Heap Leaching and Acid Mine Drainage Lecture 22


















- Slides: 38

Heap Leaching and Acid Mine Drainage Lecture 22 Fundamentals of Earth Resources Environmental Considerations L. Cathles 2008 See Chapter 4 Craig et al. , 2001

Heap leaching at Bingham Canyon, Utah • Canyons filled with mine waste • Deliberate application of water leaches Cu • Recovered by iron exchange or SXEW • 20 -25% of production is from heap leaching train Top very large mine truck pipes applying water to dump

Bingham Canyon, Utah


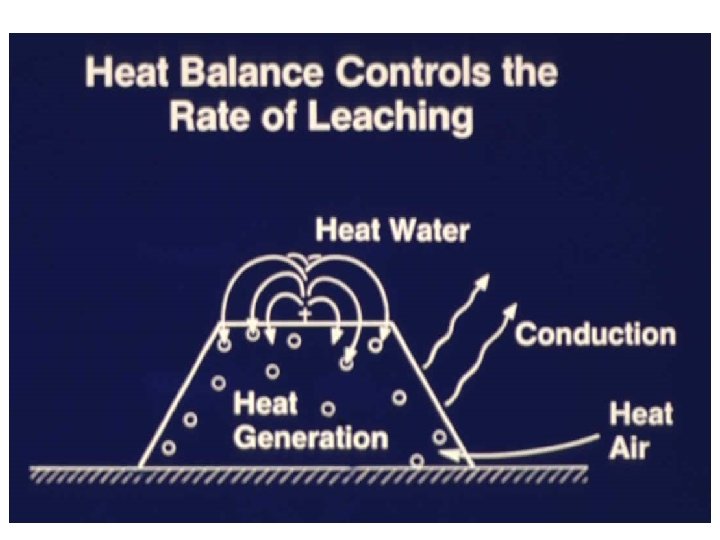
Two phase flow: Water down air up

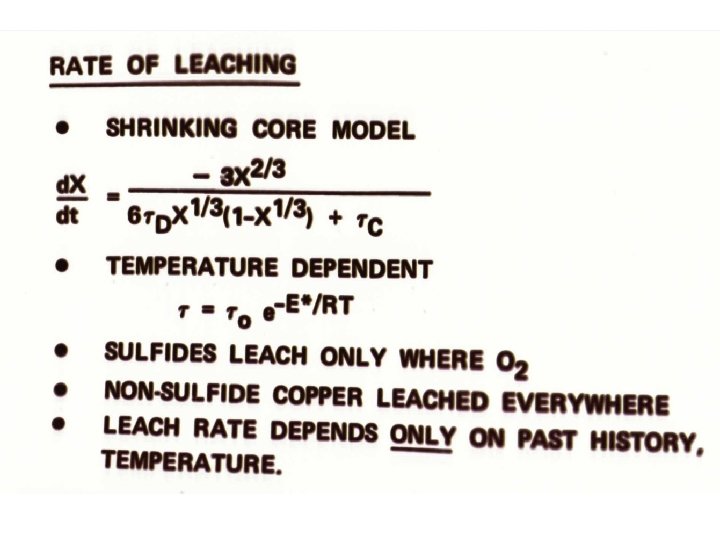
Temperature-dependent, bacteriallycatalyzed shrinking core model describes leaching kinetics

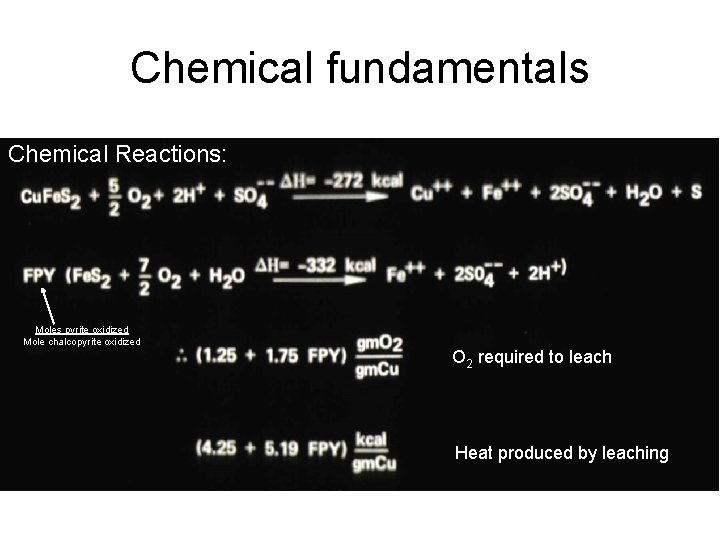
Chemical fundamentals Chemical Reactions: Moles pyrite oxidized Mole chalcopyrite oxidized O 2 required to leach Heat produced by leaching

Conversion to heat and O 2 consumption Rate of Cu leaching shrinking core model


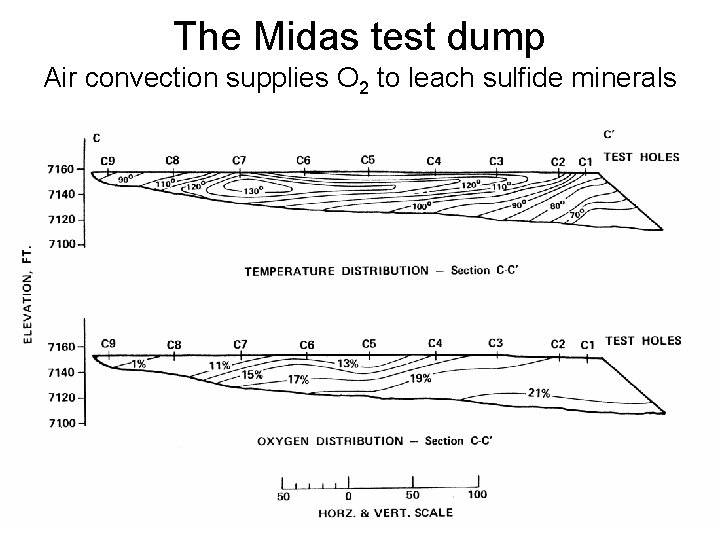
The Midas test dump Air convection supplies O 2 to leach sulfide minerals

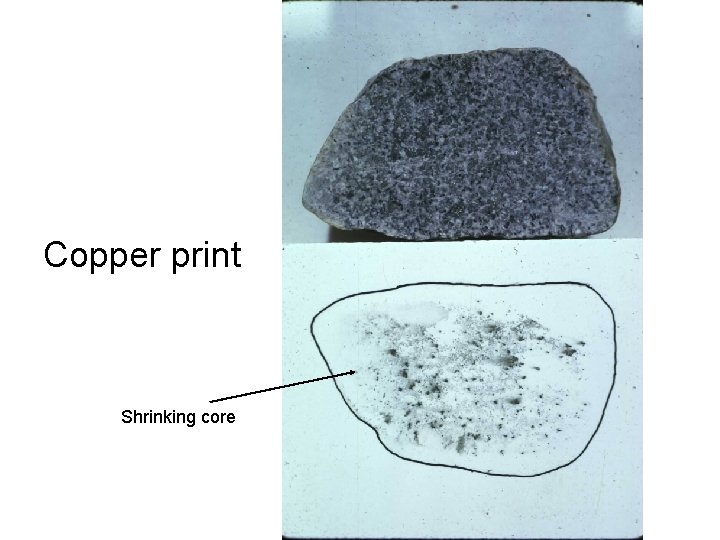
Copper print Shrinking core

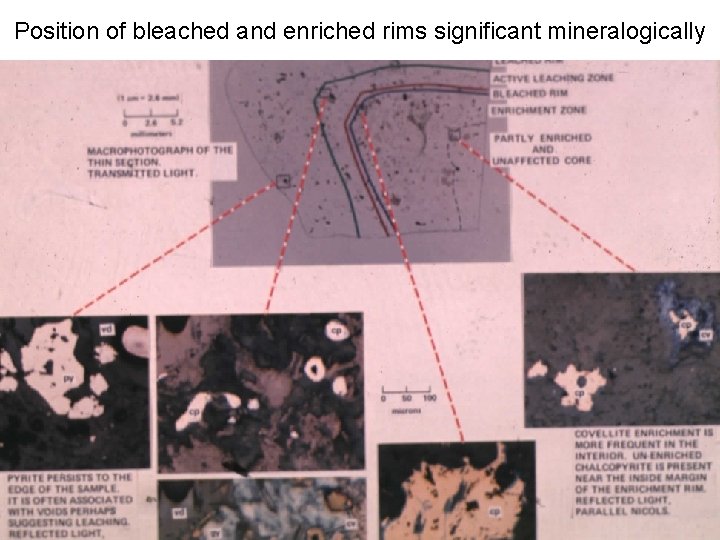
Cu Printing reveals diffusion leach rims

Bleached rim produced by acid attack Unaltered waste dark because of biotite Acid converts biotite to “sericite” producing bleached rim J. Apps experiment at Utah Research Center, KCC

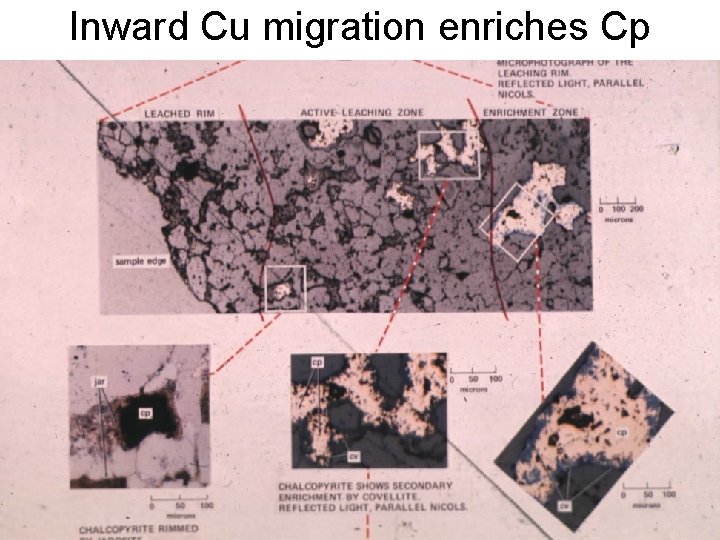
Inward Cu migration enriches Cp

Position of bleached and enriched rims significant mineralogically


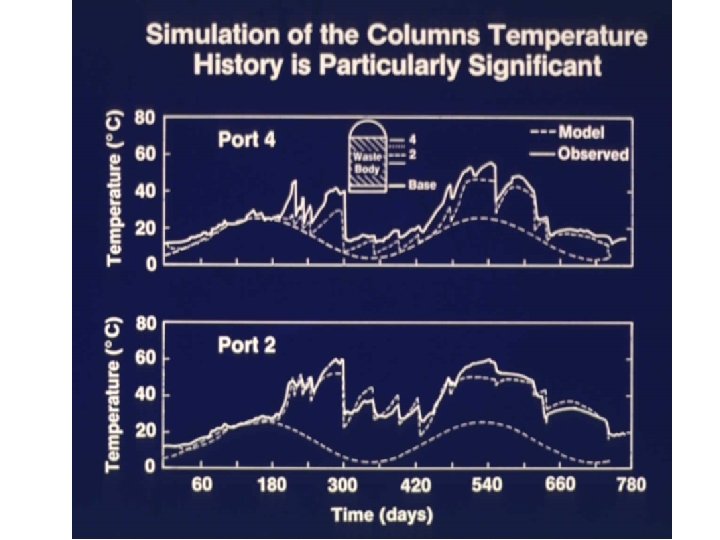
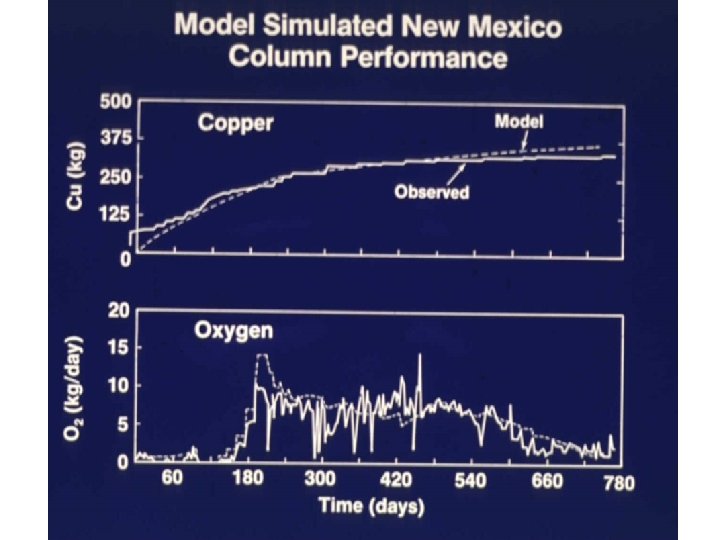
Model calibrated against test dump

Models tested in 2 -yr giant tank experiment Missile O 2 tanks in boneyard near New Mexico Institute of Mining and Technology, Socorro, New Mexico • 40’ high 10’ diameter • Double-walled stainless steel with 1’ pearlite insulation between

Loading

Waste distribution Bucket Graduate Student When full = 170 tons Chino Type 1 waste

Instrumentation




New Mexico Mining and Technology Heap Leaching Experiment Directed by Larry Muir Experiment manager David Bloss Staining of waste with rotamine B dye showed ~50% contact at end of experiment 57% Cu leached over the 2 years Contacted zones not always most leached Flow paths shifted as leaching took place



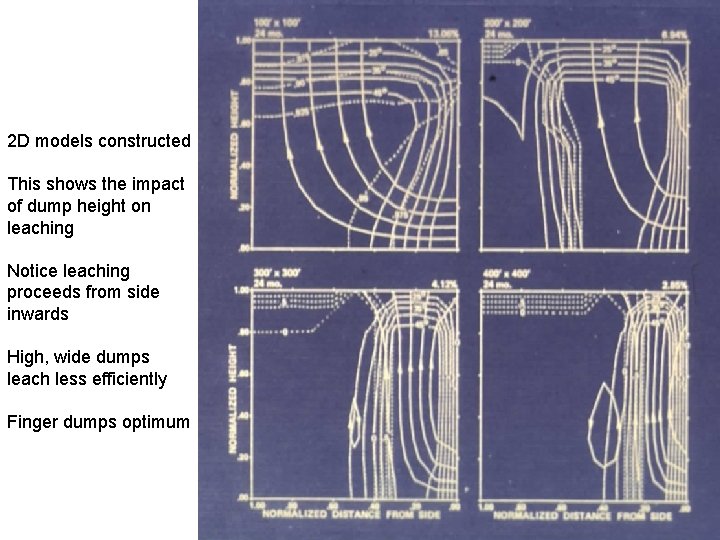
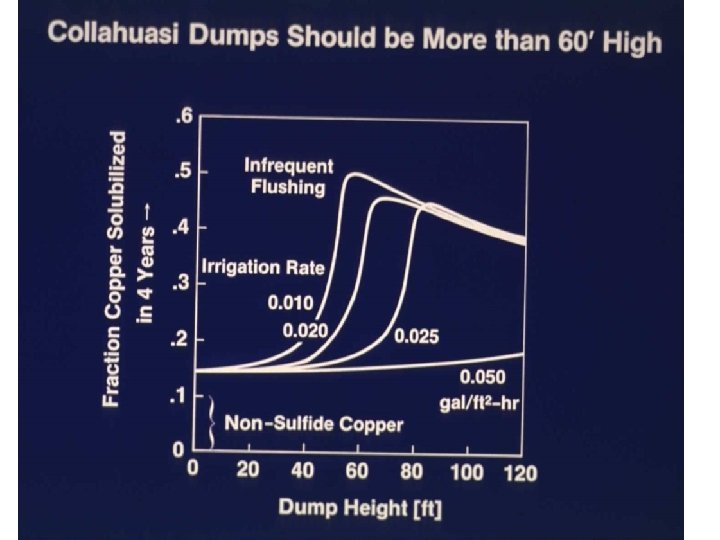
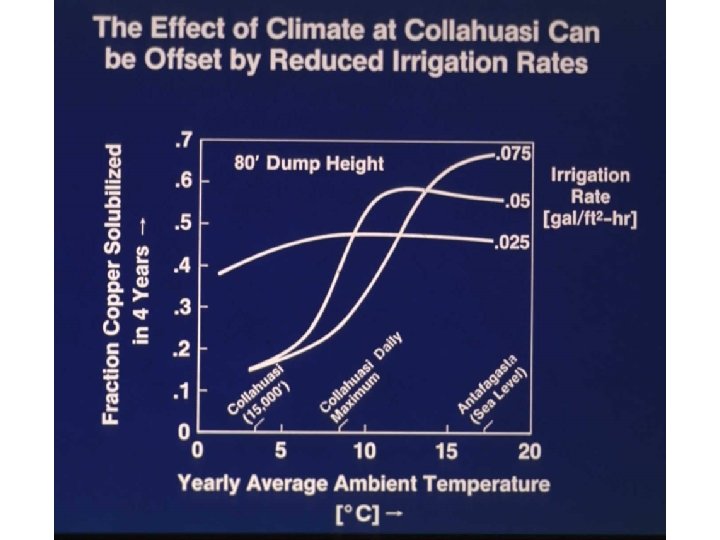
2 D models constructed This shows the impact of dump height on leaching Notice leaching proceeds from side inwards High, wide dumps leach less efficiently Finger dumps optimum

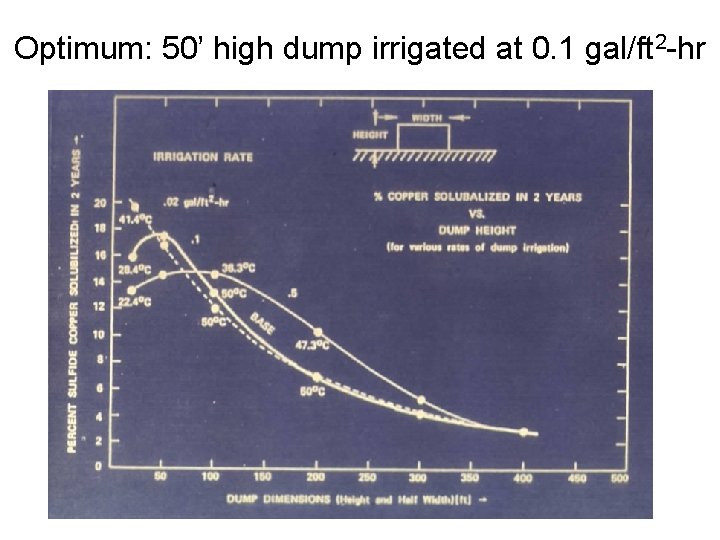
Optimum: 50’ high dump irrigated at 0. 1 gal/ft 2 -hr

Models can address what if questions



Connection to acid mine drainage

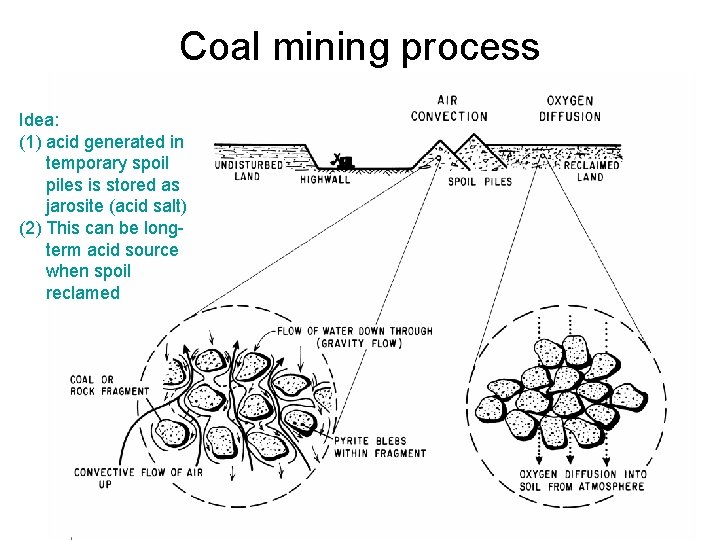
Coal mining process Idea: (1) acid generated in temporary spoil piles is stored as jarosite (acid salt) (2) This can be longterm acid source when spoil reclamed

Calculating buried acid flush time Mass balance on dissolution of jarosite (solid acid)

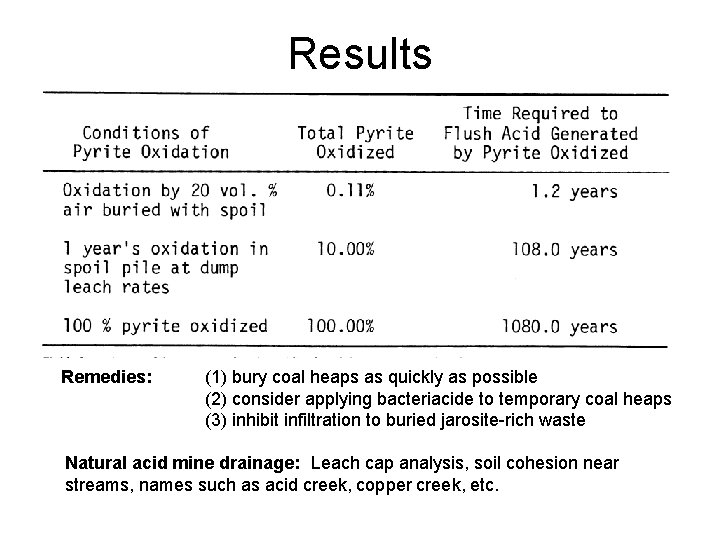
Results Remedies: (1) bury coal heaps as quickly as possible (2) consider applying bacteriacide to temporary coal heaps (3) inhibit infiltration to buried jarosite-rich waste Natural acid mine drainage: Leach cap analysis, soil cohesion near streams, names such as acid creek, copper creek, etc.

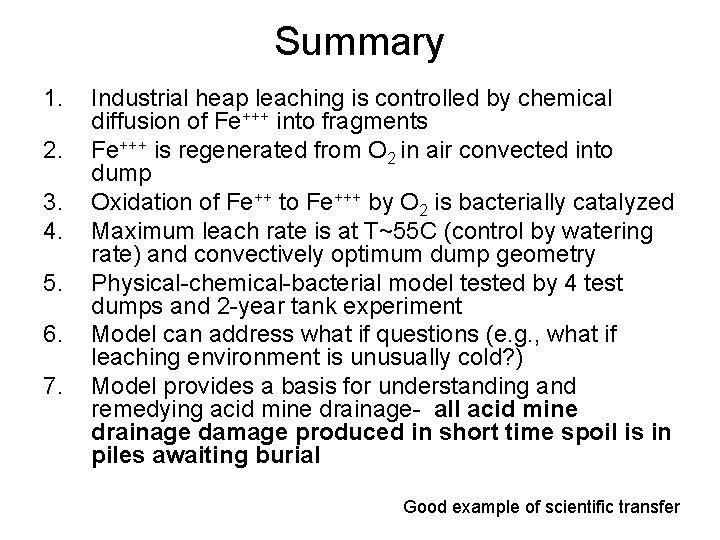
Summary 1. 2. 3. 4. 5. 6. 7. Industrial heap leaching is controlled by chemical diffusion of Fe+++ into fragments Fe+++ is regenerated from O 2 in air convected into dump Oxidation of Fe++ to Fe+++ by O 2 is bacterially catalyzed Maximum leach rate is at T~55 C (control by watering rate) and convectively optimum dump geometry Physical-chemical-bacterial model tested by 4 test dumps and 2 -year tank experiment Model can address what if questions (e. g. , what if leaching environment is unusually cold? ) Model provides a basis for understanding and remedying acid mine drainage- all acid mine drainage damage produced in short time spoil is in piles awaiting burial Good example of scientific transfer

References 1. Cathles, L. M. , 1979, Predictive capabilities of a finite difference model of copper leaching in low grade industrial sulfide waste dumps, Mathematical Geology, Vol. 11 (2), 175 -191. 2. Cathles, L. M. , 1982, Acid mine drainage, Earth and Mineral Sciences, 51(4), 37 -41.
 Which of the following best describes acid mine drainage?
Which of the following best describes acid mine drainage? Hpal autoclave
Hpal autoclave Heap vs binary heap
Heap vs binary heap Porphyrias lover meaning
Porphyrias lover meaning Stationary solid bed leaching
Stationary solid bed leaching Leaching
Leaching Leaching techniques
Leaching techniques How to get plants nitrogen
How to get plants nitrogen Zone of leaching
Zone of leaching 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Example of acid-fast bacteria
Example of acid-fast bacteria Non acid fast bacteria
Non acid fast bacteria 9-which acid is not considered a strong acid?
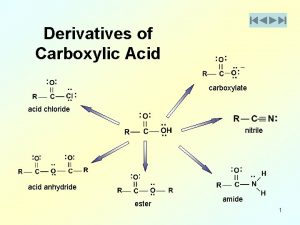
9-which acid is not considered a strong acid? Imides
Imides Lewis acid vs bronsted acid
Lewis acid vs bronsted acid Lewis acid bronsted acid
Lewis acid bronsted acid Is chloric acid a strong acid
Is chloric acid a strong acid Is chloric acid a strong acid
Is chloric acid a strong acid Acid proton donor or acceptor
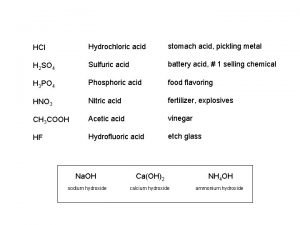
Acid proton donor or acceptor Stomach acid vs battery acid
Stomach acid vs battery acid Nitriles
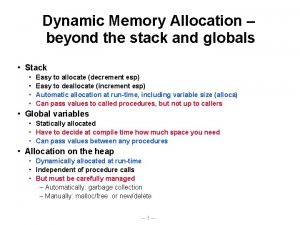
Nitriles Stack and heap memory
Stack and heap memory Saphenous vein medial malleolus
Saphenous vein medial malleolus Dwf and wwf
Dwf and wwf Mappleton coastal management
Mappleton coastal management Hunter perforator
Hunter perforator Objectives of dewatering
Objectives of dewatering Overland flow
Overland flow Retention and drainage
Retention and drainage Retention and drainage
Retention and drainage Bile color
Bile color Beach reprofiling advantages and disadvantages
Beach reprofiling advantages and disadvantages Mine reclamation before and after
Mine reclamation before and after He is mine and i am his
He is mine and i am his I acknowledge mine text analysis answers
I acknowledge mine text analysis answers Windows heap exploitation
Windows heap exploitation Binomial heap calculator
Binomial heap calculator Heap organized table
Heap organized table Apa itu heap
Apa itu heap