Electrons How are electrons organized around a nucleus






- Slides: 26

Electrons How are electrons organized around a nucleus?

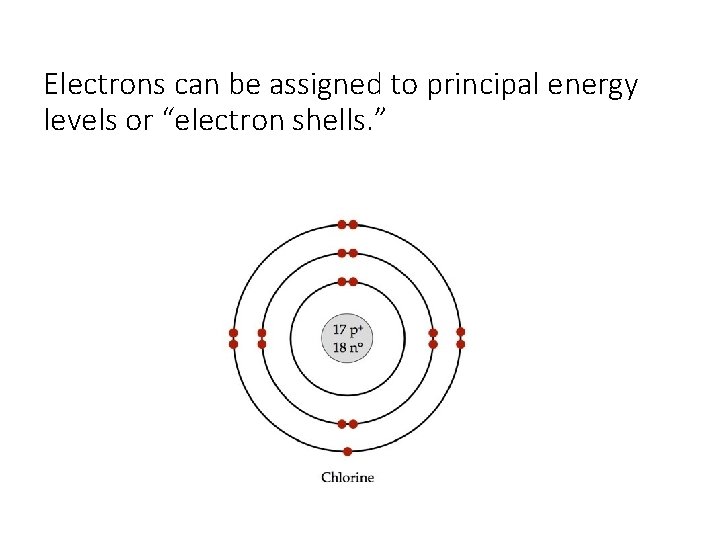
Electrons can be assigned to principal energy levels or “electron shells. ”

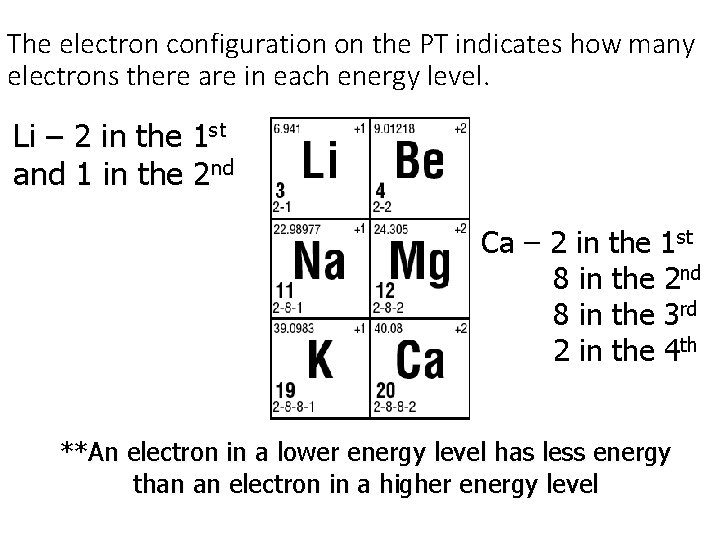
The electron configuration on the PT indicates how many electrons there are in each energy level. Li – 2 in the 1 st and 1 in the 2 nd Ca – 2 in the 1 st 8 in the 2 nd 8 in the 3 rd 2 in the 4 th **An electron in a lower energy level has less energy than an electron in a higher energy level

Vocabulary Terms: • Valence electrons – the electrons located in the highest occupied principle energy level or shell • Kernel electrons – all of the electrons that are not valence (the electrons in the inner shells)

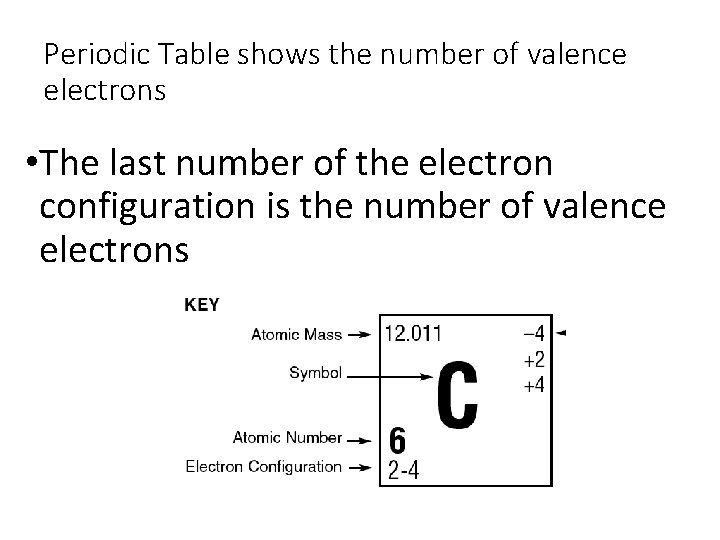
Periodic Table shows the number of valence electrons • The last number of the electron configuration is the number of valence electrons

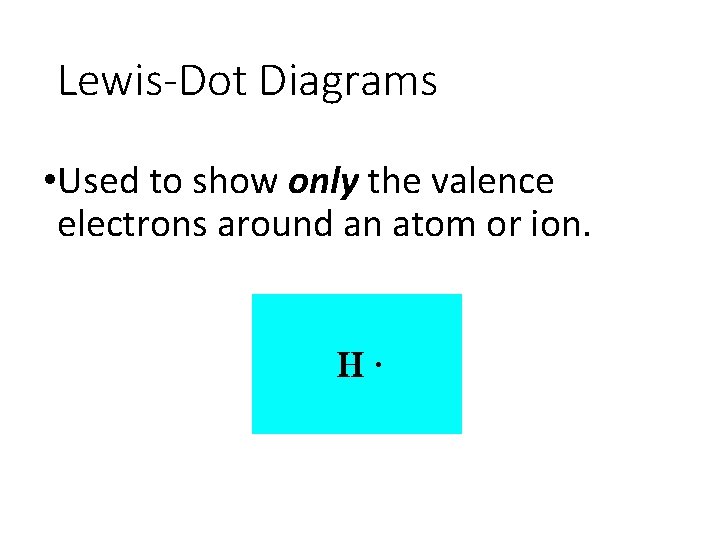
Lewis-Dot Diagrams • Used to show only the valence electrons around an atom or ion.

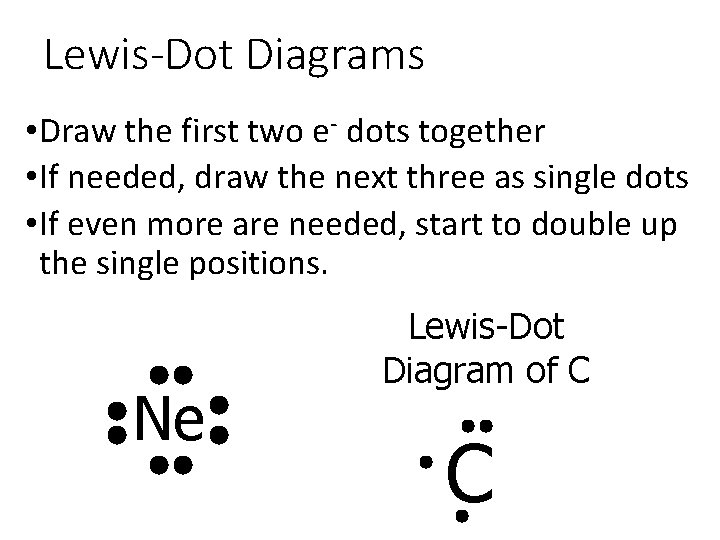
Lewis-Dot Diagrams • Draw the first two e- dots together • If needed, draw the next three as single dots • If even more are needed, start to double up the single positions. Ne Lewis-Dot Diagram of C C

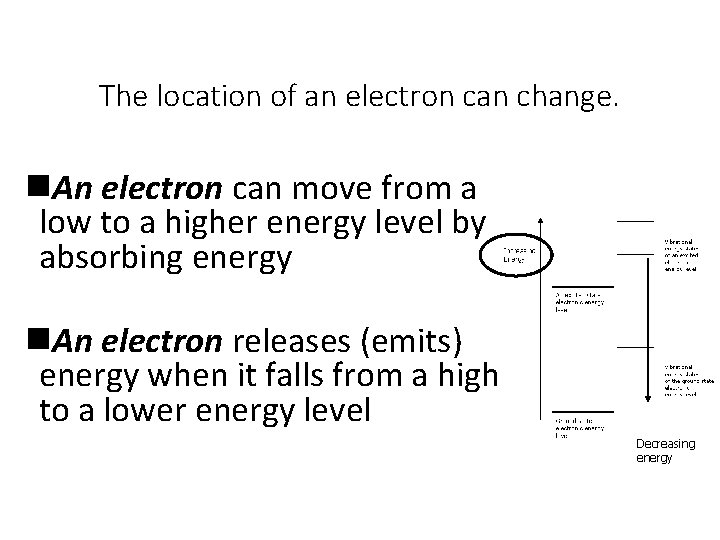
The location of an electron can change. n. An electron can move from a low to a higher energy level by absorbing energy n. An electron releases (emits) energy when it falls from a high to a lower energy level Decreasing energy

When electrons change in energy level, the atom changes in “state. ” • Ground state – the electrons are in the lowest possible energy levels (usually as close to the nucleus as possible) • The electron configuration on the Periodic Table represents the ground state • Excited state – at least one electron has absorbed energy and is in a higher energy level than it would be in the ground state

Identify an Element Based on Electron Configuration n. For atoms the total number of electrons identifies the element. n. The total number of electrons is equal to the atomic number. n. Even for atoms in an excited state, the total number of electrons will be equal to the atomic number.

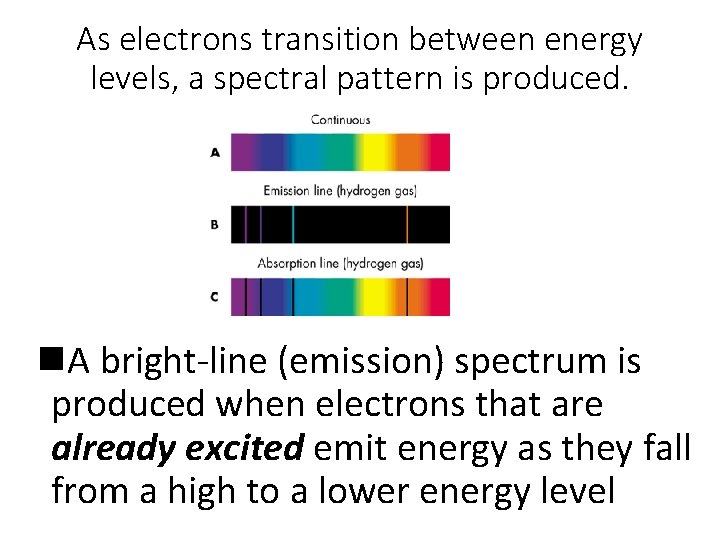
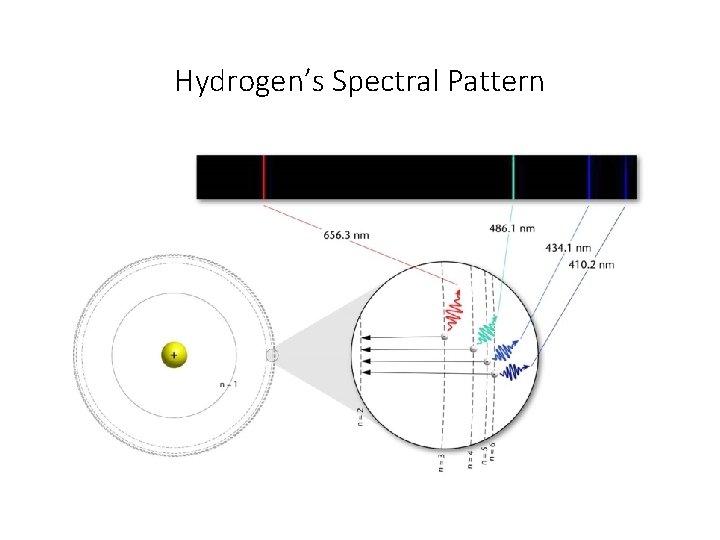
As electrons transition between energy levels, a spectral pattern is produced. n. A bright-line (emission) spectrum is produced when electrons that are already excited emit energy as they fall from a high to a lower energy level

Hydrogen’s Spectral Pattern

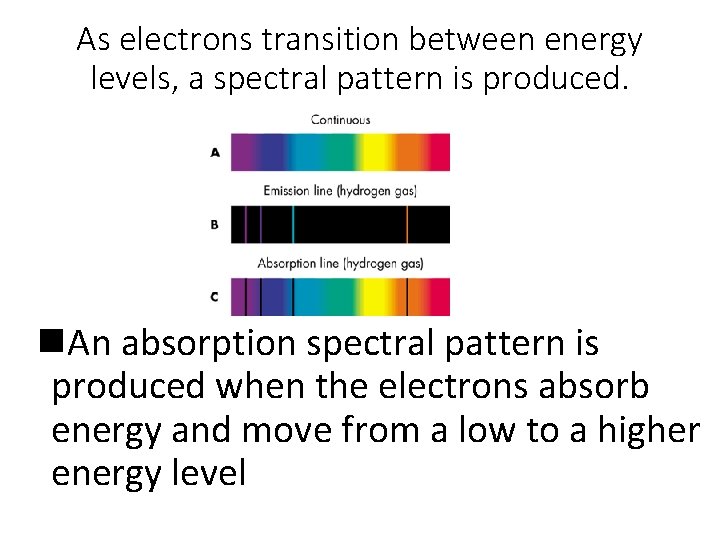
As electrons transition between energy levels, a spectral pattern is produced. n. An absorption spectral pattern is produced when the electrons absorb energy and move from a low to a higher energy level

The flame test is based on electrons’ changes in energy levels n. Electrons must first absorb energy moving from low to higher energy levels, n. Then already excited electrons emit energy as they fall from high to lower energy levels.

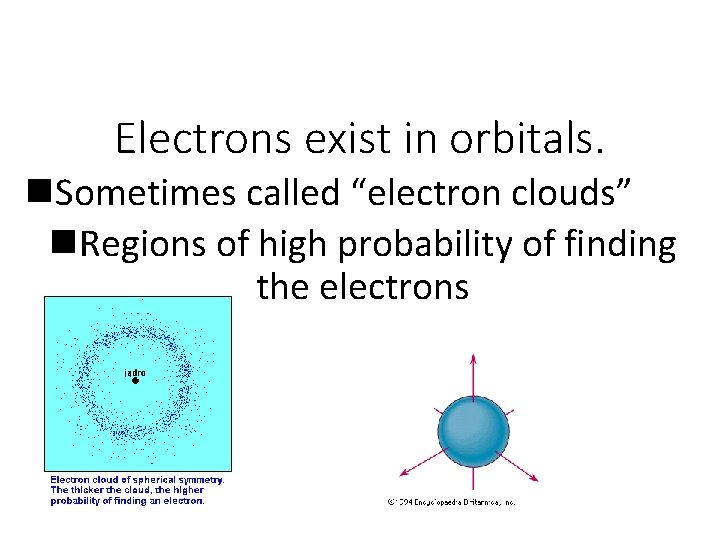
Electrons exist in orbitals. n. Sometimes called “electron clouds” n. Regions of high probability of finding the electrons

Different principal energy levels have different sublevels • 1 st energy level only has s • 2 nd energy level has s and p • 3 rd energy level has s, p, d • 4 th energy level has s, p, d, f • 5 th level + also has s, p, d, f

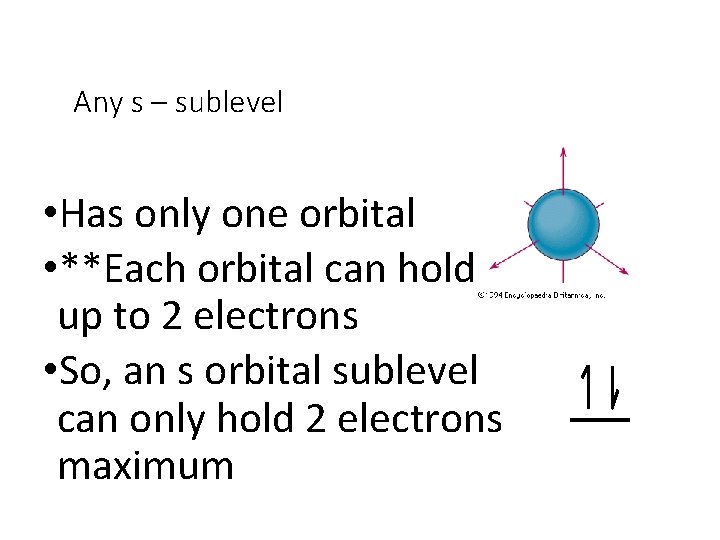
Any s – sublevel • Has only one orbital • **Each orbital can hold up to 2 electrons • So, an s orbital sublevel can only hold 2 electrons maximum

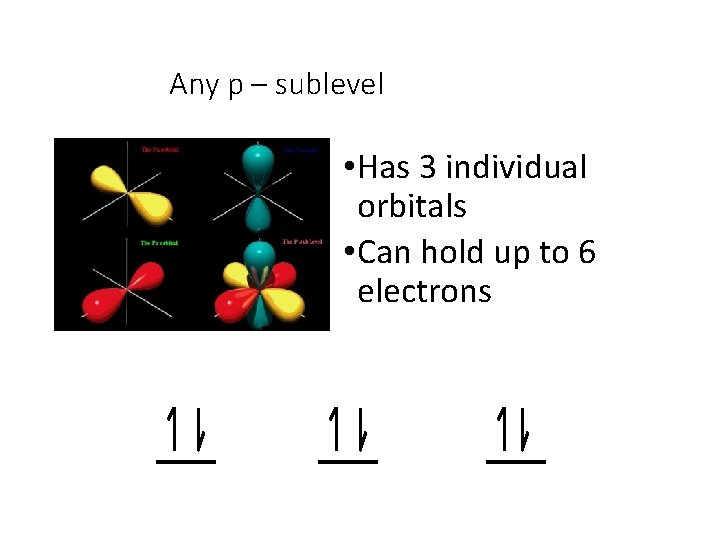
Any p – sublevel • Has 3 individual orbitals • Can hold up to 6 electrons

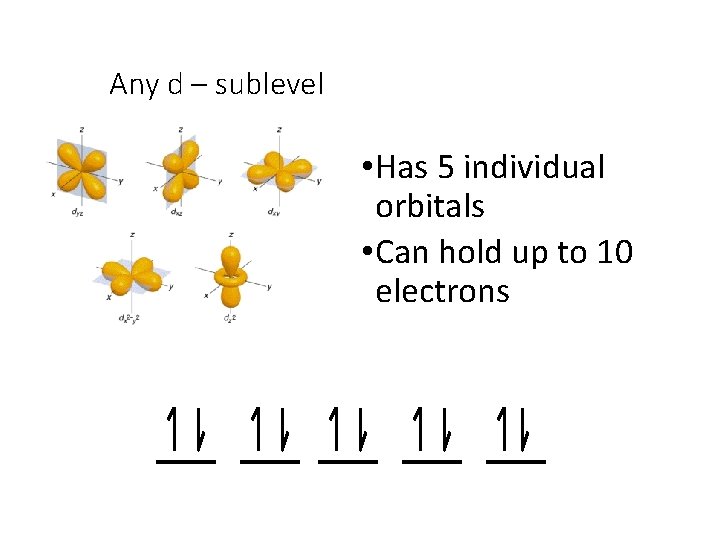
Any d – sublevel • Has 5 individual orbitals • Can hold up to 10 electrons

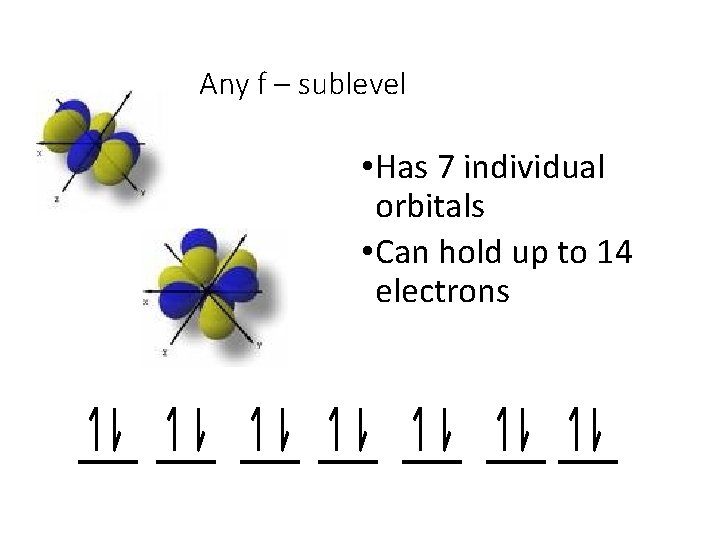
Any f – sublevel • Has 7 individual orbitals • Can hold up to 14 electrons

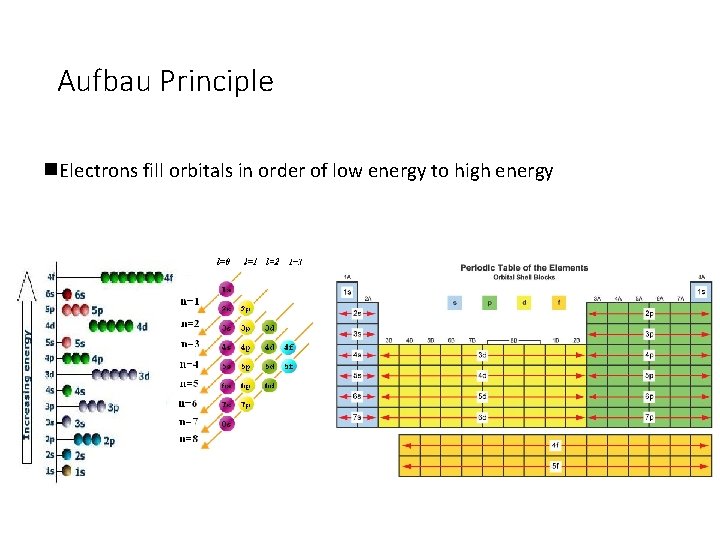
Aufbau Principle n. Electrons fill orbitals in order of low energy to high energy

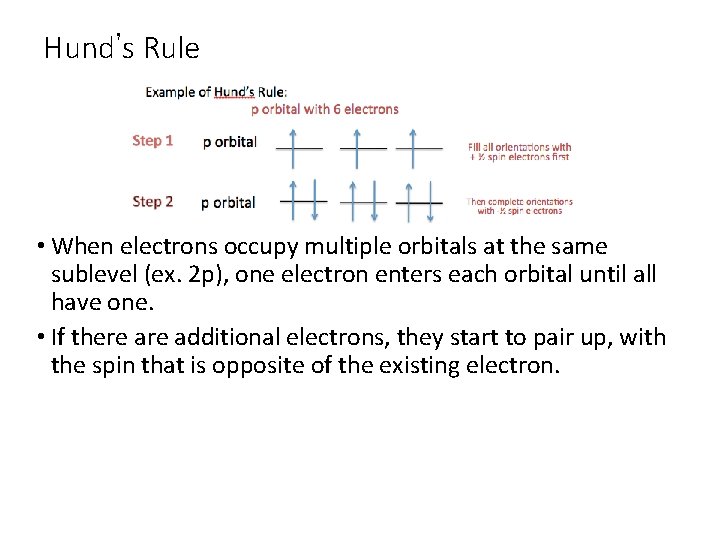
Hund’s Rule • When electrons occupy multiple orbitals at the same sublevel (ex. 2 p), one electron enters each orbital until all have one. • If there additional electrons, they start to pair up, with the spin that is opposite of the existing electron.

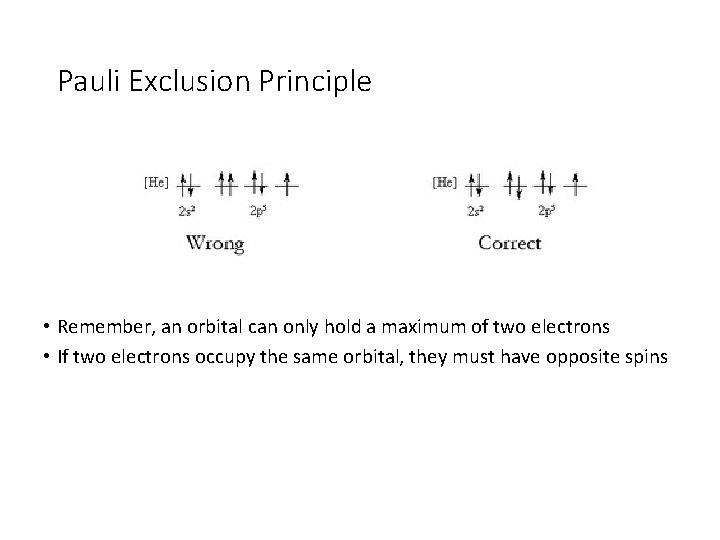
Pauli Exclusion Principle • Remember, an orbital can only hold a maximum of two electrons • If two electrons occupy the same orbital, they must have opposite spins

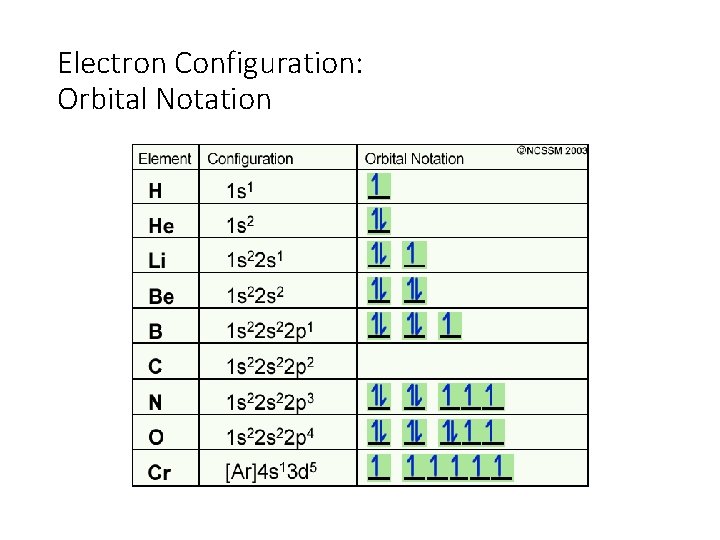
Electron Configuration: Orbital Notation

The Maximum Number of Electrons at each Principal Energy Level • You can determine the number of electrons that completely fill each PEL with the equation 2 n 2 • Plug in the PEL number as “n” • For the 1 st PEL: 2 n 2 = 2(1)2 = 2 • For the 2 nd PEL: 2 n 2 = 2(2)2 = 8 • For the 3 rd PEL: 2 n 2 = 2(3)2 = 18 • For the 4 th PEL: 2 n 2 = 2(4)2 = 32

Ground State vs Excited State using Sublevel Notation Electron Configuration • Sodium’s configuration in the ground state: • 1 s 22 p 63 s 1 • The total number of electrons is equal to the atomic number • The electrons fill the sublevels in the correct order • A possible configuration for sodium in the excited state: • 1 s 22 p 53 s 13 p 1 • The total number of electrons is still equal to the atomic number • But the electrons do not completely fill all sublevels in the correct order • For this example, 2 p should be filled with 6 electrons before electrons fill a higher sublevel