How are electrons organized in an atom Electrons





- Slides: 11

How are electrons organized in an atom?

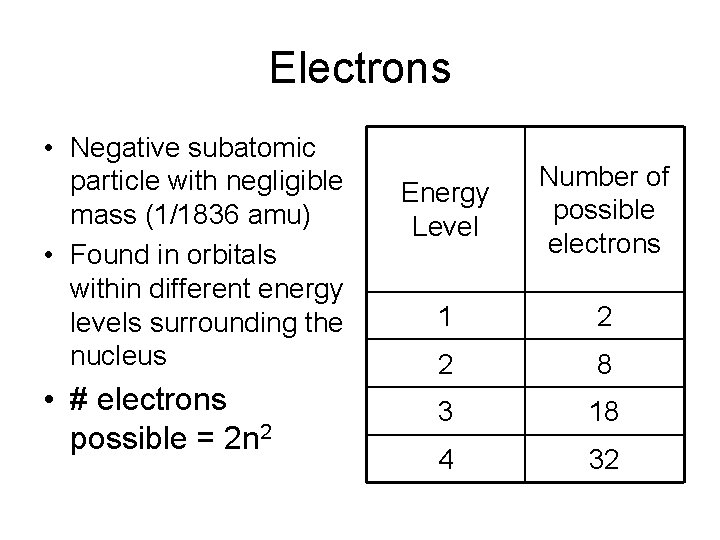
Electrons • Negative subatomic particle with negligible mass (1/1836 amu) • Found in orbitals within different energy levels surrounding the nucleus • # electrons possible = 2 n 2 Energy Level Number of possible electrons 1 2 2 8 3 18 4 32

Electron Configuration • • • Look at Carbon in periodic table 2 -4 2 electrons in first energy level 4 electrons in second energy level It has a total of 6 electrons v. All electron configurations in the periodic table are “ground state” configurations

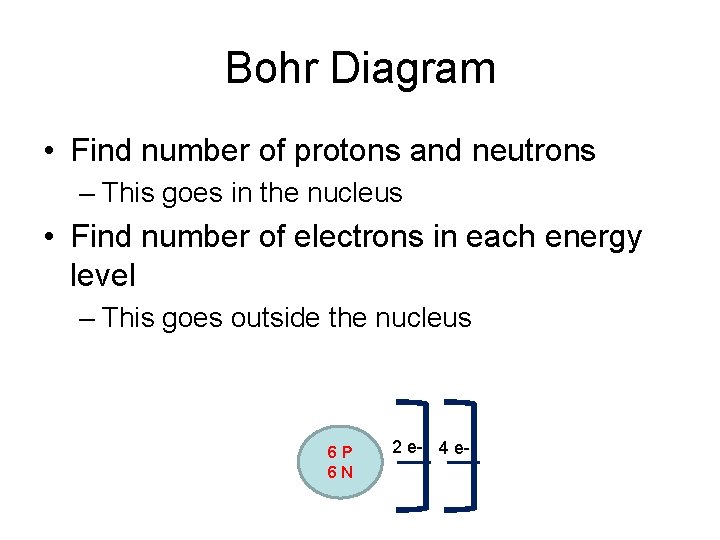
Bohr Diagram • Find number of protons and neutrons – This goes in the nucleus • Find number of electrons in each energy level – This goes outside the nucleus 6 P 6 N 2 e- 4 e-

Valence Electrons • The Valence shell is the outer most energy level • The valence electrons are the electrons in the outermost energy level v. Which energy level is the valence shell for carbon? v. How many valence electrons does carbon have in the ground state?

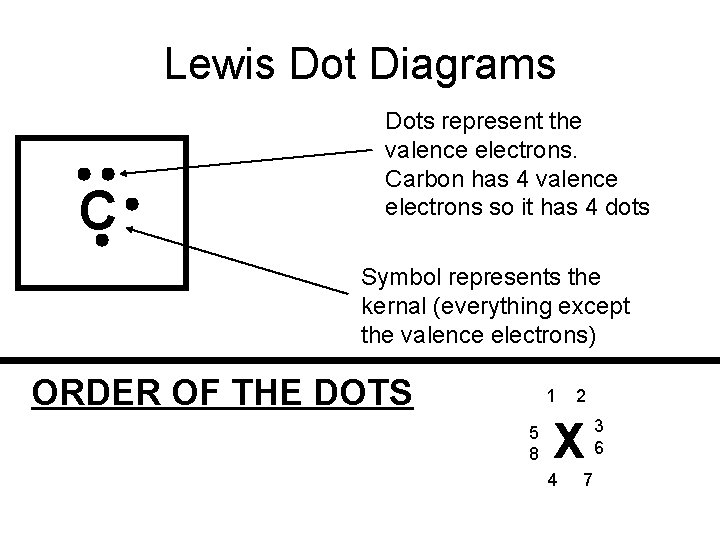
Lewis Dot Diagrams C Dots represent the valence electrons. Carbon has 4 valence electrons so it has 4 dots Symbol represents the kernal (everything except the valence electrons) ORDER OF THE DOTS 1 5 8 2 X 4 7 3 6

Practice • For each of the following write the electron configuration and Lewis dot diagram – – – Oxygen Magnesium Chlorine Copper Sulfur Sodium

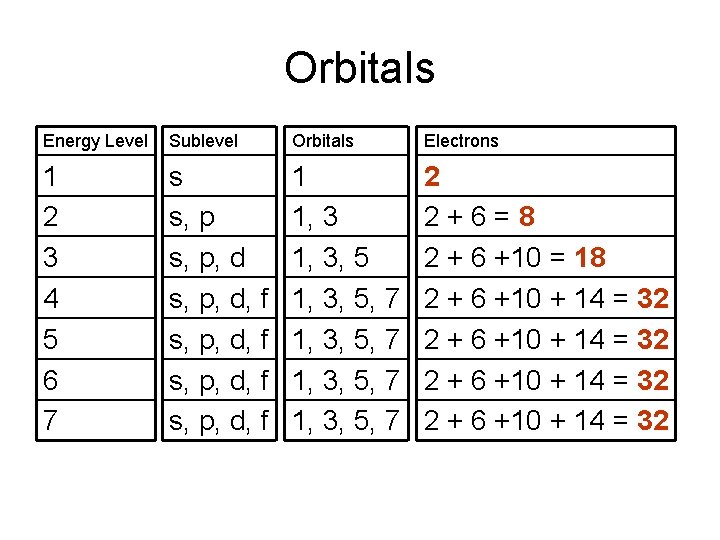
Orbitals Energy Level Sublevel Orbitals Electrons 1 2 3 4 5 6 7 s s, p, d, f s, p, d, f 1 1, 3, 5, 7 1, 3, 5, 7 2 2+6=8 2 + 6 +10 = 18 2 + 6 +10 + 14 = 32

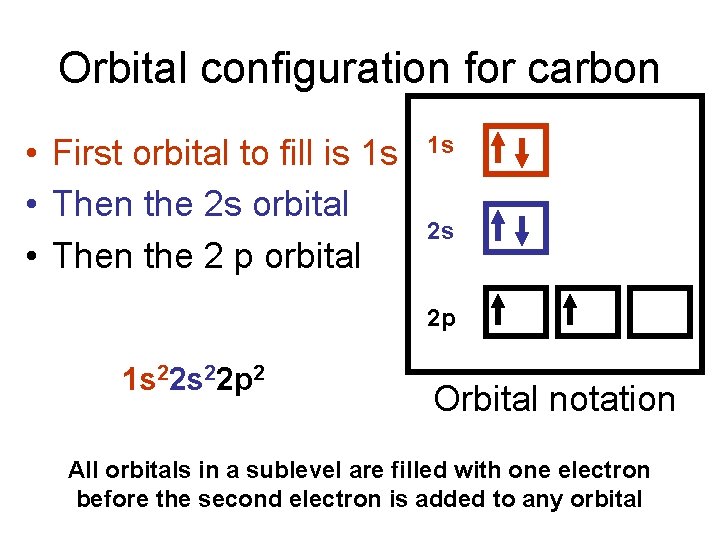
Orbital configuration for carbon • First orbital to fill is 1 s • Then the 2 s orbital • Then the 2 p orbital 1 s 2 s 2 p 1 s 22 p 2 Orbital notation All orbitals in a sublevel are filled with one electron before the second electron is added to any orbital

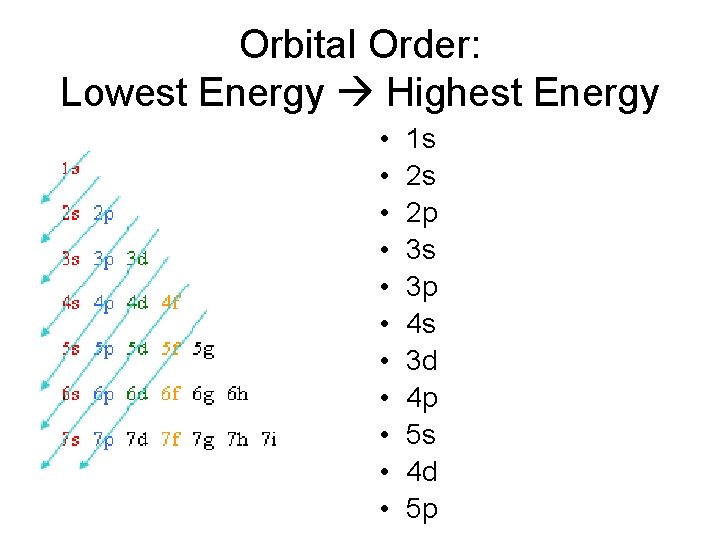
Orbital Order: Lowest Energy Highest Energy • • • 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s 4 d 5 p

Practice • For each of the following write: – Electron configuration – Electrion configuration with orbitals – Orbital notation – Give the number of valence electrons – Draw the Lewis dot diagram 1. Oxygen 3. Chlorine 5. Sulfur 2. Magnesium 4. Copper 6. Sodium