Acid Mine Drainage From Formation to Remediation CE













- Slides: 17

Acid Mine Drainage: From Formation to Remediation CE 367 - Aquatic Chemistry Julie Giardina Dominike Merle

Introduction: What is Acid Mine Drainage (AMD)? • Highly acidic water with elevated levels of dissolved metals. • Drainage from surface or deep coal or metal mines and coal refuse piles. • An important environmental issue in many areas where mining has taken place.

Sources of Acid Mine Drainage • Mining of gold, silver, copper, iron, zinc, lead (or combined metals), and coal – Past and present – During exploration, operation, and closure of mine, from the mine’s: • dewatering system • tailings disposal facilities • waste heaps – Water table rebound after pumping equipment is removed.

Process of Acid Mine Drainage • Geochemical and microbial reactions during weathering of sulfide minerals (pyrite) in coal, refuse, or mine overburden – Oxidation of sulfide minerals in the presence of air, water, and bacteria – Formation of sulfuric acid and increase in acidity – Solubilization of metals due to low p. H

A Side Note: Acid Rock Drainage • Formation of acidic waters – Occurs naturally due to weathering of sulfide minerals in rocks – Occurs at a much slower rate

Effects of Acid Mine Drainage • Water resources – Increased acidity – Depleted oxygen – Increased weathering of minerals release of heavy metals/toxic elements into stream – Precipitation of Fe(OH)3 bright orange color of water and rocks

Effects of AMD (cont’d) • Biological resources – Low p. H and oxygen content water unsuitable for aquatic life – Precipitation of Fe(OH)3 • Increased turbidity and decreased photosynthesis • Gill-clogging, smothering of bottom dwellers and food supply, and direct toxicity (benthic algae, invertebrates, and fish) • Clogging of interstitial pore space in coars aquatic substrate habitat

Effects of AMD (cont’d) • Biological resources – Elimination of aquatic plants change in channel hydraulics – Stress on other biota associated with aquatic habitats • Human resources – Corrosion of pipes, pumps, bridges, etc. – Degradation of drinking water supplies – Harm to fisheries

Chemistry of Acid Mine Drainage Reaction 1 2 Fe. S 2 + 7 O 2 + 2 H 2 O 4 Fe 2+ + 4 SO 4 + 4 H+ • weathering of pyrite in the presence of oxygen and water to produce iron(II), sulfate, and hydrogen ions Reaction 2 4 Fe 2+ + 7 O 2 + 2 H 2 O 4 Fe 3+ + 2 H 2 O • oxidation of Fe(II) to Fe(III) • rate determining step

Chemistry of AMD (cont’d) Reaction 3 2 Fe 3+ + 12 H 2 O 4 Fe(OH)3 + 12 H+ • hydrolysis of Fe(III) • precipitation of iron(III) hydroxide if p. H > 3. 5 Reaction 4 Fe. S 2 + 14 Fe 3+ + 8 H 2 O 15 Fe 2+ + 2 SO 42 - + 16 H+ • oxidation of additional pyrite (from steps 1 and 2) by Fe(III) -- here iron is the oxidizing agent, not oxygen • cyclic and self-propagating step

Chemistry of AMD (cont’d) Overall Reaction 4 Fe. S 2 + 15 O 2 + 14 H 2 O 4 Fe(OH)3 + 8 H 2 SO 4

Typical Case: Manila Creek, VA • Iron content: 567 mg/L, p. H: 3. 5, flow from mine of 42 GPM. • Wetlands were used to increase p. H. • p. H increased to 5. 1, iron contents reduced to 67 mg/L.

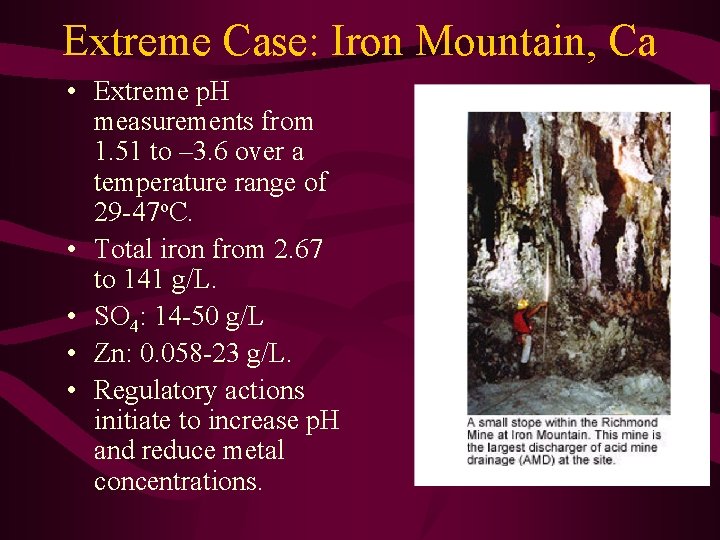
Extreme Case: Iron Mountain, Ca • Extreme p. H measurements from 1. 51 to – 3. 6 over a temperature range of 29 -47 o. C. • Total iron from 2. 67 to 141 g/L. • SO 4: 14 -50 g/L • Zn: 0. 058 -23 g/L. • Regulatory actions initiate to increase p. H and reduce metal concentrations.

Remediation • Use of acid generating rocks to segregate/blend waste. • Bacteria Desulfovibrio and Desulfotomaculum – SO 4 -2 + 2 CH 2 O = H 2 S + 2 HCO 3 - • Alkaline Materials (Ca. CO 3, Na. OH, Na. HCO 3, anhydrous ammonia). – Ca. CO 3 + H+ = Ca+2 + HCO 3 • Soil, clay, synthetic covers. • Chemical additives

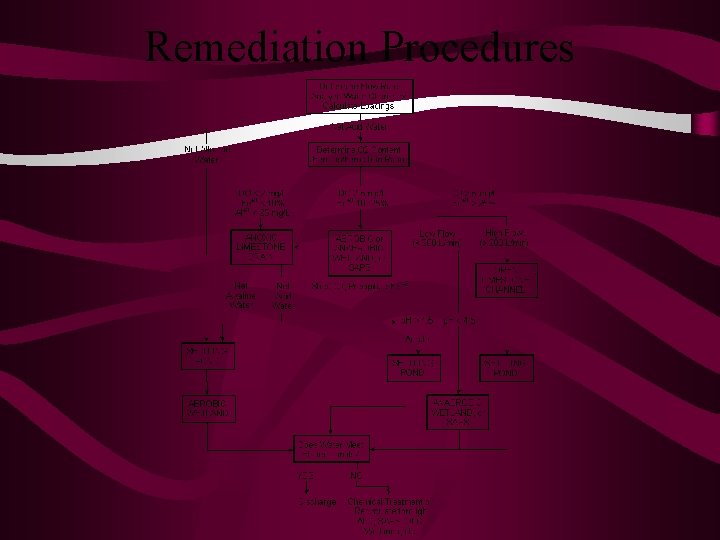
Remediation Procedures

Future/Ongoing Research • Prediction of acid generation – Acidbase accounting – Weathering tests – Computer models • Prevention/Mitigation – Rock phosphate to inhibit pyrite oxidation. – Coatings and sealant to inhibit acid production. – Improve time for bactericide leaching. – Encapsulation of pyrite material.

Conclusions • AMD is an environmental problem results from the oxidation of pyrite by bacteria air, and water. • Oxidation of pyrite decrease p. H and increase concentrations of dissolve metals in water. • The latter results in the pollution of water, which can be harmful for the environment and living species. • Several methods such as wetlands have been done to increase p. H and decrease metal concentrations in water. • AMD research continues in order to find better ways to mitigate pollution and reduce the overall effects in the environment such as global warming.
 Which of the following best describes acid mine drainage?
Which of the following best describes acid mine drainage? Alliteration in porphyria's lover
Alliteration in porphyria's lover Defect remediation
Defect remediation Network access quarantine control
Network access quarantine control Sox remediation software
Sox remediation software Ada remediation
Ada remediation Ckla assessment and remediation guide
Ckla assessment and remediation guide Dlp incident management process
Dlp incident management process Remediation plan in change lifecycle
Remediation plan in change lifecycle Remediation of the struggling medical learner
Remediation of the struggling medical learner Bolter grusin remediation
Bolter grusin remediation Mold removal somerset county
Mold removal somerset county Site remediation toronto
Site remediation toronto Formation initiale vs formation continue
Formation initiale vs formation continue Monoprotic acid
Monoprotic acid Non acid fast bacteria
Non acid fast bacteria 9-which acid is not considered a strong acid?
9-which acid is not considered a strong acid? Lewis acid bronsted acid
Lewis acid bronsted acid

































