Hadron Radiotherapy Pavel Kundrt Institute of Physics Academy








- Slides: 30

Hadron Radiotherapy Pavel Kundrát Institute of Physics, Academy of Sciences of the Czech Republic, Prague Pavel. Kundrat@fzu. cz

Outline l l Introduction Hadron radiotherapy l l Principles Technical requirements Existing centres Treatment planning, mathematical modelling

Introduction l Cancer: l l l 2 nd major cause of death A form of cancer is diagnosed to every 3 rd person Czech Rep. : 60 000 new tumours/year (10 mil. inhabitants) Cure rate: 45 – 50% only Surgery, radiotherapy , Surgery, chemotherapy Strategies: l l l Early detection, improved diagnostics Improved local treatment Improved systemic treatments

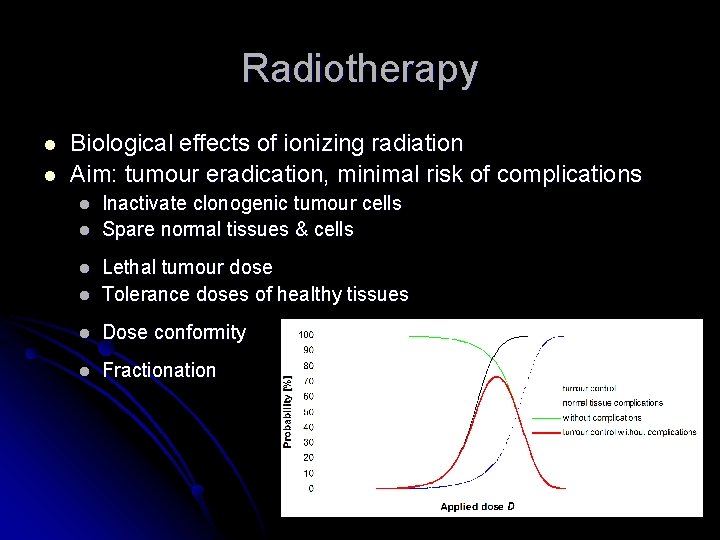
Radiotherapy l l Biological effects of ionizing radiation Aim: tumour eradication, minimal risk of complications l l Inactivate clonogenic tumour cells Spare normal tissues & cells l Lethal tumour dose Tolerance doses of healthy tissues l Dose conformity l Fractionation l

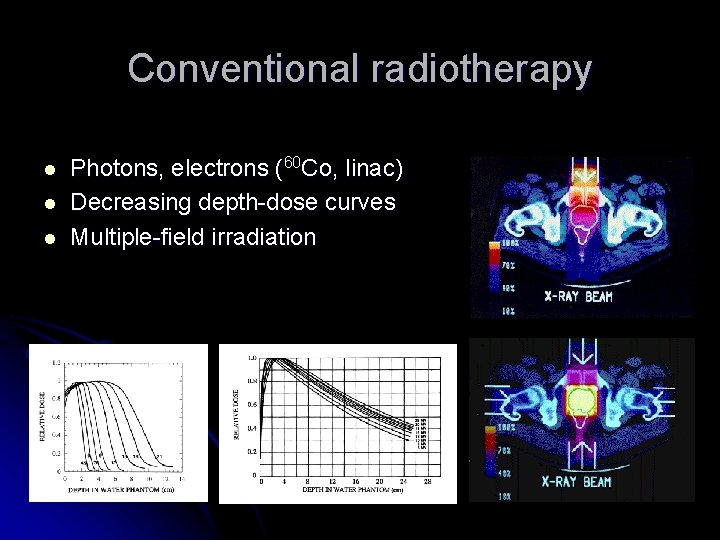
Conventional radiotherapy l l l Photons, electrons (60 Co, linac) Decreasing depth-dose curves Multiple-field irradiation

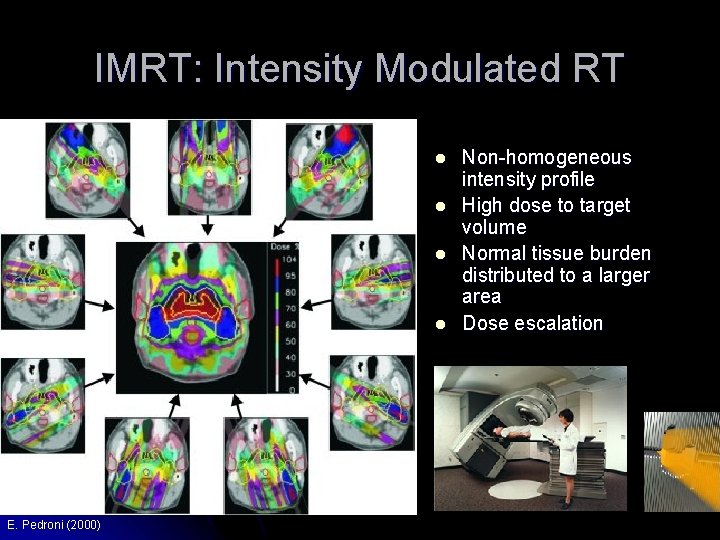
IMRT: Intensity Modulated RT l l E. Pedroni (2000) Non-homogeneous intensity profile High dose to target volume Normal tissue burden distributed to a larger area Dose escalation

Hadron radiotherapy

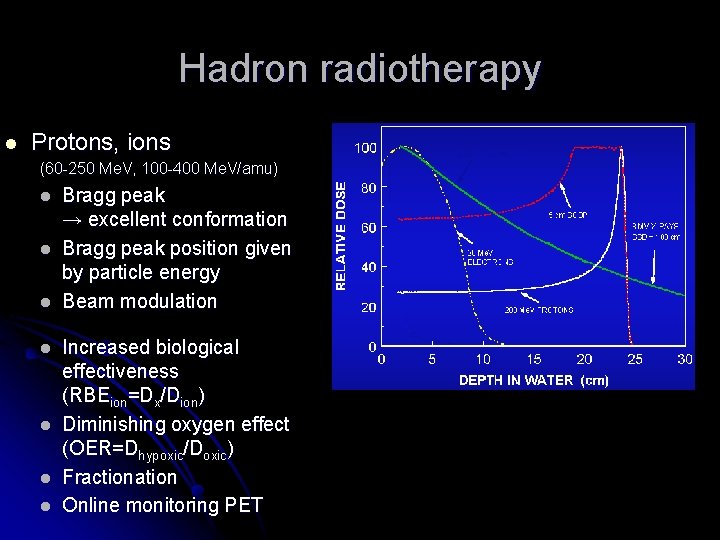
Hadron radiotherapy l Protons, ions (60 -250 Me. V, 100 -400 Me. V/amu) l l l l Bragg peak → excellent conformation Bragg peak position given by particle energy Beam modulation Increased biological effectiveness (RBEion=Dx/Dion) Diminishing oxygen effect (OER=Dhypoxic/Doxic) Fractionation Online monitoring PET

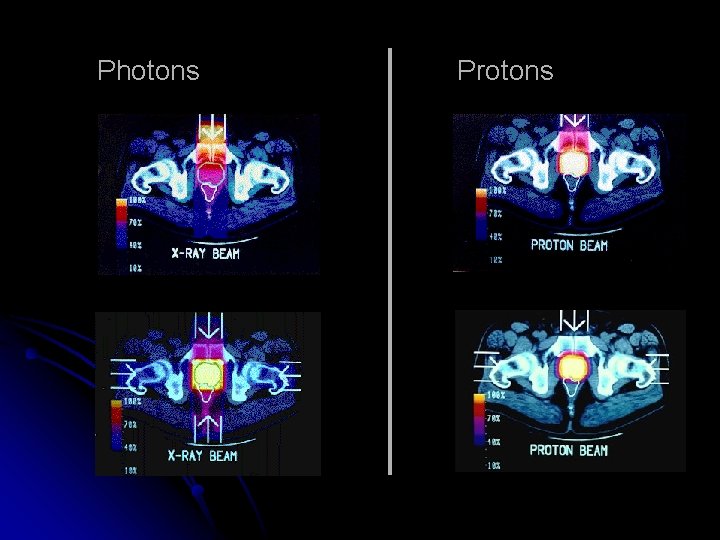
Photons Protons

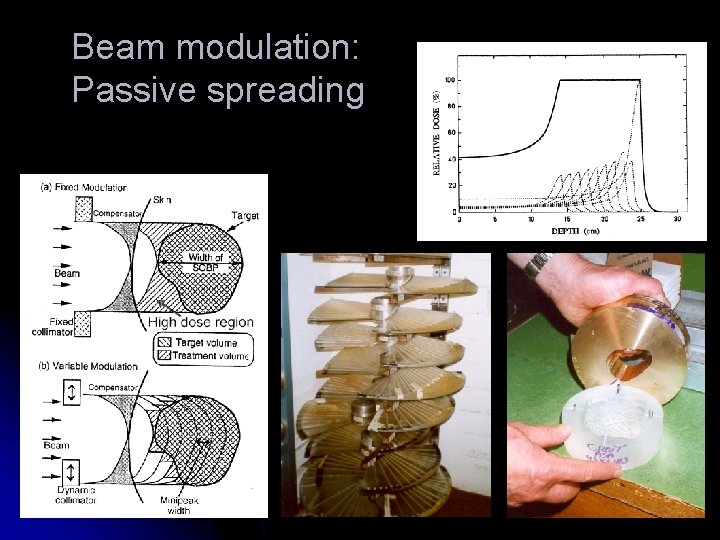
Beam modulation: Passive spreading

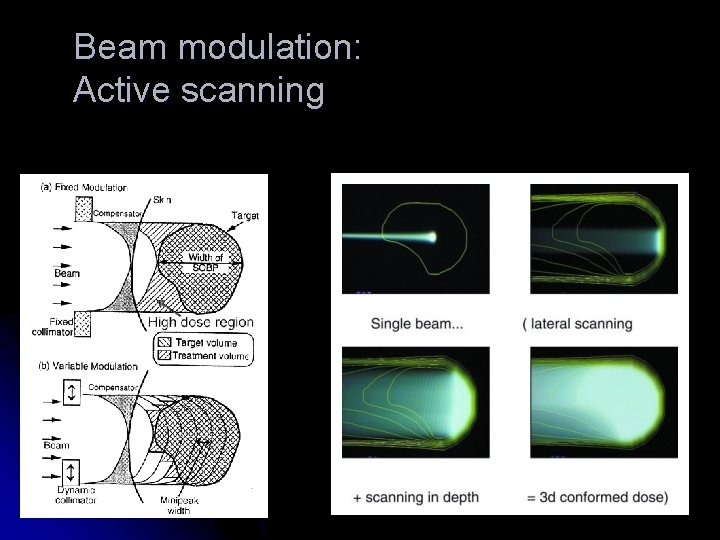
Beam modulation: Active scanning

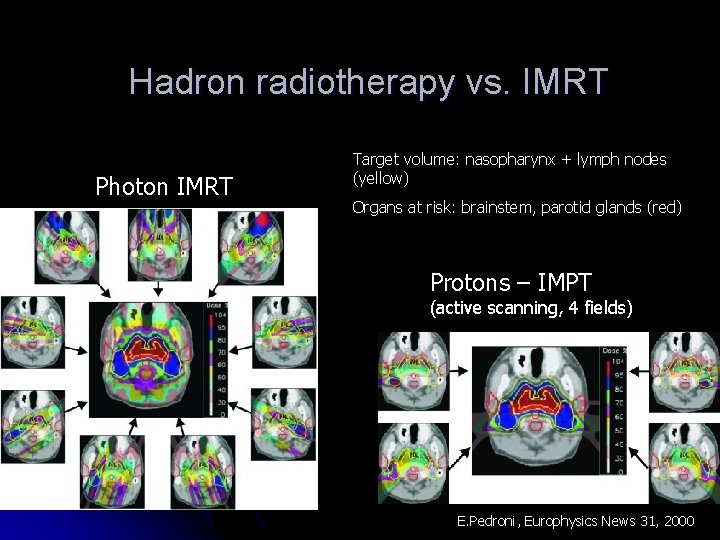
Hadron radiotherapy vs. IMRT Photon IMRT Target volume: nasopharynx + lymph nodes (yellow) Organs at risk: brainstem, parotid glands (red) Protons – IMPT (active scanning, 4 fields) E. Pedroni, Europhysics News 31, 2000

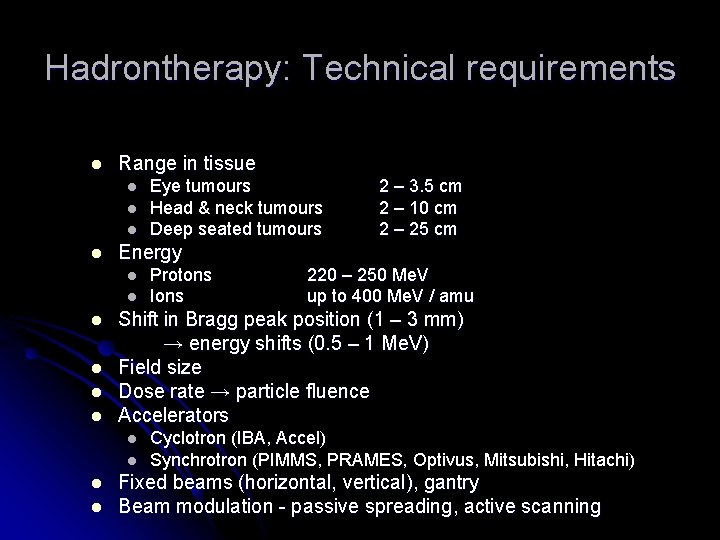
Hadrontherapy: Technical requirements l Range in tissue l l l l l Protons Ions 220 – 250 Me. V up to 400 Me. V / amu Shift in Bragg peak position (1 – 3 mm) → energy shifts (0. 5 – 1 Me. V) Field size Dose rate → particle fluence Accelerators l l 2 – 3. 5 cm 2 – 10 cm 2 – 25 cm Energy l l Eye tumours Head & neck tumours Deep seated tumours Cyclotron (IBA, Accel) Synchrotron (PIMMS, PRAMES, Optivus, Mitsubishi, Hitachi) Fixed beams (horizontal, vertical), gantry Beam modulation - passive spreading, active scanning

Hadrontherapy worldwide l Nuclear physics centres l head & neck tumours eye tumours NSCLC lung cancer prostate cancer Berkeley, Harvard (USA), Dubna, Moscow (Russia), PSI (Switzerland), Nice, Orsay (France) → medical facilities Loma Linda (CA, USA) 1991 HIMAC Chiba (Japan) 1994 NCC Kashiwa (Japan) 1998 NPTC Boston (USA) 2001 Wan Jie PTC (China) 2004 RPTC Munich (Germany) 2006 l l l Approx. 25 centres + 20 in planning (USA, Europe, Japan) Almost 50000 patients (protons + ions) Potential patients: 1000 -3000 per year / 10 mil. inhabitants Main indications: l Clinical results: 5 yrs. local control photon vs. hadron RT chordomas 17 -50% vs. 73 -83% chondrosarcomas 50 -60% vs. 90 -98% l Costs: initial costs 70 -120 mil. € (1000 pt/y) about 20 000 € / patient approx. 2 -3 x more expensive than photon RT, about the same as surgery, by far less expensive than chemotherapy l Particle Therapy Co-Operative Group (PTCOG) http: //ptcog. web. psi. ch/

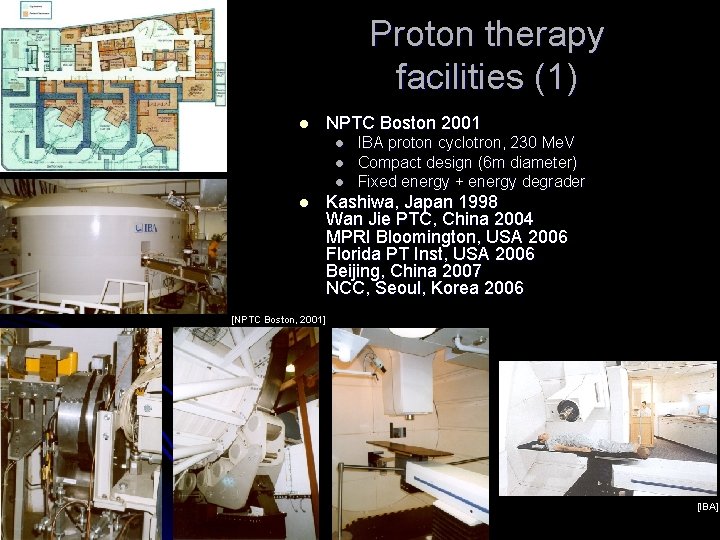
Proton therapy facilities (1) l NPTC Boston 2001 l l IBA proton cyclotron, 230 Me. V Compact design (6 m diameter) Fixed energy + energy degrader Kashiwa, Japan 1998 Wan Jie PTC, China 2004 MPRI Bloomington, USA 2006 Florida PT Inst, USA 2006 Beijing, China 2007 NCC, Seoul, Korea 2006 [NPTC Boston, 2001] [IBA]

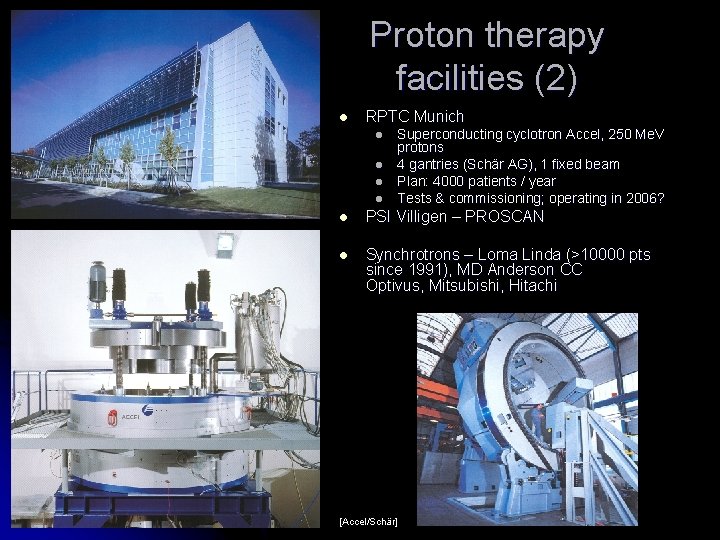
Proton therapy facilities (2) l RPTC Munich l l Superconducting cyclotron Accel, 250 Me. V protons 4 gantries (Schär AG), 1 fixed beam Plan: 4000 patients / year Tests & commissioning; operating in 2006? l PSI Villigen – PROSCAN l Synchrotrons – Loma Linda (>10000 pts since 1991), MD Anderson CC Optivus, Mitsubishi, Hitachi [Accel/Schär]

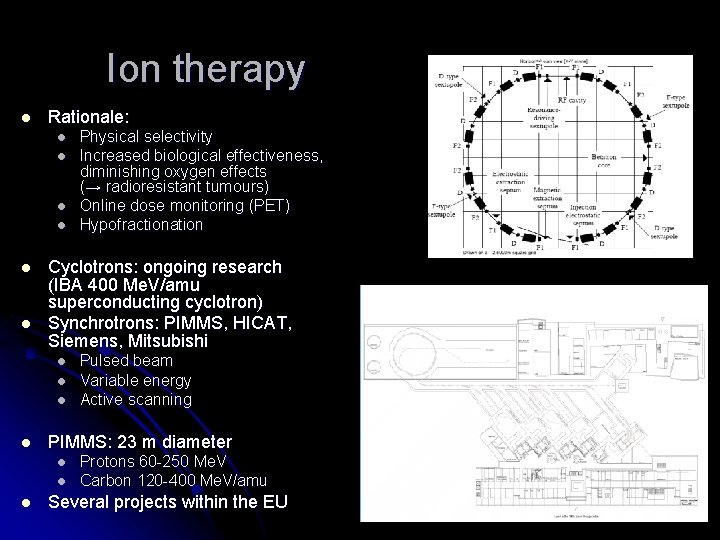
Ion therapy l Rationale: l l l Cyclotrons: ongoing research (IBA 400 Me. V/amu superconducting cyclotron) Synchrotrons: PIMMS, HICAT, Siemens, Mitsubishi l l Pulsed beam Variable energy Active scanning PIMMS: 23 m diameter l l l Physical selectivity Increased biological effectiveness, diminishing oxygen effects (→ radioresistant tumours) Online dose monitoring (PET) Hypofractionation Protons 60 -250 Me. V Carbon 120 -400 Me. V/amu Several projects within the EU

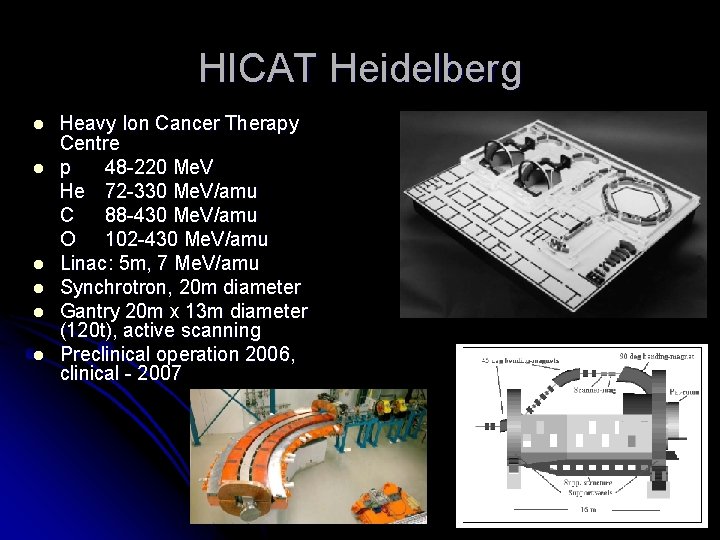
HICAT Heidelberg l l l Heavy Ion Cancer Therapy Centre p 48 -220 Me. V He 72 -330 Me. V/amu C 88 -430 Me. V/amu O 102 -430 Me. V/amu Linac: 5 m, 7 Me. V/amu Synchrotron, 20 m diameter Gantry 20 m x 13 m diameter (120 t), active scanning Preclinical operation 2006, clinical - 2007

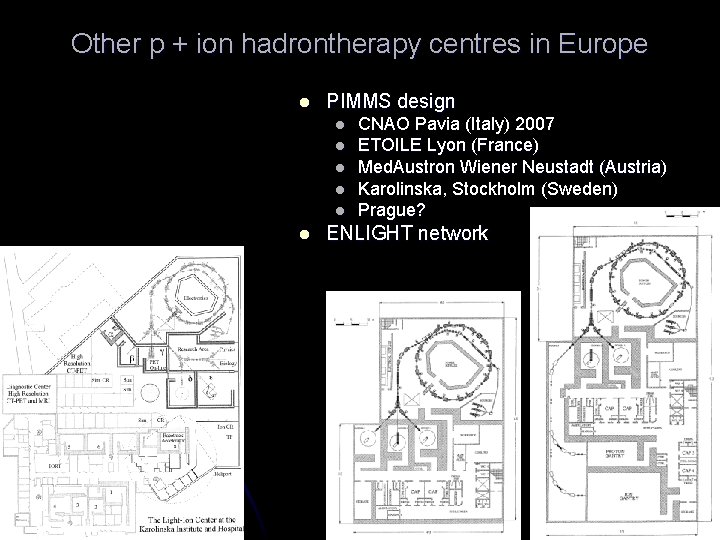
Other p + ion hadrontherapy centres in Europe l PIMMS design l l l CNAO Pavia (Italy) 2007 ETOILE Lyon (France) Med. Austron Wiener Neustadt (Austria) Karolinska, Stockholm (Sweden) Prague? ENLIGHT network

ENLIGHT l European Network for Light Ion Hadron Therapy, European Commission, QLG 1 -CT-2002 -01574, 2002 -2005 ESTRO, CERN, EORTC, GSI Darmstadt, DKFZ Heidelberg, GHIP Heidelberg, TERA, Karolinska Institutet, ETOILE Project, Med-Austron, FZR Rossendorf, Linköping University, Hospital Virgen de la Macarena, Charles University in Prague l Workpackages: l l Epidemiology and patient selection Design and conduct of clinical trials Preparation, delivery and dosimetry of ion beams l l l l Preparation of Ion Beams Dosimetry of Ion Beams Treatment Planning Accelerator Technology Radiation biology In-situ monitoring with positron emission tomography Health-Economic Assessment ENLIGHT++ proposal (2006)

Summary – Hadron therapy l l l Excellent dose conformity Proven clinical benefits in several tumours/locations Approx. 1000 – 3000 pts/year/10 mil. inhabitants would benefit from HT Ion therapy: radioresistant tumours Costs: initial ~ 70 -120 M€, per pt ~ 20 k€

Biological effects of ionizing radiation: Mechanism and its modelling

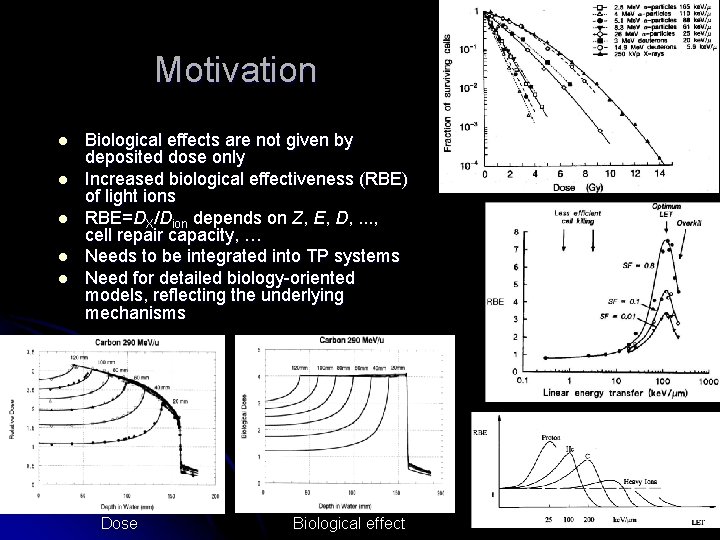
Motivation l l l Biological effects are not given by deposited dose only Increased biological effectiveness (RBE) of light ions RBE=DX/Dion depends on Z, E, D, . . . , cell repair capacity, … Needs to be integrated into TP systems Need for detailed biology-oriented models, reflecting the underlying mechanisms Dose Biological effect

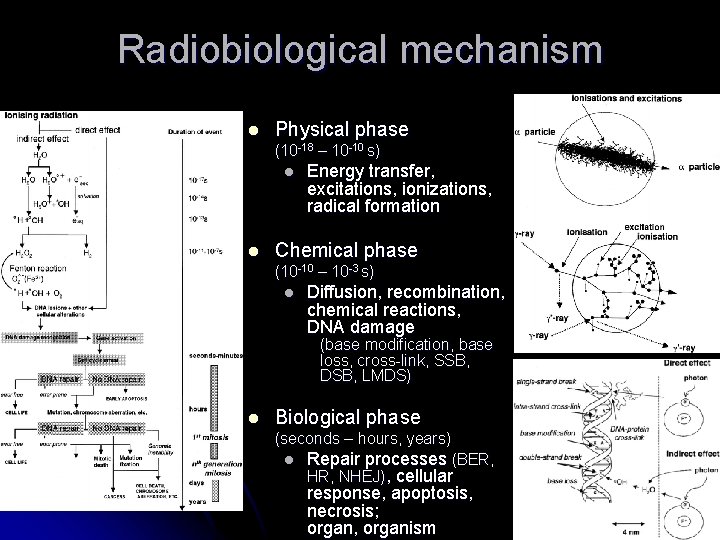
Radiobiological mechanism l Physical phase (10 -18 – 10 -10 s) l l Energy transfer, excitations, ionizations, radical formation Chemical phase (10 -10 – 10 -3 s) l Diffusion, recombination, chemical reactions, DNA damage (base modification, base loss, cross-link, SSB, DSB, LMDS) l Biological phase (seconds – hours, years) l Repair processes (BER, HR, NHEJ), cellular response, apoptosis, necrosis; organ, organism

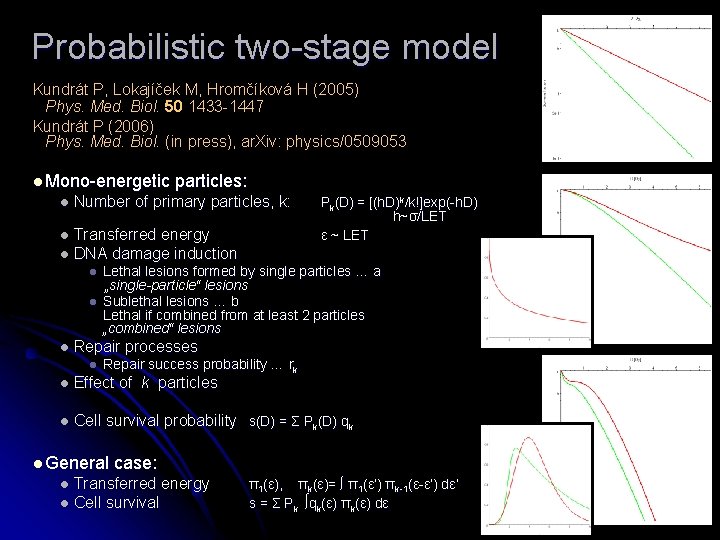
Probabilistic two-stage model Kundrát P, Lokajíček M, Hromčíková H (2005) Phys. Med. Biol. 50 1433 -1447 Kundrát P (2006) Phys. Med. Biol. (in press), ar. Xiv: physics/0509053 l Mono-energetic particles: l Number of primary particles, k: l l Transferred energy DNA damage induction l l l Pk(D) = [(h. D)k/k!]exp(-h. D) h~σ/LET ε ~ LET Lethal lesions formed by single particles … a „single-particle“ lesions Sublethal lesions … b Lethal if combined from at least 2 particles „combined“ lesions Repair processes l Repair success probability … rk Effect of k particles l Cell survival probability s(D) = Σ Pk(D) qk l l General case: l l Transferred energy Cell survival π1(ε), πk(ε)= ∫ π1(ε’) πk-1(ε-ε’) dε’ s = Σ Pk ∫qk(ε) πk(ε) dε

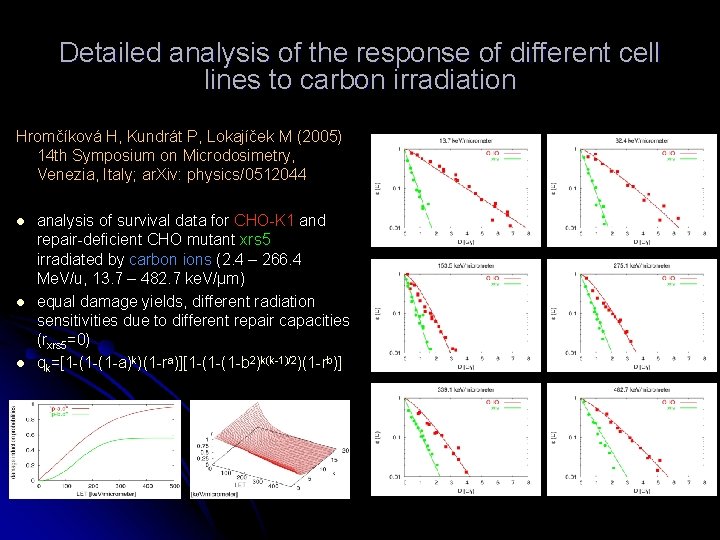
Detailed analysis of the response of different cell lines to carbon irradiation Hromčíková H, Kundrát P, Lokajíček M (2005) 14 th Symposium on Microdosimetry, Venezia, Italy; ar. Xiv: physics/0512044 l l l analysis of survival data for CHO-K 1 and repair-deficient CHO mutant xrs 5 irradiated by carbon ions (2. 4 – 266. 4 Me. V/u, 13. 7 – 482. 7 ke. V/μm) equal damage yields, different radiation sensitivities due to different repair capacities (rxrs 5=0) qk=[1 -(1 -(1 -a)k)(1 -ra)][1 -(1 -(1 -b 2)k(k-1)/2)(1 -rb)]

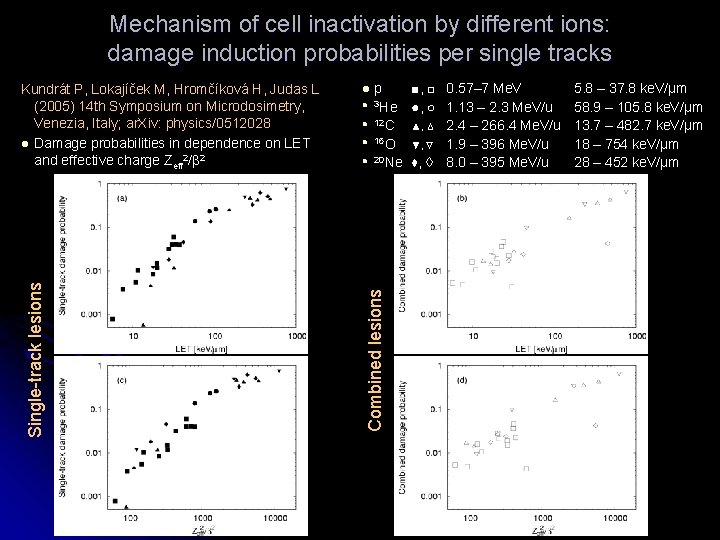
Mechanism of cell inactivation by different ions: damage induction probabilities per single tracks l l l p ■, □ 0. 57– 7 Me. V 3 He ●, ○ 1. 13 – 2. 3 Me. V/u 12 C ▲, Δ 2. 4 – 266. 4 Me. V/u 16 O ▼, 1. 9 – 396 Me. V/u 20 Ne ♦, 8. 0 – 395 Me. V/u Combined lesions Single-track lesions Kundrát P, Lokajíček M, Hromčíková H, Judas L (2005) 14 th Symposium on Microdosimetry, Venezia, Italy; ar. Xiv: physics/0512028 l Damage probabilities in dependence on LET and effective charge Zeff 2/β 2 5. 8 – 37. 8 ke. V/μm 58. 9 – 105. 8 ke. V/μm 13. 7 – 482. 7 ke. V/μm 18 – 754 ke. V/μm 28 – 452 ke. V/μm

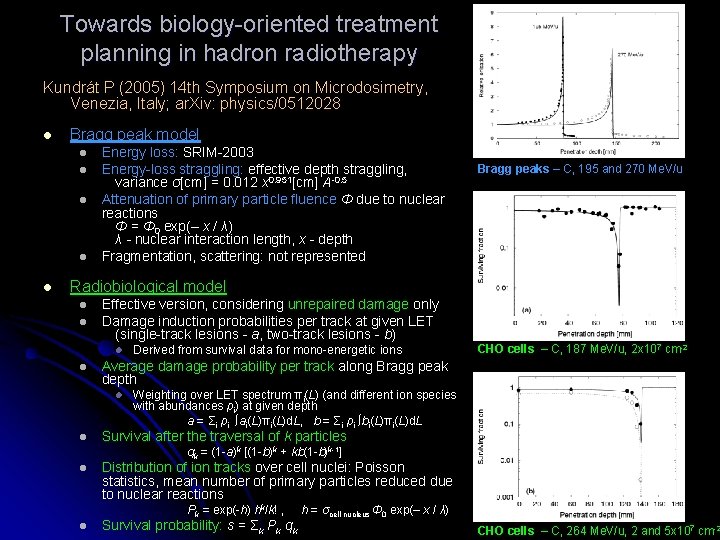
Towards biology-oriented treatment planning in hadron radiotherapy Kundrát P (2005) 14 th Symposium on Microdosimetry, Venezia, Italy; ar. Xiv: physics/0512028 l Bragg peak model l l Energy loss: SRIM-2003 Energy-loss straggling: effective depth straggling, variance σ[cm] = 0. 012 x 0. 951[cm] A-0. 5 Attenuation of primary particle fluence Φ due to nuclear reactions Φ = Φ 0 exp(– x / λ) λ - nuclear interaction length, x - depth Fragmentation, scattering: not represented Bragg peaks – C, 195 and 270 Me. V/u Radiobiological model l l Effective version, considering unrepaired damage only Damage induction probabilities per track at given LET (single-track lesions - a, two-track lesions - b) l l CHO cells – C, 187 Me. V/u, 2 x 107 cm-2 Average damage probability per track along Bragg peak depth l l Derived from survival data for mono-energetic ions Weighting over LET spectrum πi(L) (and different ion species with abundances ρi) at given depth a = Σi ρi ∫ai(L)πi(L)d. L, b = Σi ρi ∫bi(L)πi(L)d. L Survival after the traversal of k particles qk = (1 -a)k [(1 -b)k + kb(1 -b)k-1] l Distribution of ion tracks over cell nuclei: Poisson statistics, mean number of primary particles reduced due to nuclear reactions Pk = exp(-h) hk/k! , h = σcell nucleus Φ 0 exp(– x / λ) l Survival probability: s = Σk Pk qk CHO cells – C, 264 Me. V/u, 2 and 5 x 107 cm-2

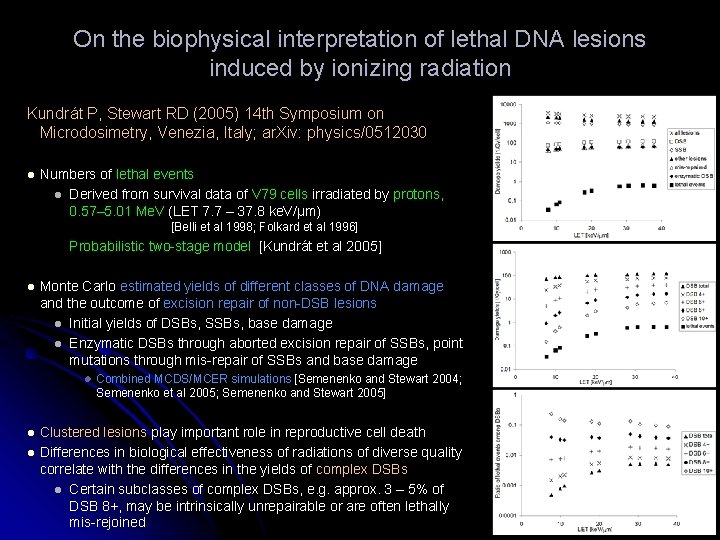
On the biophysical interpretation of lethal DNA lesions induced by ionizing radiation Kundrát P, Stewart RD (2005) 14 th Symposium on Microdosimetry, Venezia, Italy; ar. Xiv: physics/0512030 l Numbers of lethal events l Derived from survival data of V 79 cells irradiated by protons, 0. 57– 5. 01 Me. V (LET 7. 7 – 37. 8 ke. V/µm) [Belli et al 1998; Folkard et al 1996] Probabilistic two-stage model [Kundrát et al 2005] l Monte Carlo estimated yields of different classes of DNA damage and the outcome of excision repair of non-DSB lesions l Initial yields of DSBs, SSBs, base damage l Enzymatic DSBs through aborted excision repair of SSBs, point mutations through mis-repair of SSBs and base damage l l l Combined MCDS/MCER simulations [Semenenko and Stewart 2004; Semenenko et al 2005; Semenenko and Stewart 2005] Clustered lesions play important role in reproductive cell death Differences in biological effectiveness of radiations of diverse quality correlate with the differences in the yields of complex DSBs l Certain subclasses of complex DSBs, e. g. approx. 3 – 5% of DSB 8+, may be intrinsically unrepairable or are often lethally mis-rejoined

Summary – Modelling l P 2 S model l l Biology-oriented model DNA damage & repair Systematic description of survival curves → TCP, NTCP models Future work l l l Relate damage induction to track structure Interpretation of lethal events TCP, NTCP models Pavel. Kundrat@fzu. cz
 Kundrt
Kundrt Mayneord factor equation
Mayneord factor equation Ssd and sad technique in radiotherapy
Ssd and sad technique in radiotherapy Radiotherapy
Radiotherapy Pterigiums
Pterigiums Radiotherapy
Radiotherapy Siemens radiotherapy
Siemens radiotherapy Hadron calorimeter
Hadron calorimeter Hadron collider
Hadron collider Hadron collider
Hadron collider Hadron
Hadron Hadron
Hadron Nuclear safety institute of the russian academy of sciences
Nuclear safety institute of the russian academy of sciences Skobeltsyn institute of nuclear physics
Skobeltsyn institute of nuclear physics Budker institute of nuclear physics
Budker institute of nuclear physics Petersburg nuclear physics institute
Petersburg nuclear physics institute Kirchhoff institute for physics
Kirchhoff institute for physics Aip style
Aip style Institute of physics ascr
Institute of physics ascr Budker institute of nuclear physics
Budker institute of nuclear physics Electromagnetism khan academy
Electromagnetism khan academy Pavel pablo gregorić
Pavel pablo gregorić Pavel buividovich
Pavel buividovich Pavel batista
Pavel batista Pavel pudlak
Pavel pudlak Lidl platba stravenkami
Lidl platba stravenkami Pavel brych
Pavel brych Pavel motloch
Pavel motloch Pavel sonnenschein
Pavel sonnenschein A treia calatorie misionara a lui pavel
A treia calatorie misionara a lui pavel Mgr. pavel pražák
Mgr. pavel pražák