Glargine Lantus 1562007 Dr HK Pang Endocrinologist Endocrine









- Slides: 15

Glargine (Lantus®) 15/6/2007. Dr. HK Pang. Endocrinologist, Endocrine team. Department of medicine. PYNEH

What is insulin Glargine (Lantus®) • A type of modified insulin. 1. Glargine (Lantus®) 2. Detemir ( Levemir®) – with property of the “Ideal” Long-Acting Insulin.

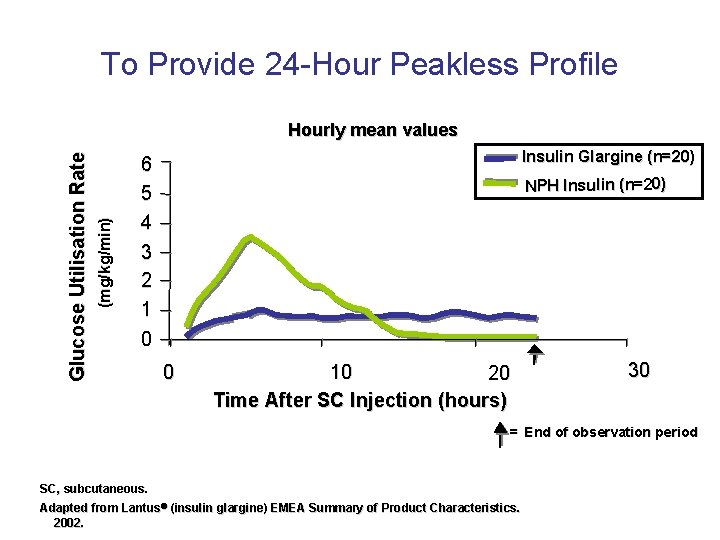
What is the “Ideal” Long-Acting Insulin? • Should have the property of – 1 injection daily 24 hours basal insulin coverage. – No peaks (peakless) provide an ideal, constant level of basal insulin – mimicks the physiological basal insulin level.

“Older” generation of insulin with peak, and cannot last for 24 hrs……

To Provide 24 -Hour Peakless Profile (mg/kg/min) Glucose Utilisation Rate Hourly mean values Insulin Glargine (n=20) 6 5 4 3 2 1 0 NPH Insulin (n=20) 0 10 20 Time After SC Injection (hours) 30 = End of observation period SC, subcutaneous. Adapted from Lantus (insulin glargine) EMEA Summary of Product Characteristics. 2002.

1. Insulin Glargine (Lantus®)

Glargine (Lantus®) • Has the property of an “ideal” basal insulin – A recombinant human insulin analogue 1 • A basal (long-acting) insulin 1 • Relatively constant peakless profile over 24 hours 1, 2 • Once-daily SC administration 1 1. Lantus® (insulin glargine) EMEA Summary of Product Characteristics. 2002. ® 2. Lantus receives European approval for pediatric use. Aventis Pharma Web site. Available at: http: //www. aventis. no/nyheter/nyheter_lantus_eu_approval_pediatric. shtml. Accessed March 19, 2003.

Glargine (Lantus®) • Modification of the insulin molecule makes it acquires the property of the “ ideal”. …

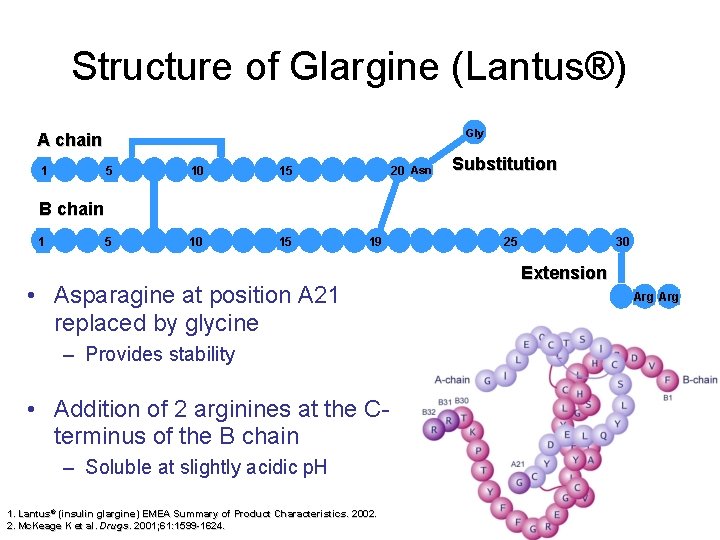
Structure of Glargine (Lantus®) Gly A chain 1 5 10 15 5 10 10 15 15 20 Asn Substitution B chain 1 19 • Asparagine at position A 21 replaced by glycine – Provides stability • Addition of 2 arginines at the Cterminus of the B chain – Soluble at slightly acidic p. H 1. Lantus® (insulin glargine) EMEA Summary of Product Characteristics. 2002. 2. Mc. Keage K et al. Drugs. 2001; 61: 1599 -1624. 25 30 Extension Arg

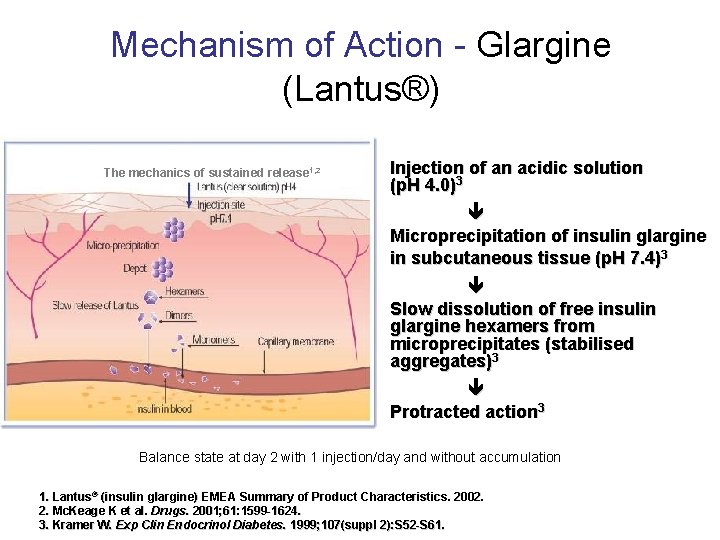
Mechanism of Action - Glargine (Lantus®) The mechanics of sustained release 1, 2 Injection of an acidic solution (p. H 4. 0)3 Microprecipitation of insulin glargine in subcutaneous tissue (p. H 7. 4)3 Slow dissolution of free insulin glargine hexamers from microprecipitates (stabilised aggregates)3 Protracted action 3 Balance state at day 2 with 1 injection/day and without accumulation 1. Lantus® (insulin glargine) EMEA Summary of Product Characteristics. 2002. 2. Mc. Keage K et al. Drugs. 2001; 61: 1599 -1624. 3. Kramer W. Exp Clin Endocrinol Diabetes. 1999; 107(suppl 2): S 52 -S 61.

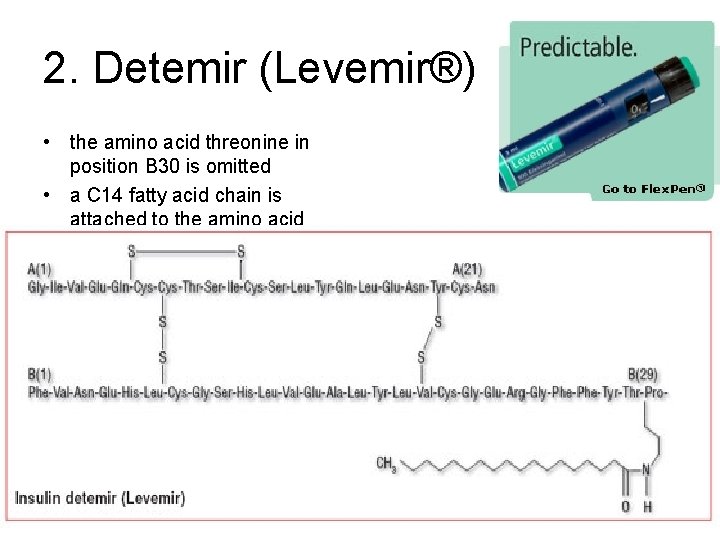
2. Detemir (Levemir®) • the amino acid threonine in position B 30 is omitted • a C 14 fatty acid chain is attached to the amino acid B 29, imparting its long-acting properties


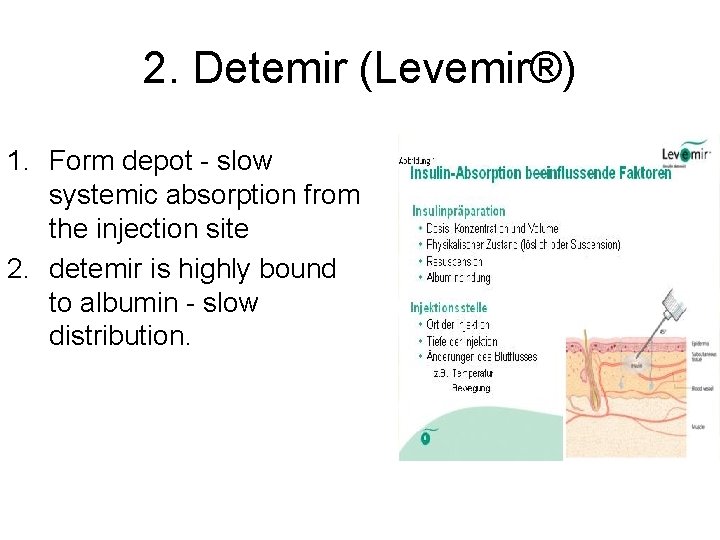
2. Detemir (Levemir®) 1. Form depot - slow systemic absorption from the injection site 2. detemir is highly bound to albumin - slow distribution.


END Thanks
 1562007 color
1562007 color Humalog nursing implications
Humalog nursing implications Insulin glargine drugbank
Insulin glargine drugbank Tresiba pen
Tresiba pen Perbedaan humalog dan humalog mix
Perbedaan humalog dan humalog mix Tresiba to lantus conversion pharmacist letter
Tresiba to lantus conversion pharmacist letter Human actrapid sliding scale
Human actrapid sliding scale Syringe
Syringe Accarbose
Accarbose Lovenox robholland
Lovenox robholland Glargin inzulin
Glargin inzulin Lantus dosage
Lantus dosage Endocrinologist newcastle
Endocrinologist newcastle Dr ponder endocrinologist
Dr ponder endocrinologist Dexamethasone suppression test
Dexamethasone suppression test Endocrinologist in parbhani
Endocrinologist in parbhani