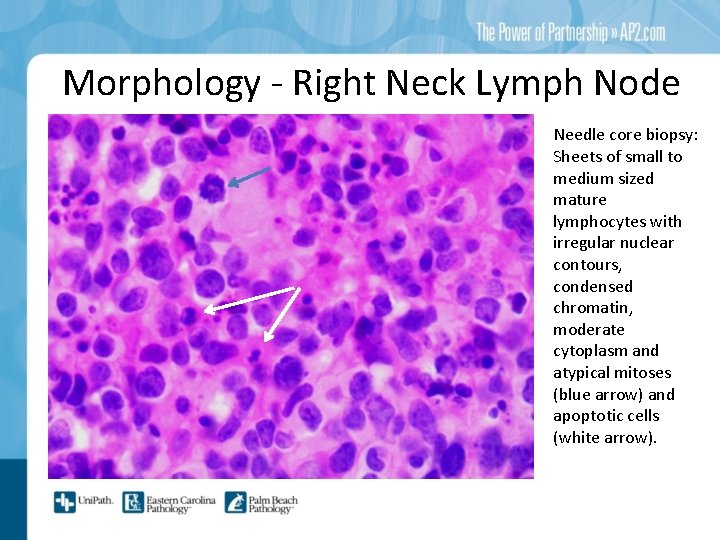
Morphology - Right Neck Lymph Node Needle core biopsy: Sheets of small to medium sized mature lymphocytes with irregular nuclear contours, condensed chromatin, moderate cytoplasm and atypical mitoses (blue arrow) and apoptotic cells (white arrow).

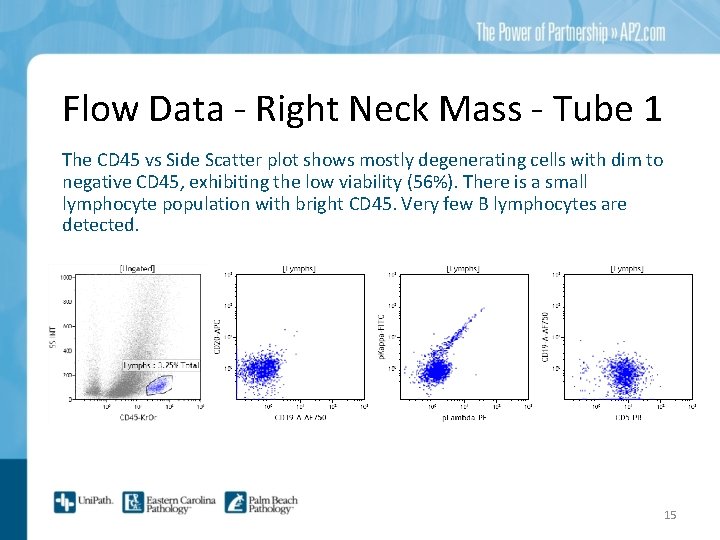
Flow Data - Right Neck Mass - Tube 1 The CD 45 vs Side Scatter plot shows mostly degenerating cells with dim to negative CD 45, exhibiting the low viability (56%). There is a small lymphocyte population with bright CD 45. Very few B lymphocytes are detected. 15

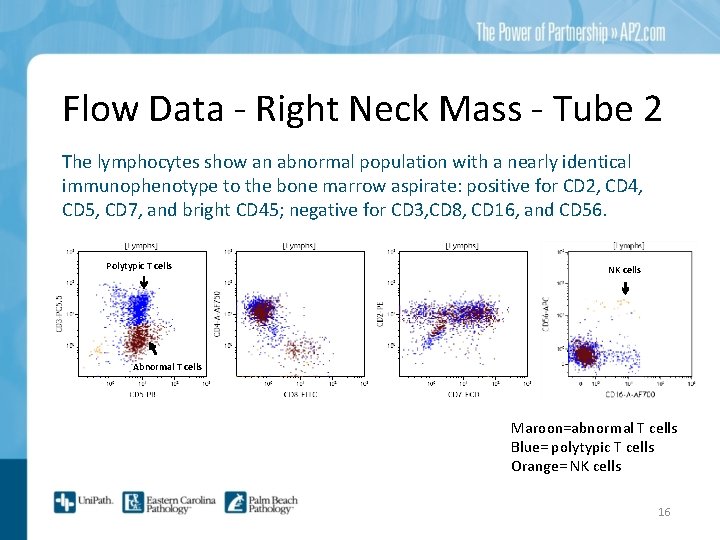
Flow Data - Right Neck Mass - Tube 2 The lymphocytes show an abnormal population with a nearly identical immunophenotype to the bone marrow aspirate: positive for CD 2, CD 4, CD 5, CD 7, and bright CD 45; negative for CD 3, CD 8, CD 16, and CD 56. Polytypic T cells NK cells Abnormal T cells Maroon=abnormal T cells Blue= polytypic T cells Orange= NK cells 16

The right neck mass was treated with palliative radiation. Approximately one month later, the patient presented with a lower extremity deep venous thrombosis and a rapidly increasing white blood cell count (up to 51, 000, with 20% lymphocytes). Therefore, peripheral blood was submitted for flow cytometry analysis. PERIPHERAL BLOOD

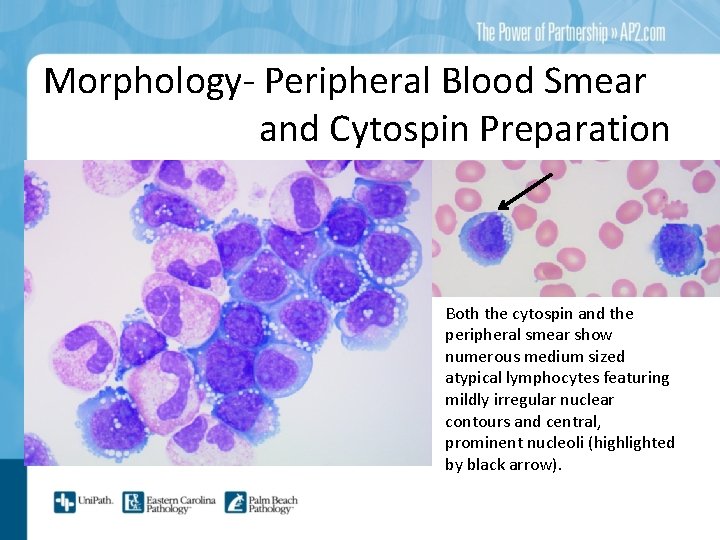
Morphology- Peripheral Blood Smear and Cytospin Preparation Both the cytospin and the peripheral smear show numerous medium sized atypical lymphocytes featuring mildly irregular nuclear contours and central, prominent nucleoli (highlighted by black arrow).

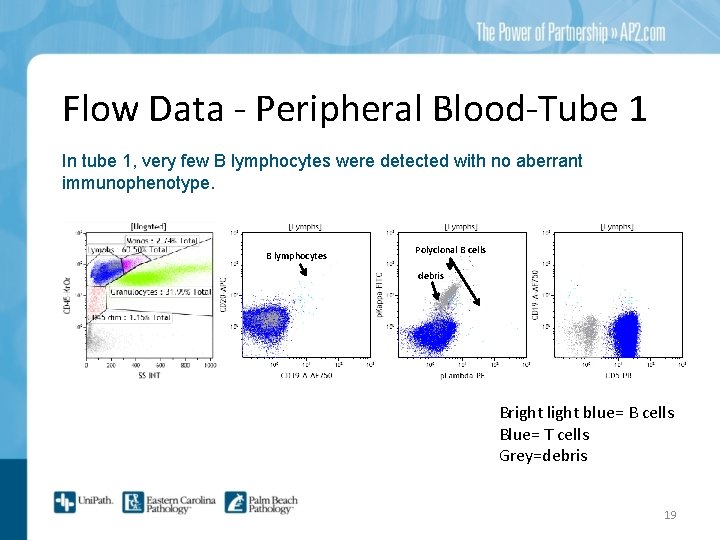
Flow Data - Peripheral Blood-Tube 1 In tube 1, very few B lymphocytes were detected with no aberrant immunophenotype. B lymphocytes Polyclonal B cells debris Bright light blue= B cells Blue= T cells Grey=debris 19

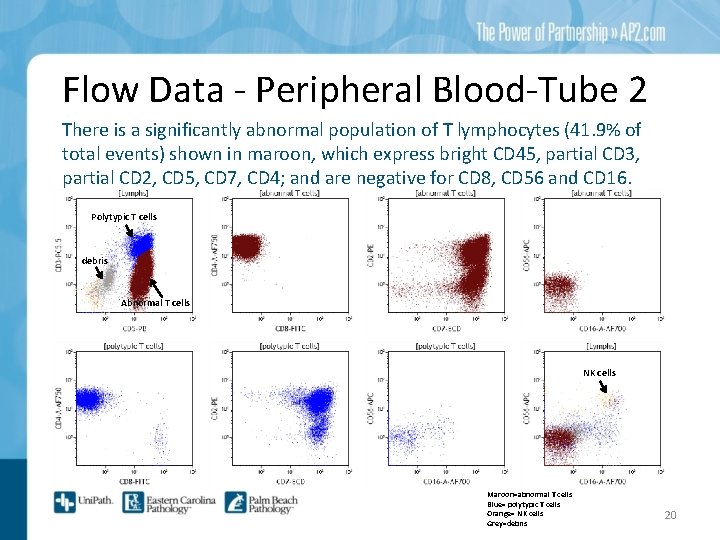
Flow Data - Peripheral Blood-Tube 2 There is a significantly abnormal population of T lymphocytes (41. 9% of total events) shown in maroon, which express bright CD 45, partial CD 3, partial CD 2, CD 5, CD 7, CD 4; and are negative for CD 8, CD 56 and CD 16. Polytypic T cells debris Abnormal T cells NK cells Maroon=abnormal T cells Blue= polytypic T cells Orange= NK cells Grey=debris 20

Final Diagnosis Recurrent T-Prolymphocytic Leukemia (T-PLL) • Following a 2 nd allogeneic, matched, unrelated bone marrow transplant, a persistent small abnormal T-cell population was detected in marrow and tissues (0. 57% and 2. 11% respectively). • A peripheral blood drawn 90 days post transplant exhibited full relapse with the aberrant T-cell population growing to 41. 90% of total events. • Key immunophenotypic findings across all specimens included negative to partial/dim surface CD 3; and CD 2, CD 4, CD 5, CD 7, bright CD 45 positive; CD 8, CD 16, CD 56 negative. • Morphology consistently showed medium-sized atypical lymphocytes with slightly dispersed chromatin, 1 -3 variably prominent nucleoli, irregular nuclear contours, and moderate lightly basophilic cytoplasm. • The patient previously showed a complex abnormal karyotype with a derivative chromosome 14 from a t(14; 14)(q 11; q 32), which is the second most common cytogenetic abnormality seen in T-PLL (10% of patients). FISH for TCRAD rearrangement was positive in the relapsed specimens, c/w persistence of the rearrangement of the TCRAD locus at 14 q 11. 2. 21

T-Prolymphoctyic Leukemia (T-PLL) WHO Classification/Definition • An aggressive T-cell leukemia characterized by a proliferation of small to medium-sized prolymphocytes. Epidemiology • Represents only 2% of mature lymphocytic leukemias in adults over 30. • Median age of incidence is 65 years, with a range of 30 -94 years. Sites of Involvement • Peripheral blood and bone marrow are the major sites of involvement. • Infiltrates may also be seen in the spleen, liver, and skin. Clinical Features • Anemia, thrombocytopenia, and absolute lymphocytosis. • Hepatosplenomegaly and generalized lymphadenopathy are common. • Skin infiltration in 20% of cases, with serous effusions appearing in few cases. 22

T-Prolymphoctyic Leukemia Diagnostic Testing • Peripheral Blood/Bone Marrow Morphology – Abundant small to medium-sized lymphs. – Non-granular basophilic cytoplasm. – Round, oval, or markedly irregular nuclei and visible nucleolus. • Immunophenotype is consistent with that of peripheral T-cells – Negative for Td. T, CD 1 a, CD 16, CD 56; Positive for CD 2, CD 3 (may be weak to negative on membrane), CD 5, CD 7, bright CD 45. – CD 4+8 - (60% of cases), CD 4+8+ (25% of cases), CD 4 -8+ (15% of cases). – CD 52 testing may be requested to determine treatment. • Cytogenetic testing – Rearrangement between T-cell receptor genes beta and gamma. – Inv (14)(q 11 q 32) in 80% of cases; t(14; 14)(q 11; q 32) in 10% of cases – Trisomy 8 q, idic (8 p 11), and t(8; 8)(p 11 -12; q 12) in 70 -80% of cases. 23

T-Prolymphoctyic Leukemia Prognosis • Aggressive disease course with a median survival of less than 1 year. • Chronic courses may accelerate after 2 to 3 years. • Patient declined furtherapy and opted for hospice care. Treatment Options • Monoclonal antibody therapy with anti-CD 52 (alemtuzumab). Has a median survival of 20 months. • Autologous or allogeneic stem cell transplant following successful immunotherapy and remission. Median survival of 48 months. 24

References • Dearden C. B- and T-cell prolymphocytic leukemia: antibody approaches. Hematology Am Soc Hematol Educ Program. 2012; 2012: 645 -51. doi: 10. 1182/asheducation-2012. 1. 645. Review. Pub. Med PMID: 23233647. • Dearden C. How I treat prolymphocytic leukemia. Blood. 2012 Jul 19; 120(3): 538 -51. doi: 10. 1182/blood-2012 -01 -380139. Epub 2012 May 30. Pub. Med PMID: 22649104. • Foucar K. Mature T-cell leukemias including T-prolymphocytic leukemia, adult T-cell leukemia/lymphoma, and Sézary syndrome. Am J Clin Pathol. 2007 Apr; 127(4): 496 -510. Pub. Med PMID: 17369126. • Graham RL, Cooper B, Krause JR. T-cell prolymphocytic leukemia. Proc (Bayl Univ Med Cent). 2013 Jan; 26(1): 19 -21. Pub. Med PMID: 23382603; Pub. Med Central PMCID: PMC 3523759 • Swerdlow, Steven H. “T-cell prolymphocytic leukemia. " WHO classification of tumours of haematopoietic and lymphoid tissues. 4 th ed. Lyon, France: International Agency for Research on Cancer, 2008. 270 -271. 25