Glycolysis II 110509 Front half of glycolysis Aldolase









- Slides: 26

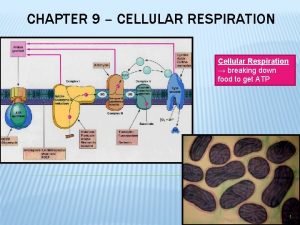
Glycolysis II 11/05/09


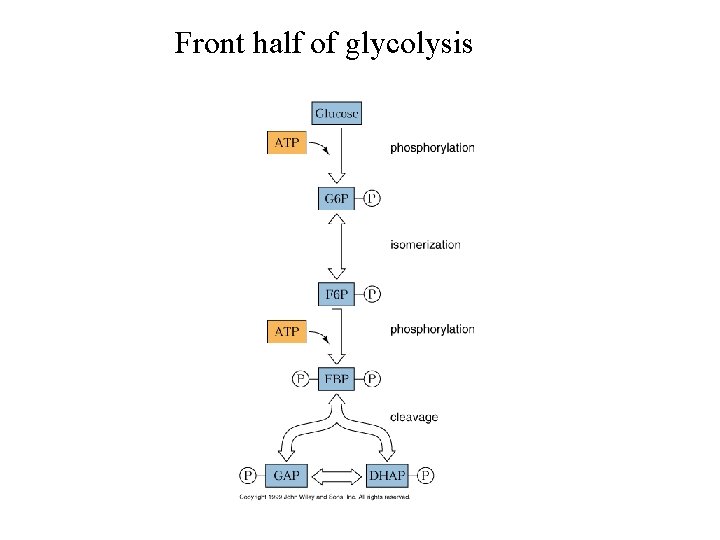
Front half of glycolysis

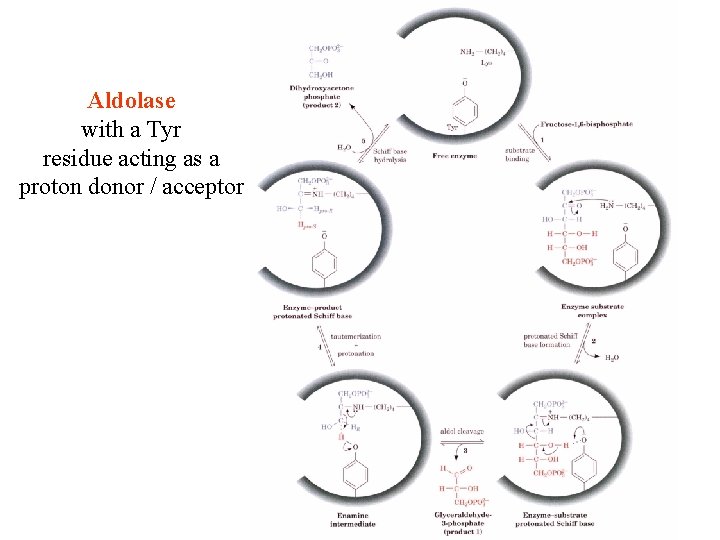
Aldolase with a Tyr residue acting as a proton donor / acceptor

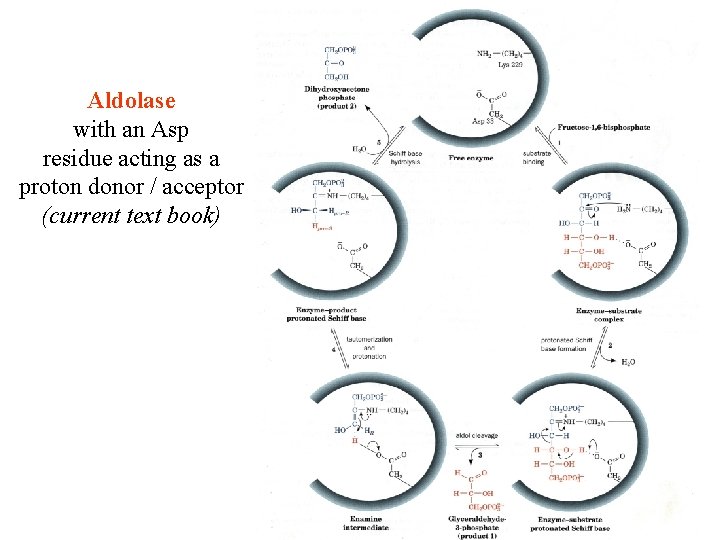
Aldolase with an Asp residue acting as a proton donor / acceptor (current text book)

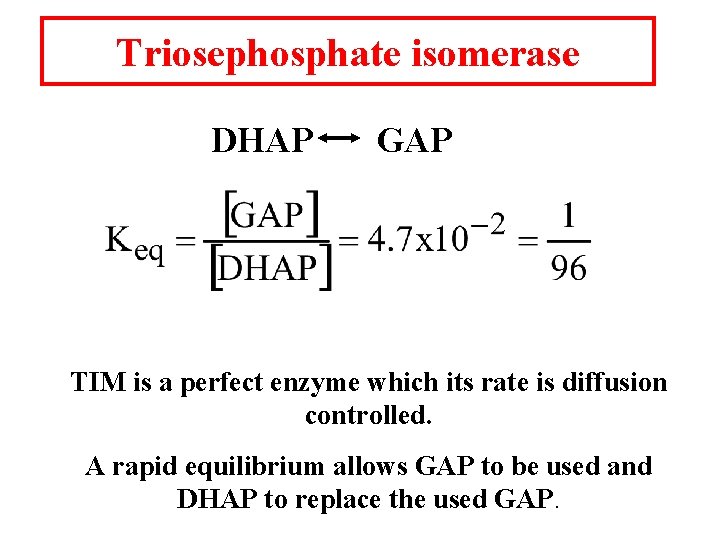
Triosephosphate isomerase DHAP GAP TIM is a perfect enzyme which its rate is diffusion controlled. A rapid equilibrium allows GAP to be used and DHAP to replace the used GAP.

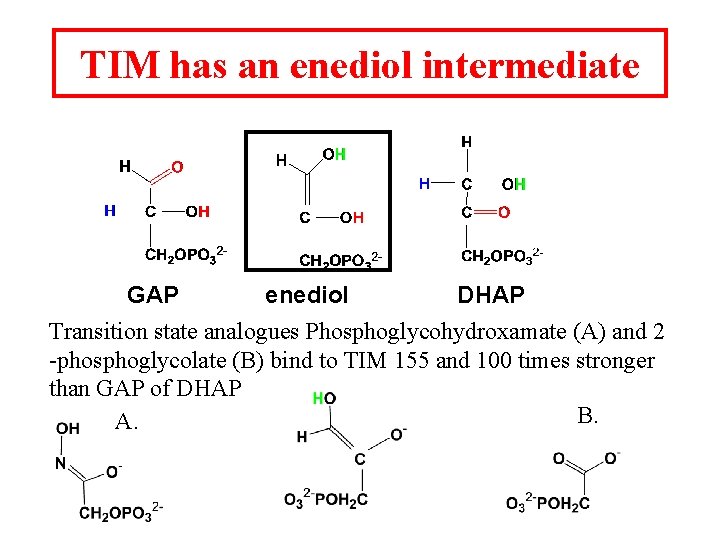
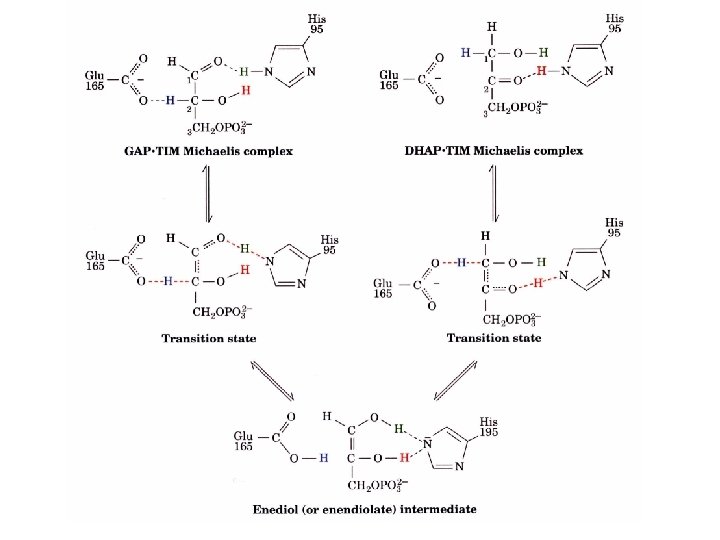
TIM has an enediol intermediate GAP enediol DHAP Transition state analogues Phosphoglycohydroxamate (A) and 2 -phosphoglycolate (B) bind to TIM 155 and 100 times stronger than GAP of DHAP B. A.

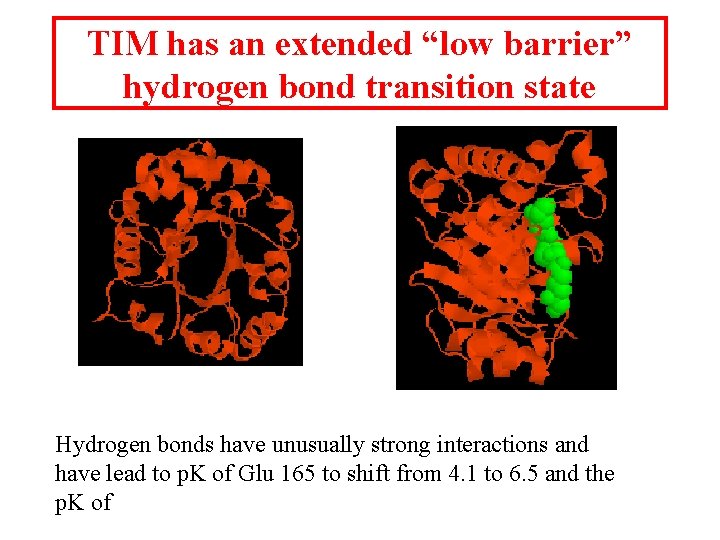
TIM has an extended “low barrier” hydrogen bond transition state Hydrogen bonds have unusually strong interactions and have lead to p. K of Glu 165 to shift from 4. 1 to 6. 5 and the p. K of


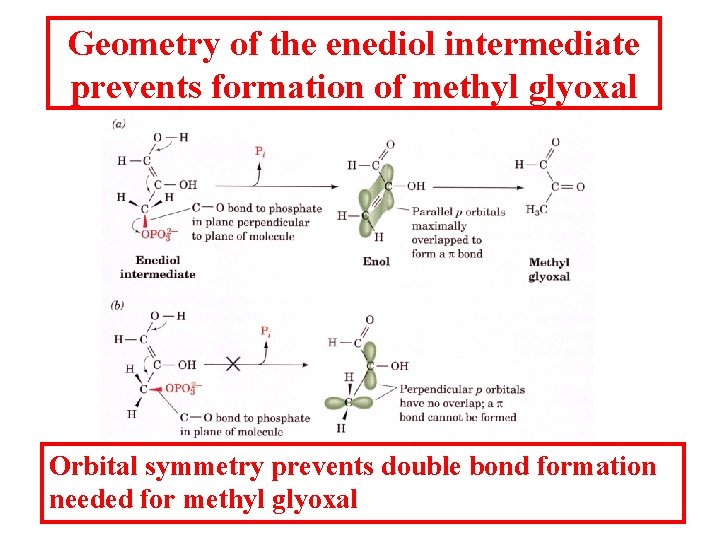
Geometry of the enediol intermediate prevents formation of methyl glyoxal Orbital symmetry prevents double bond formation needed for methyl glyoxal

The second half of glycolysis

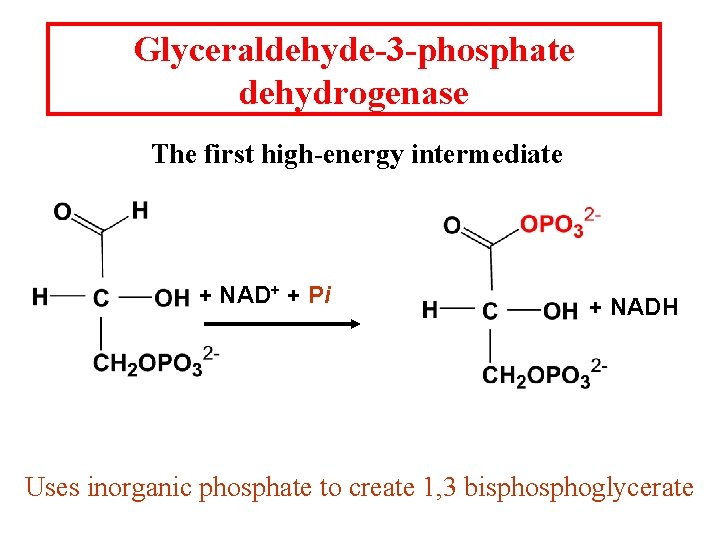
Glyceraldehyde-3 -phosphate dehydrogenase The first high-energy intermediate + NAD+ + Pi + NADH Uses inorganic phosphate to create 1, 3 bisphoglycerate

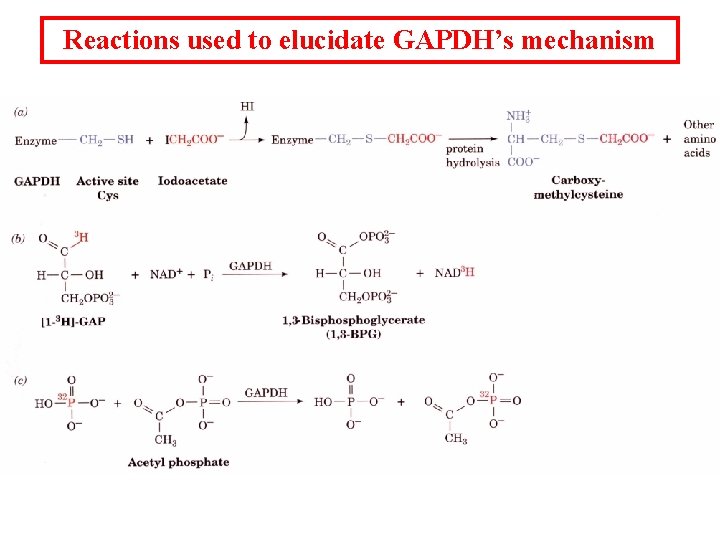
Reactions used to elucidate GAPDH’s mechanism


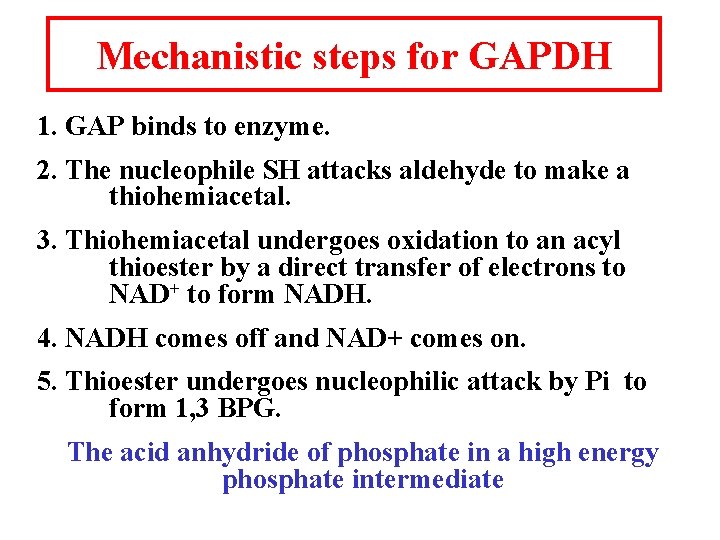
Mechanistic steps for GAPDH 1. GAP binds to enzyme. 2. The nucleophile SH attacks aldehyde to make a thiohemiacetal. 3. Thiohemiacetal undergoes oxidation to an acyl thioester by a direct transfer of electrons to NAD+ to form NADH. 4. NADH comes off and NAD+ comes on. 5. Thioester undergoes nucleophilic attack by Pi to form 1, 3 BPG. The acid anhydride of phosphate in a high energy phosphate intermediate

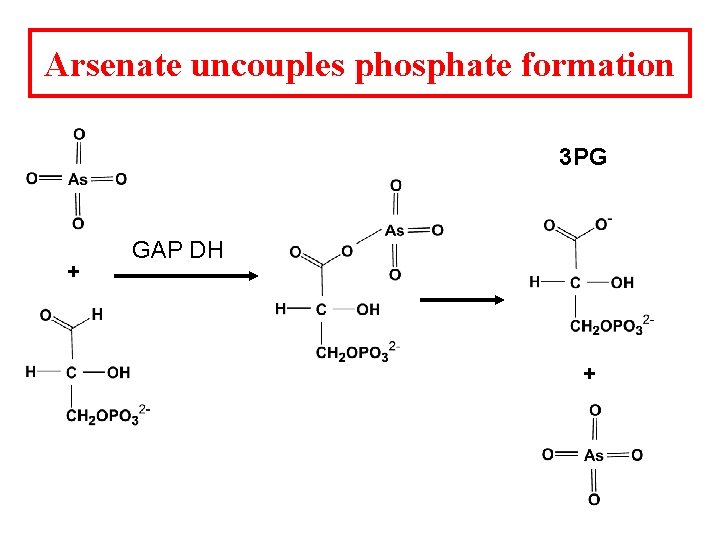
Arsenate uncouples phosphate formation 3 PG + GAP DH +

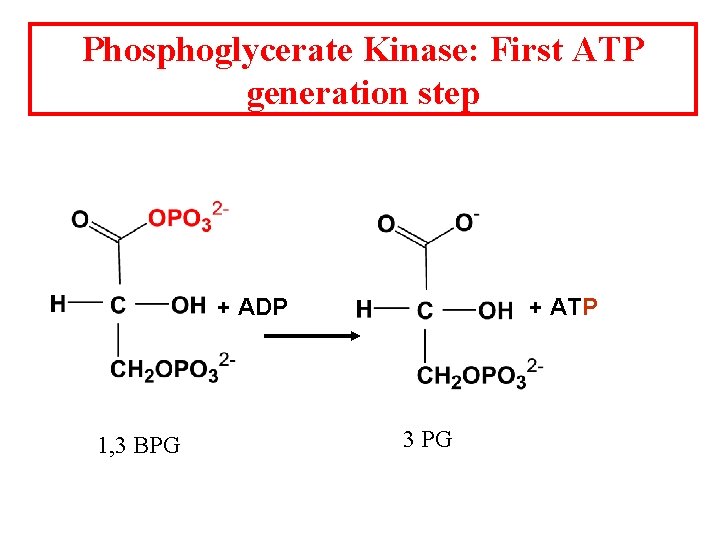
Phosphoglycerate Kinase: First ATP generation step + ADP 1, 3 BPG + ATP 3 PG

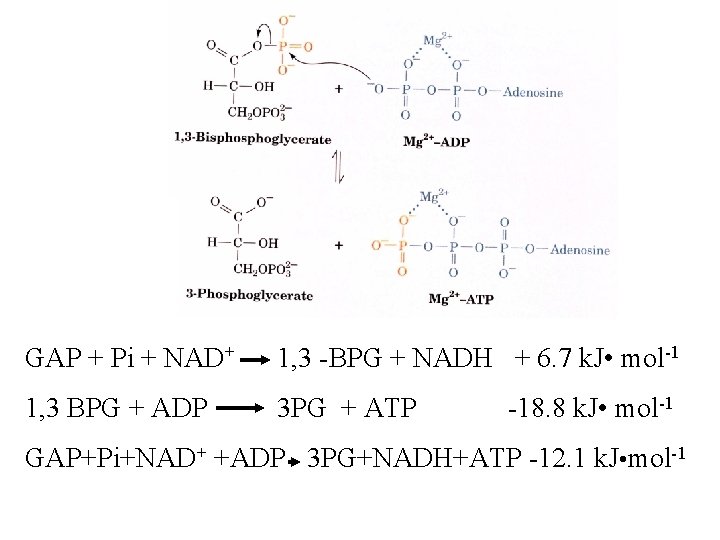
GAP + Pi + NAD+ 1, 3 -BPG + NADH + 6. 7 k. J • mol-1 1, 3 BPG + ADP 3 PG + ATP -18. 8 k. J • mol-1 GAP+Pi+NAD+ +ADP 3 PG+NADH+ATP -12. 1 k. J • mol-1

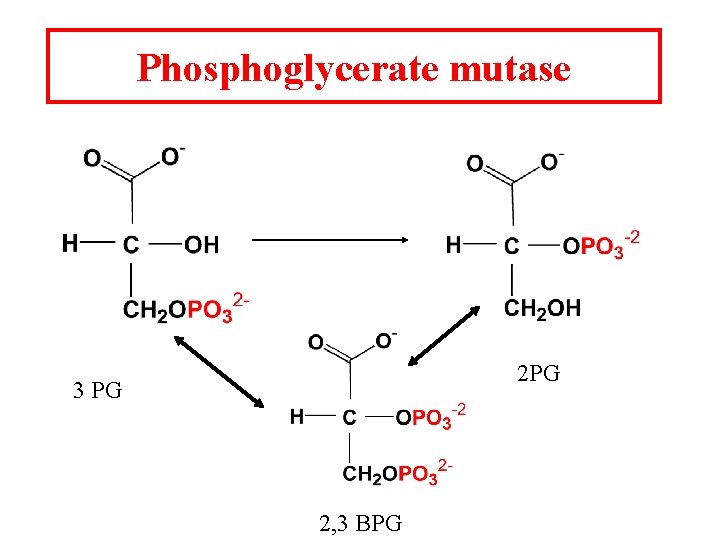
Phosphoglycerate mutase 2 PG 3 PG 2, 3 BPG

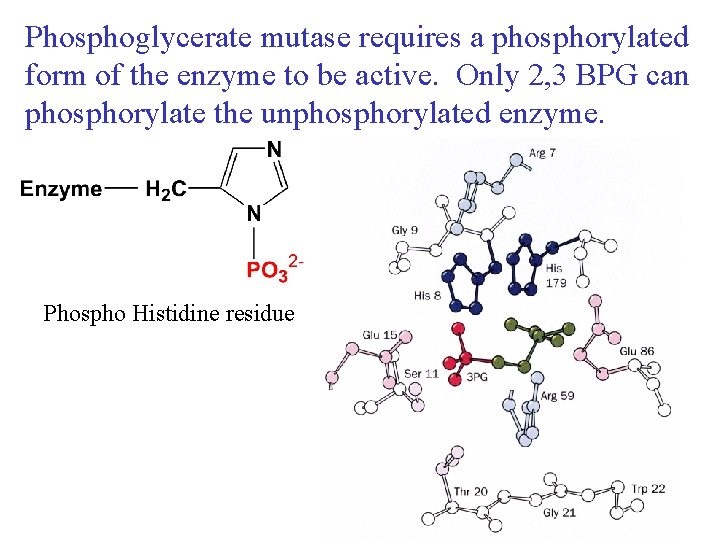
Phosphoglycerate mutase requires a phosphorylated form of the enzyme to be active. Only 2, 3 BPG can phosphorylate the unphosphorylated enzyme. Phospho Histidine residue


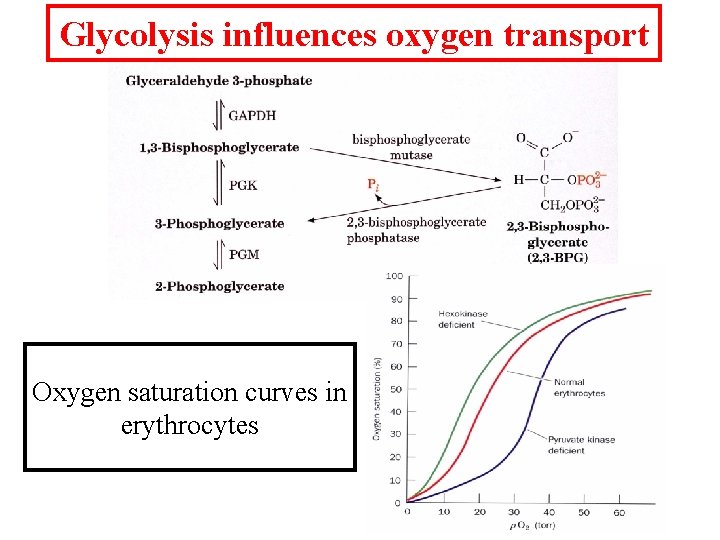
Glycolysis influences oxygen transport Oxygen saturation curves in erythrocytes

Enolase generation of a second “high energy” intermediate + H 2 O 2 Phosphoglycerate Phosphoenol pyruvate


Pyruvate kinase: Second ATP generation step

Next Lecture Tuesday 11/10/09 Glycolysis III
 Aldolase function in glycolysis
Aldolase function in glycolysis Mulciber roman god
Mulciber roman god Half empty or half full
Half empty or half full Half man half horse name
Half man half horse name Clasp classification
Clasp classification A pair of tickets questions and answers
A pair of tickets questions and answers Gerald croft
Gerald croft Half woman half snake greek mythology
Half woman half snake greek mythology Tasklet_hi_schedule
Tasklet_hi_schedule Retainer partial denture
Retainer partial denture Delphi
Delphi Narnia half geit
Narnia half geit Mythological creature half man half horse
Mythological creature half man half horse A picture of a warm front
A picture of a warm front Cover page of school magazine
Cover page of school magazine Dead front vs live front transformer
Dead front vs live front transformer Investment phase of glycolysis
Investment phase of glycolysis Glycolysis
Glycolysis Glycolysis phases
Glycolysis phases Amp glycolysis
Amp glycolysis Defination of atp
Defination of atp Insulin and glycolysis
Insulin and glycolysis Glucosis
Glucosis Glycolysis ib biology
Glycolysis ib biology Glycolysis in muscle cells
Glycolysis in muscle cells Krebs cycle atp yield
Krebs cycle atp yield Glycolysis atp yield
Glycolysis atp yield