Stroke Hyperglycemia Insulin Network Effort SHINE Trial Protocol


























































- Slides: 83

Stroke Hyperglycemia Insulin Network Effort (SHINE) Trial Protocol Training NIH-NINDS U 01 NSO 69498

The Problem • Over 750, 000 strokes/ year (~80% ischemic) • ~30 -50% hyperglycemic on admission • Hyperglycemia associated w/ worse clinical outcome • Hypoglycemia bad for ischemic brain • Unknown if Rx of hyperglycemia improves outcome • Unknown if risks of aggressive Rx outweigh benefit • Stroke community deals with hyperglycemic acute stroke patients every day without evidence on what is best

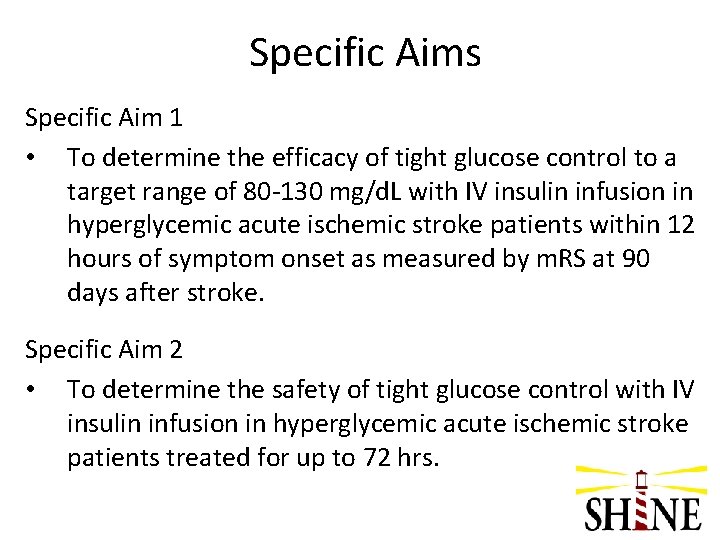
Specific Aims Specific Aim 1 • To determine the efficacy of tight glucose control to a target range of 80 -130 mg/d. L with IV insulin infusion in hyperglycemic acute ischemic stroke patients within 12 hours of symptom onset as measured by m. RS at 90 days after stroke. Specific Aim 2 • To determine the safety of tight glucose control with IV insulin infusion in hyperglycemic acute ischemic stroke patients treated for up to 72 hrs.

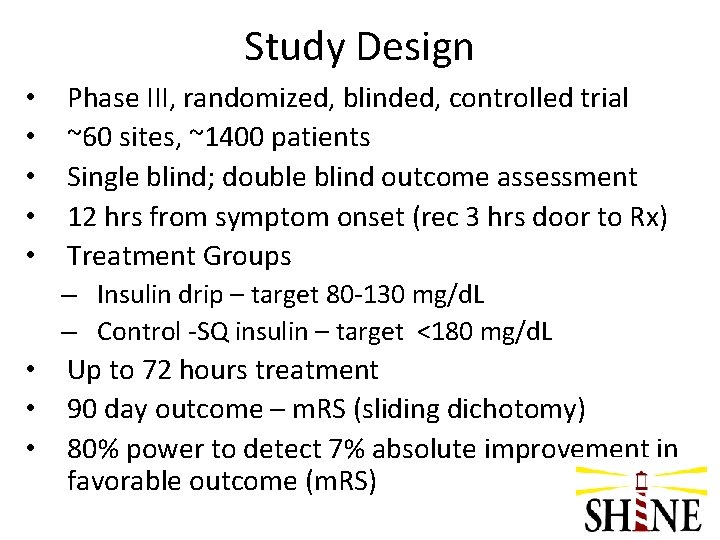
Study Design • • • Phase III, randomized, blinded, controlled trial ~60 sites, ~1400 patients Single blind; double blind outcome assessment 12 hrs from symptom onset (rec 3 hrs door to Rx) Treatment Groups – Insulin drip – target 80 -130 mg/d. L – Control -SQ insulin – target <180 mg/d. L • • • Up to 72 hours treatment 90 day outcome – m. RS (sliding dichotomy) 80% power to detect 7% absolute improvement in favorable outcome (m. RS)

Study Innovation • Response Adaptive Randomization (RAR) • Gluco. Stabilizer – electronic decision support tool • Responder Analysis (sliding dichotomy) – successful outcome based on enrollment stroke severity – allows consideration of expected outcome to be considered in determination of favorable outcome

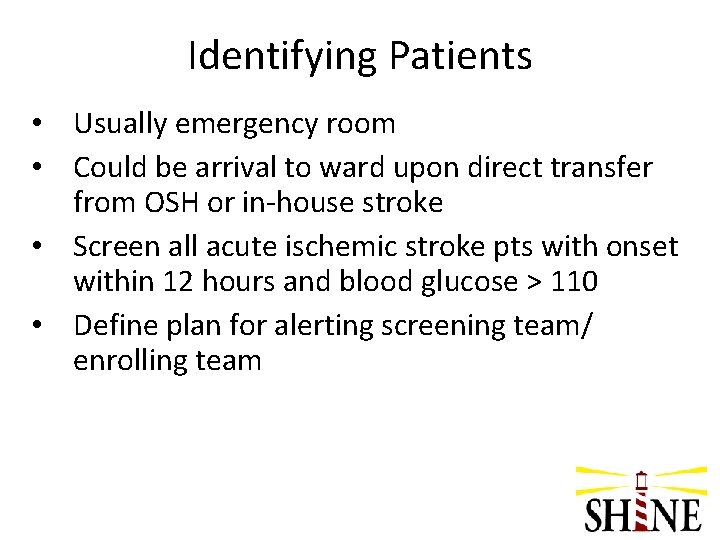
Identifying Patients • Usually emergency room • Could be arrival to ward upon direct transfer from OSH or in-house stroke • Screen all acute ischemic stroke pts with onset within 12 hours and blood glucose > 110 • Define plan for alerting screening team/ enrolling team

Consent – General Concepts • Use standard consent procedures per local regulatory requirements • Written informed consent with process documented • No emergency exemption • Randomization recommended but not required to occur w/in 3 hours of arrival to enrolling ED (hospital)

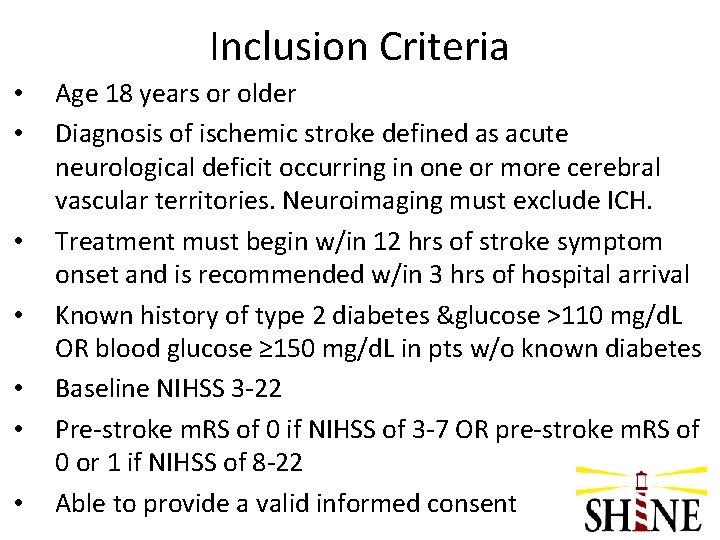
Inclusion Criteria • • Age 18 years or older Diagnosis of ischemic stroke defined as acute neurological deficit occurring in one or more cerebral vascular territories. Neuroimaging must exclude ICH. Treatment must begin w/in 12 hrs of stroke symptom onset and is recommended w/in 3 hrs of hospital arrival Known history of type 2 diabetes &glucose >110 mg/d. L OR blood glucose ≥ 150 mg/d. L in pts w/o known diabetes Baseline NIHSS 3 -22 Pre-stroke m. RS of 0 if NIHSS of 3 -7 OR pre-stroke m. RS of 0 or 1 if NIHSS of 8 -22 Able to provide a valid informed consent

Exclusion Criteria • Known history of type 1 diabetes • Substantial neurological or psychiatric illness that would confound neurological or outcome assessment • Received experimental therapy for enrollment stroke. IV t. PA (up to 4. 5 hrs), IA t. PA &IA therapies including FDA cleared devices allowed. Non FDA cleared devices excluded. • Pregnant or breast-feeding • Other serious conditions that make pt unlikely to survive 90 days • Inability to follow protocol or return for 90 day f/u • Renal dialysis

Randomization– General Concepts • Web. DCU-based randomization • Randomization time is start of treatment time • Must be randomized within 12 hours of symptom onset (recommended but not required within 3 hours of arrival to enrolling hospital) • RAR – Response Adaptive Randomization • Start out 1: 1 (flip of coin) • RAR – higher chance of being enrolled in treatment group that has better outcomes

Treatment Groups - General Concepts Control Group • BG target 80 -179 mg/d. L • SQ insulin (regular human) per sliding scale • IV saline drip (to mimic IV insulin drip to maintain blind) • Q 1 hr glucose checks for first four hours • Q 3 hr glucose checks after 1 st 4 hrs– but insulin given only at 06: 00, 12: 00, 18: 00 and 24: 00 if indicated • Basal insulin only for Level 3 indicated

Treatment Groups - General Concepts Intervention Group • BG target 80 -130 mg/d. L • Glucose checks and IV insulin (regular human) drip adjustments per Gluco. Stabilizer (computer decision support tool) • For pts who are PO or on bolus tube feeds, SQ meal insulin (rapid acting analog) per meal carbohydrate diet • For pts who are NPO or on continuous tube feeds, SQ saline administered (to mimic SQ insulin to maintain blind)

Control Group

Control Group Initiation of Treatment Enter orders/notify pharmacy Start SHINE laptop and select Control Group Re-check glucose when IV saline is ready Start IV saline infusion per protocol (displayed on control treatment screen) & adjust as needed w/ EACH glucose check • Check glucose once to start and then q 1 hr x 4 hrs, then q 3 hr (03: 00, 06: 00, 09: 00, 12: 00, 15: 00, 18: 00, 21: 00 and 24: 00) • Give insulin per sliding scale ONLY at 06: 00, 12: 00, 18: 00 and 24: 00 • •

Getting Started

Control Group Computer Screen

Control Group Documenting

Control Group Continuation of Treatment • Check glucose per study sliding scale schedule • Give SQ insulin only at the designated times if indicated by the sliding scale (≥ 180 mg/d. L) • All patients start at Level 1 for first 24 hrs (opportunity to advance to more aggressive glucose control at 24 & 48 hrs if indicated) • Adjust IV saline rate if indicated after each glucose check • Same procedure whether eating or not

Shifting from Q 1 to Q 3 hour glucose checks in control group • First glucose check for SHINE study treatment protocol as soon as saline bag ready to drip • Q 1 hr check for first 4 glucose checks (one check to start and then four more) • After the 4 th Q 1 hr check, start glucose checks on Q 3 hr schedule, but skip 1 st scheduled check if <1 hr from previous check unless it is a SQ insulin dosing time

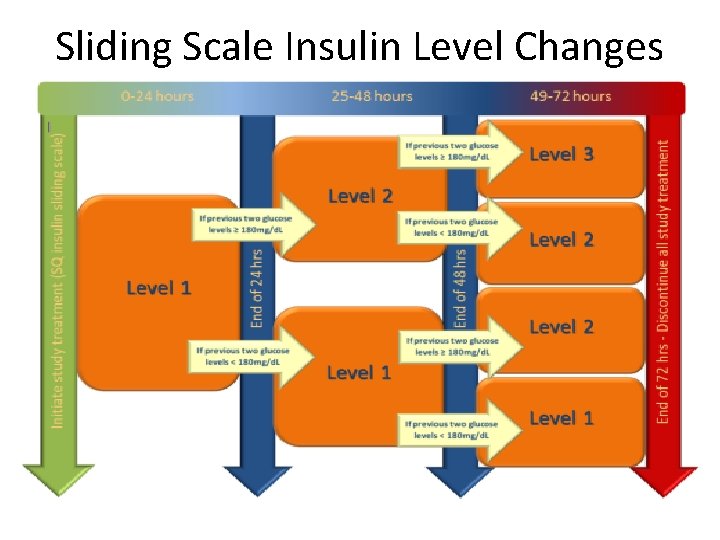
Sliding Scale Insulin Level Changes • All patients on Level 1 for first 24 hours (start time is time of randomization) • Advance level only if indicated at 24 & 48 hrs – At end of 1 st 24 hrs, advance to Level 2 only if the latest 2 glucose levels ≥ 180 mg/d. L; otherwise stay on Level 1 – At end of 48 hrs, advance to next level (2 or 3) only if the latest 2 glucose levels ≥ 180 mg/d. L; otherwise stay on current level – Level 3 includes one-time SQ basal insulin (Lantus) given at end of 48 hrs (sliding scale insulin doses for Level 3 are the same as Level 2) • Do not backwards/go down a level unless safety monitor recommends

Sliding Scale Insulin Level Changes

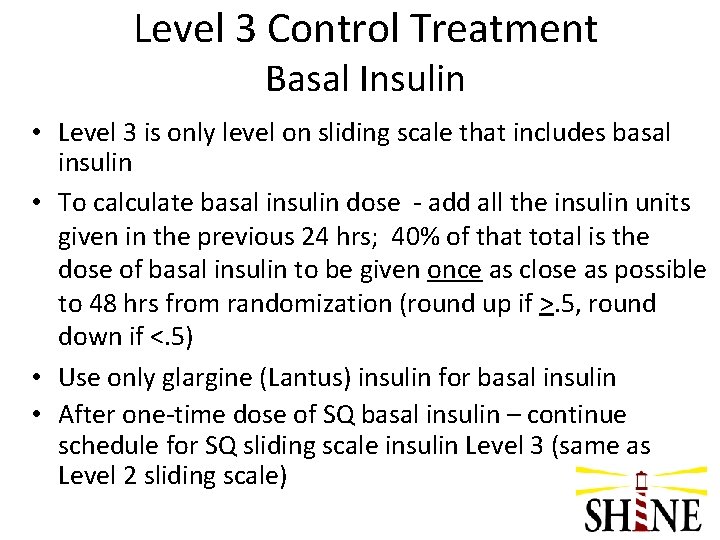
Level 3 Control Treatment Basal Insulin • Level 3 is only level on sliding scale that includes basal insulin • To calculate basal insulin dose - add all the insulin units given in the previous 24 hrs; 40% of that total is the dose of basal insulin to be given once as close as possible to 48 hrs from randomization (round up if >. 5, round down if <. 5) • Use only glargine (Lantus) insulin for basal insulin • After one-time dose of SQ basal insulin – continue schedule for SQ sliding scale insulin Level 3 (same as Level 2 sliding scale)

Control Group - Meals • 60 gram carbohydrate diet per meal should be ordered • NO estimate of meal consumption required and NO meal insulin given in control group (only SQ sliding scale insulin at 06: 00, 12: 00, 18: 00 and 24: 00 if indicated) • Pts should eat only after their glucose check and the SQ insulin has been given as needed at 6: 00, 12: 00, and 18: 00. (If meal arrives before these times, hold until time for glucose check and insulin dose if indicated) • Only protocol-approved snacks during the 72 hour SHINE treatment protocol

Intervention Group

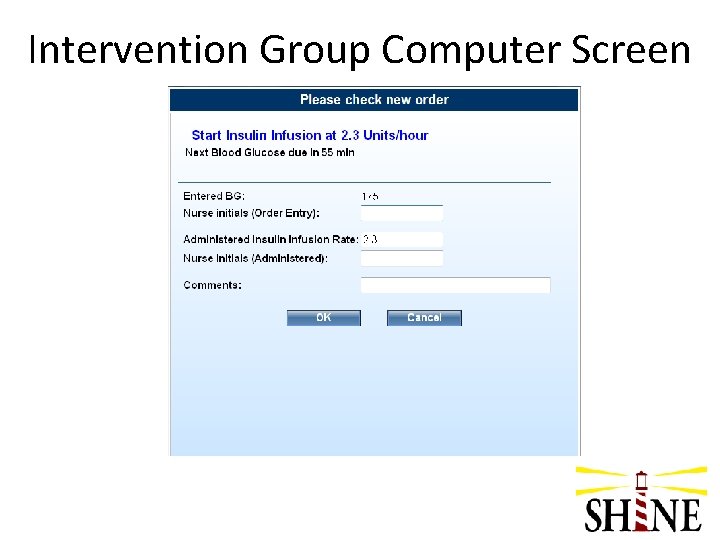
Intervention Group Initiation of Treatment • Enter orders/notify pharmacy • Start SHINE laptop and select Intervention Group • Re-check glucose when IV insulin infusion is ready • Start IV insulin infusion per Gluco. Stabilizer recommendation

Getting Started

Intervention Group Computer Screen

Intervention Group Continuation of Treatment • Recheck and enter glucose in Gluco. Stabilizer • Adjust IV insulin drip per Gluco. Stabilizer recommendation • For pts that are PO or receiving bolus tube feeds, give meal insulin per protocol • For pts that are NPO or receiving continuous tube feeds, give SQ saline 0. 05 cc after check closest to 09: 00 and 21: 00 to simulate SQ insulin injections in control group

Intervention Group Meals • Order 60 gram carbohydrate diet for breakfast, lunch & dinner for pts who are eating or on bolus tube feeds – PO - Allow 20 minutes from start of eating – then estimate the proportion of meal consumed – Bolus tube feeds – estimate proportion of bolus given • SQ meal insulin (rapid acting analog) given based on proportion of meal consumed & dose recommended per Gluco. Stabilizer • Only protocol-approved snacks during 72 hour SHINE study treatment period

Intervention Group SQ Meal Insulin for PO pts • Meal consumed – all or nearly all – Enter 60 carb meal into Gluco. Stabilizer – Give dose of meal insulin recommended • Meal consumed– none or nearly none – DO NOT make an entry into Gluco. Stabilizer – DO NOT give meal insulin • Meal consumed– partial or in between – Enter 30 carb meal into Gluco. Stabilizer – Give dose of meal insulin recommended

Intervention Group Meal Insulin Computer Screens

Data Entry • Standard chart documentation is required per site procedure • Additionally, glucose and study treatment data will be entered in control treatment screen of study laptop

Special situations Hypoglycemia Pauses in study protocol Discontinuing study protocol < 72 hours Transition from study protocol

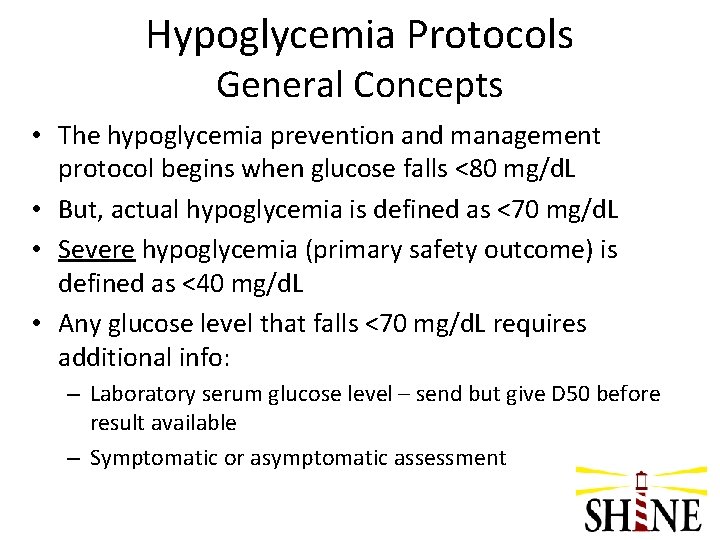
Hypoglycemia Protocols General Concepts • The hypoglycemia prevention and management protocol begins when glucose falls <80 mg/d. L • But, actual hypoglycemia is defined as <70 mg/d. L • Severe hypoglycemia (primary safety outcome) is defined as <40 mg/d. L • Any glucose level that falls <70 mg/d. L requires additional info: – Laboratory serum glucose level – send but give D 50 before result available – Symptomatic or asymptomatic assessment

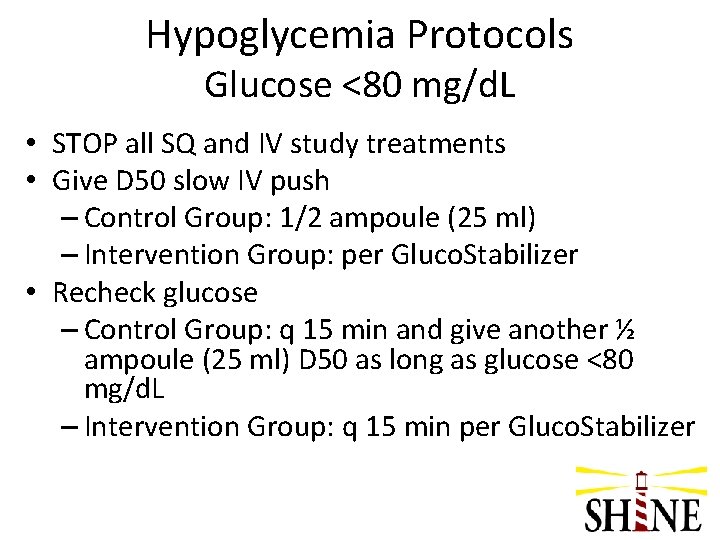
Hypoglycemia Protocols Glucose <80 mg/d. L • STOP all SQ and IV study treatments • Give D 50 slow IV push – Control Group: 1/2 ampoule (25 ml) – Intervention Group: per Gluco. Stabilizer • Recheck glucose – Control Group: q 15 min and give another ½ ampoule (25 ml) D 50 as long as glucose <80 mg/d. L – Intervention Group: q 15 min per Gluco. Stabilizer

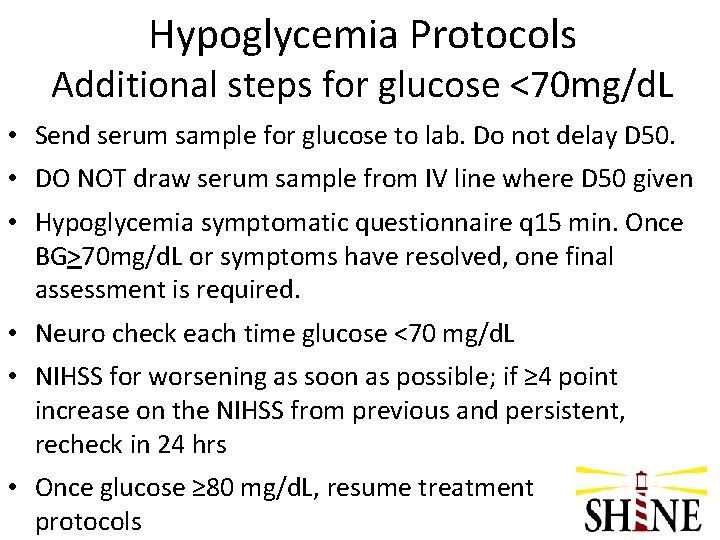
Hypoglycemia Protocols Additional steps for glucose <70 mg/d. L • Send serum sample for glucose to lab. Do not delay D 50. • DO NOT draw serum sample from IV line where D 50 given • Hypoglycemia symptomatic questionnaire q 15 min. Once BG>70 mg/d. L or symptoms have resolved, one final assessment is required. • Neuro check each time glucose <70 mg/d. L • NIHSS for worsening as soon as possible; if ≥ 4 point increase on the NIHSS from previous and persistent, recheck in 24 hrs • Once glucose ≥ 80 mg/d. L, resume treatment protocols

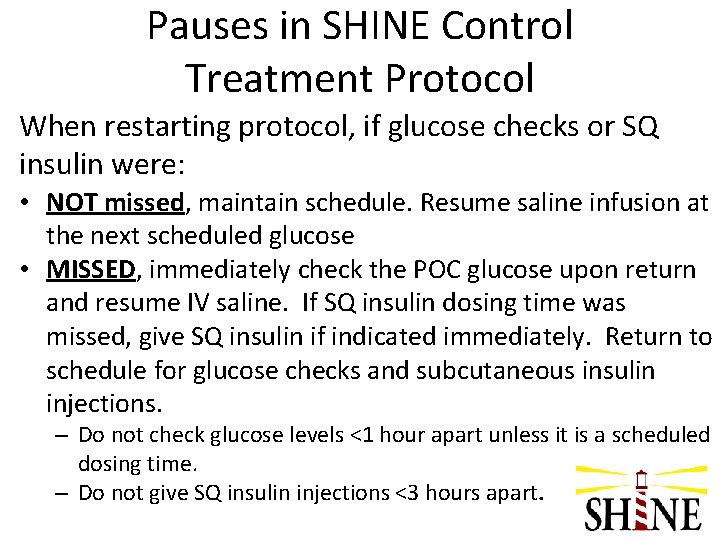
Pauses in SHINE Control Treatment Protocol When restarting protocol, if glucose checks or SQ insulin were: • NOT missed, maintain schedule. Resume saline infusion at the next scheduled glucose • MISSED, immediately check the POC glucose upon return and resume IV saline. If SQ insulin dosing time was missed, give SQ insulin if indicated immediately. Return to schedule for glucose checks and subcutaneous insulin injections. – Do not check glucose levels <1 hour apart unless it is a scheduled dosing time. – Do not give SQ insulin injections <3 hours apart.

Pauses in SHINE Intervention Treatment Protocol • When able to restart protocol, recheck glucose • Use result of glucose check to resume IV insulin drip – If IV drip off for <3 hrs, use “Resume” option in Gluco. Stabilizer – If IV drip off for ≥ 3 hrs, use “Start New Drip” option in Gluco. Stabilizer • If a meal is eaten late, give SQ meal insulin with meal per protocol • If SQ saline dose was missed (NPO or continuous tube feed pts) • Give 0. 05 cc SQ saline • If next saline dosing time in <3 hrs, then skip it

NUTRITIONAL STATUS SHINE TREATMENT GROUP Study treatment by group and IV saline IV insulin nutritional status plus Eating PO meals or Bolus Tube Feeds NPO or Continuous Tube Feeds INTERVENTION GROUP CONTROL GROUP Subcutaneous meal insulin injections Subcutaneous sliding scale insulin injections How much IV saline? Per Sliding Scale Control Treatment Screen How many units SQ meal insulin? Per Gluco. Stabilizer recommendation based on proportion of meal consumed given 20 minutes after start of meal 3 x/day @ 0600, 1200, & 1800 How many units SQ insulin? Per Sliding Scale Control Treatment Screen Finger stick glucose check @ 0300, 0600, 0900, 1200, 1500, 1800, 2100, 2400 (Insulin dosing only @0600, 1200, 1800, & 2400) IV insulin plus Subcutaneous saline injections IV saline plus Subcutaneous sliding scale insulin injections How much IV Insulin? Per Gluco. Stabilizer recommendation How much IV Insulin? As per recommendation of Gluco. Stabilizer How much SQ saline? 0. 05 ml of SQ saline @ time of glucose check nearest 0900 and 2100 How much IV saline? Per Sliding Scale Control Treatment Screen How many units of SQ insulin? Per Sliding Scale Control Treatment Screen Finger stick glucose check @ 0300, 0600, 0900, 1200, 1500, 1800, 2100, 2400 (Insulin dosing only @0600, 1200, 1800, & 2400)

Discontinuation of SHINE Treatment • Any study pt clinically ready for hospital discharge prior to 72 hrs of treatment may be discontinued from SHINE protocol (not a protocol deviation) • 6 hrs prior to discharge – D/C SHINE study infusion (insulin or saline) – D/C SHINE sliding scale SQ insulin protocol • Gluco. Stabilizer Drip Weaning Report (24 hr insulin total) available for review for intervention group only • Any subsequent SQ insulin Rx at discretion of treating physician should be >3 hrs after last SHINE SQ insulin • Oral diabetes Rx per treating team

Transition to Standard Care • Per ADA guidelines scheduled subcutaneous insulin that delivers basal, nutritional and correction components is preferred. • Consider that oral agents are not recommended in hospitalized patients, but may be initiated or resumed in anticipation of discharge per ADA guidelines. • Consider individualized discharge planning per ADA guidelines

Summary – Clinical Study Outcomes • Primary Study Outcome – 3 month m. RS • Primary Safety Outcome – frequency of severe hypoglycemia (<40 mg/d. L) in intervention group versus control group • Additional Outcomes – 6 week phone call – m. RS by phone, SAEs – 3 month - BI, NIHSS, QOL

Outcome Visits • Assessor for outcomes visits must be blinded to treatment • Visits • 6 week visit – by phone – m. RS and SAEs • 3 month visit – in person – m. RS, NIHSS, BI, SSQOL – SAEs – Unblinding survey (patient and investigator)

Bedding for SHINE patients

Bedding for SHINE Patients • Hospitals have different regulations for unit/level of care required for insulin drip • For the 72 hours of study treatment, all patients must be bedded in a location that would support and allow delivery of insulin drip therapy. • Must not differentially bed intervention and control patients as this will bias the study due to differential level of care

Bedding for SHINE Patients • If your hospital supports insulin drip therapy in ICU, step down, stroke unit & floor level settings: – May bed any pt in any one of these insulin drip supporting environments according to clinical need • If your hospital supports insulin drip therapy in ICU, step down, stroke unit BUT NOT in floor level settings: – May only bed any pt in ICU, SDU, ASU and may not bed any pt on the floor during the 72 hr study treatment period regardless of treatment group (intervention or control)

Bedding for SHINE Patients If your hospital ONLY supports insulin drip therapy in ICU then: • All SHINE patients must be cared for in ICU for 72 hours unless discharged OR • There must be a special plan in place with your institution to manage this

Pharmacy

Treatment Assignment • Randomization generated by study team in Web. DCU • Study team provides copy of treatment assignment to pharmacy (may be faxed, scanned or delivered in person)

Study Treatment by Group IV Infusion Subcutaneous Injections Control Group Intervention Group Normal saline 0. 9% Sodium Chloride Human regular insulin Humulin R, Novolin R Rapid acting analog insulin lispro (Humalog), aspart (Novolog) or glulisine (Apidra) AND OR Basal insulin (Level 3 only) glargine (Lantus) Normal saline 0. 9% Sodium Chloride

Control Group – Study Treatment Control Group IV infusion Normal saline Per sliding scale (continuous) Subcutaneous injections Human regular insulin Per sliding scale (@ 06: 00, 12: 00, 18: 00 & 24: 00) AND Basal insulin (Level 3 only) 40% of insulin requirement during previous 24 hrs (@ ~48 hrs)

Intervention Group – Study Treatment Intervention Group IV infusion Human regular insulin (1: 1) Per Gluco. Stabilizer (continuous) Subcutaneous injections Rapid acting analog insulin (meal insulin) Per Gluco. Stabilizer @ ~06: 00, 12: 00 & 18: 00 OR Normal saline 0. 05 m. L @ ~09: 00 & 21: 00

D 50 for Hypoglycemia Prevention and Management • D 50 stored to allow immediate availability • Glucose <80 mg/d. L • Control group - 25 ml (1/2 amp) • Intervention group - individualized dose per Gluco. Stabilizer

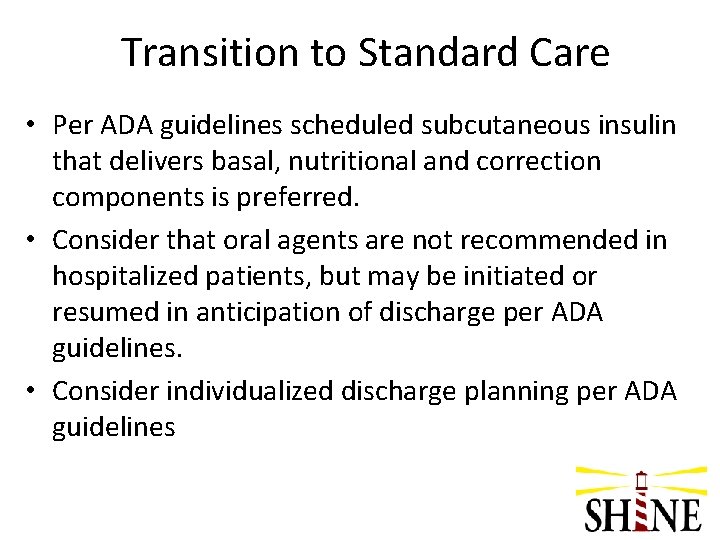
Control Screens – Orange Background

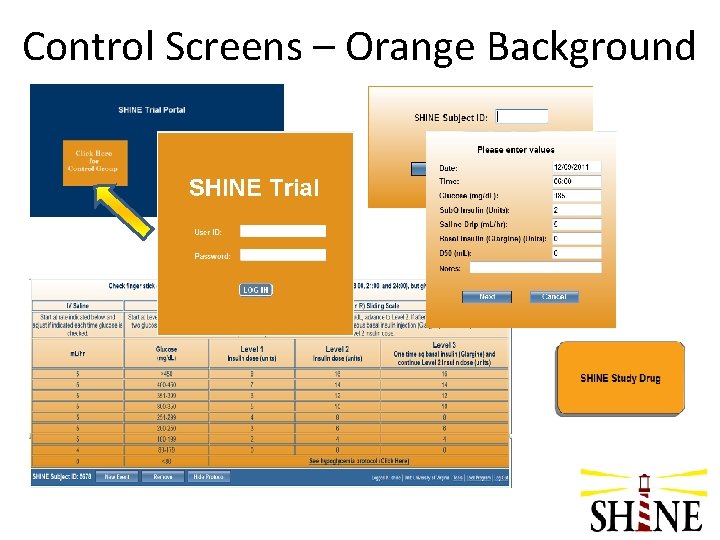
Intervention Screens – Blue Background

Site Pharmacy Plan • Establish site-specific pharmacy plan prior to initiation • Retain one empty infusion bag for each study patient for monitoring

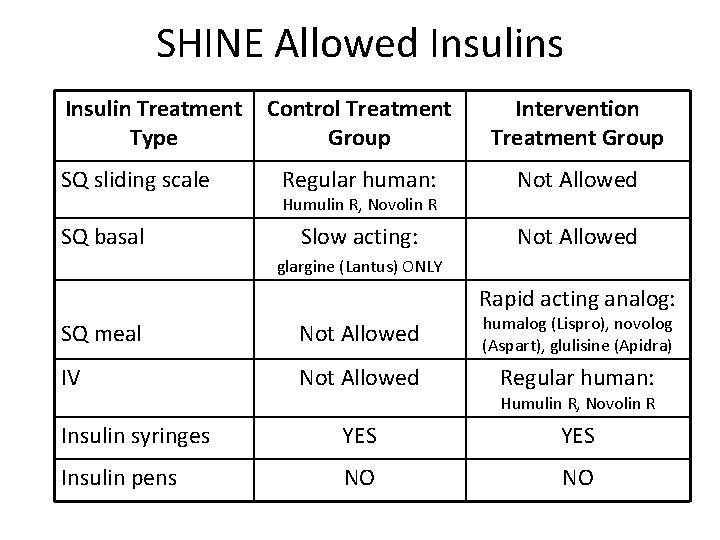
SHINE Allowed Insulins Insulin Treatment Type SQ sliding scale SQ basal Control Treatment Group Intervention Treatment Group Regular human: Not Allowed Slow acting: Not Allowed Humulin R, Novolin R glargine (Lantus) ONLY Rapid acting analog: SQ meal Not Allowed humalog (Lispro), novolog (Aspart), glulisine (Apidra) IV Not Allowed Regular human: Humulin R, Novolin R Insulin syringes YES Insulin pens NO NO

Lead Nurse Role

Role of the Lead Nurse • Work closely to advise research team on training • Assist the nursing staff in implementing study protocol • “Super-user” of protocol and Gluco. Stabilizer • Anticipate issues during study treatment and provide input as study team develops resources • Give feedback to research team

Clinical nurses play pivotal role • Initiate treatment – drip and SQ injections • Check glucose per Gluco. Stabilizer or control sliding scale schedule • Review level of sliding scale in control group • Meals - estimate consumption & administer meal insulin dosing (intervention group only) • Maintain blind for patient and family • Initiate hypoglycemia prevention & management (glucose <80 mg/d. L) • Transition of care from shift to shift • Manage pauses in study treatment • Transition off study protocol

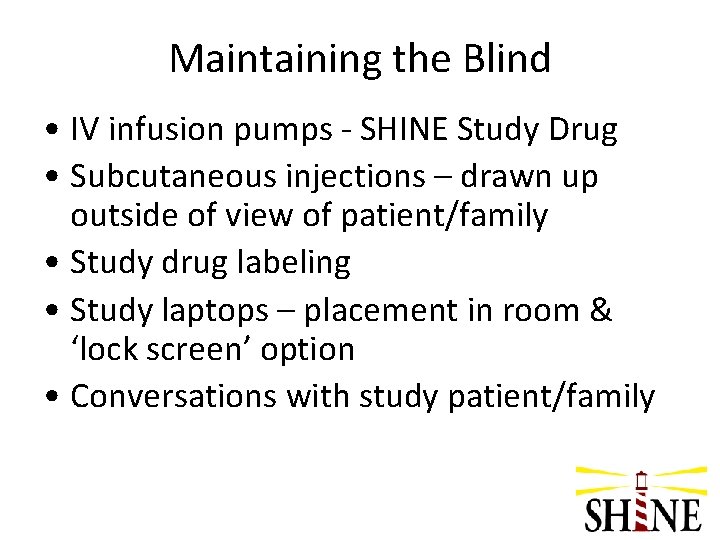
Maintaining the Blind • IV infusion pumps - SHINE Study Drug • Subcutaneous injections – drawn up outside of view of patient/family • Study drug labeling • Study laptops – placement in room & ‘lock screen’ option • Conversations with study patient/family

Regulatory requirements and Site monitoring

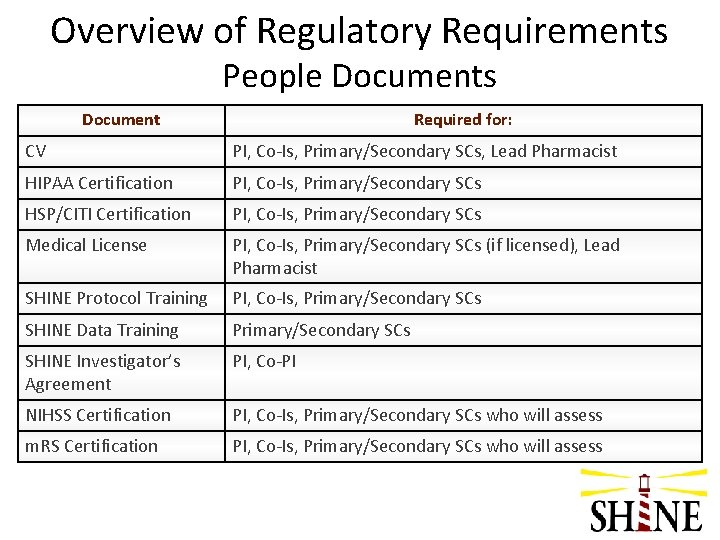
Overview of Regulatory Requirements People Documents Document Required for: CV PI, Co-Is, Primary/Secondary SCs, Lead Pharmacist HIPAA Certification PI, Co-Is, Primary/Secondary SCs HSP/CITI Certification PI, Co-Is, Primary/Secondary SCs Medical License PI, Co-Is, Primary/Secondary SCs (if licensed), Lead Pharmacist SHINE Protocol Training PI, Co-Is, Primary/Secondary SCs SHINE Data Training Primary/Secondary SCs SHINE Investigator’s Agreement PI, Co-PI NIHSS Certification PI, Co-Is, Primary/Secondary SCs who will assess m. RS Certification PI, Co-Is, Primary/Secondary SCs who will assess

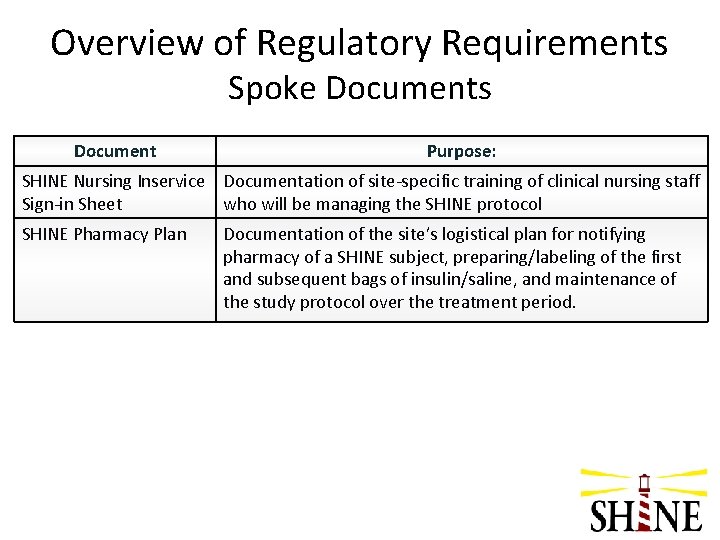
Overview of Regulatory Requirements Spoke Documents Document Purpose: SHINE Nursing Inservice Documentation of site-specific training of clinical nursing staff Sign-in Sheet who will be managing the SHINE protocol SHINE Pharmacy Plan Documentation of the site’s logistical plan for notifying pharmacy of a SHINE subject, preparing/labeling of the first and subsequent bags of insulin/saline, and maintenance of the study protocol over the treatment period.

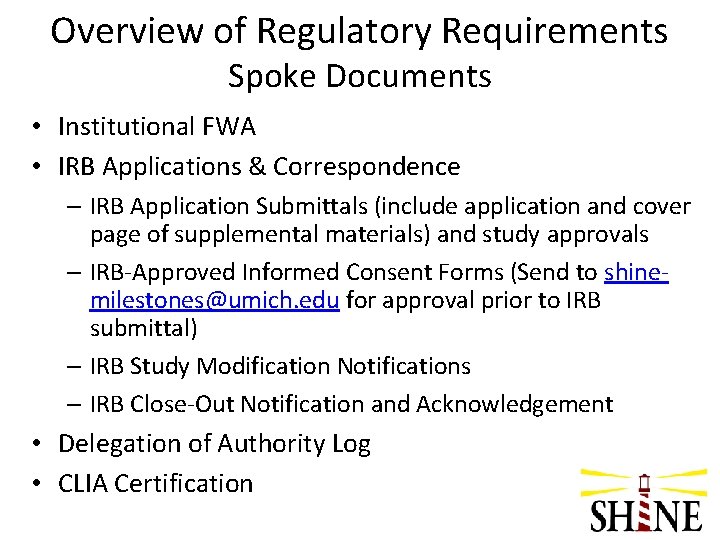
Overview of Regulatory Requirements Spoke Documents • Institutional FWA • IRB Applications & Correspondence – IRB Application Submittals (include application and cover page of supplemental materials) and study approvals – IRB-Approved Informed Consent Forms (Send to shinemilestones@umich. edu for approval prior to IRB submittal) – IRB Study Modification Notifications – IRB Close-Out Notification and Acknowledgement • Delegation of Authority Log • CLIA Certification

Personnel Changes • Study team members must be listed on the Delegation of Authority Log – please keep DOA log on Web. DCU current • Study team members must be assigned roles in Web. DCU Project Spoke Team Member table • Regulatory Parameters Document lists specific requirements • Nursing staff and pharmacy staff (with the exception of the lead pharmacist/pharmacy contact) do not need to be added to the DOA log or the Project Spoke Team Member table.

Regulatory Readiness • Site should notify CCC when regulatory ready after IRB approval is received and all regulatory docs are uploaded • CCC confirms required documents & schedules readiness call w/ key personnel from SHINE & site study team. • Site will complete Readiness Checklist and provide prior to readiness call. • Following the call, if no action items required, the site will be notified that they are released to begin enrollment. • If action items are required, these must be resolved prior to being released to enrollment.

Site Monitoring Schedule • Study Initiation Visit – Readiness Call • Routine Interim Monitoring Visits – At least one visit per year • Close-Out Visit

Site Monitoring Schedule • Timing of visits First visit to each site after subject #1 enrolled and completed treatment period

Reporting Adverse Events

Reporting Adverse Events • Adverse Events (AEs) are “. . . any untoward medical occurrence in a subject that was not previously identified which does not necessarily have a causal relationship to the study drug…” • Events existing prior to randomization should not be reported as AEs, unless there is a change in severity • AEs (both serious and non-serious) are reported on the Adverse Event CRF

Reporting Adverse Events • Report the diagnosis, not the symptoms: Fever, cough, chest pain, crackles = pneumonia • Death, surgery, intubation, etc. are not adverse events. They are outcomes of adverse events

Reporting Adverse Events All AEs will be coded centrally using Med. DRA 1 AE per CRF Avoid abbreviations/colloquialisms AEs that can’t be coded will be queried All AEs must be reported through completion of study treatment. • All SAEs must be reported through End of Study. • • •

Serious Adverse Events fatal life-threatening result in hospitalization/prolongation of hospitalization • result in disability/congenital anomaly • • • OR • require intervention to prevent permanent impairment or damage

Data Entry Timelines for AEs • Non-serious AEs must be entered and submitted into Web. DCUTM within 5 days of data collection • SAEs must be entered and submitted into Web. DCUTM within 24 hours of the time that the study team receives knowledge of the event

Reporting SAEs • To assist in review by medical safety monitors, SAEs require additional information: – – Detailed description of the event Relevant tests/laboratory data Relevant history and pre-existing conditions Concomitant meds • Do not identify any subject, physician, or institution by name.

Reporting SAEs • Site data enters and submits AE CRF into Web. DCUTM • Automatic e-mail notification to Project Manager • PM reviews narrative - If CRF is sufficient, an automatic email notification will be sent to the Internal Quality and Safety Reviewer (IQSR)

Reporting SAEs • IMM reviews narrative - If AE data is sufficient, an automatic email notification will be sent to the external Independent Safety Monitor (ISM Thomas Bleck, MD) • ISM reviews the event and indicates whether the event is serious and unexpected • PM closes review process

SAE Reporting • DSMB requires expedited reporting of all SAEs • Site PIs are responsible for reporting the SAE to their IRB according to local requirements • Site PIs responsible for submitting follow-up information into Web. DCUTM as it becomes available.

Hypoglycemia Reporting • Site PI must report to ISM if a subject has 3 or more episodes of hypoglycemia within a 24 hour period. • Call the SHINE study hotline (800 -915 -7320 ext 2) • ISM will determine if the level of sliding scale insulin should be adjusted or if insulin drip protocol should discontinued

Study Contacts - Emergencies Category Contact Name Business Hours Number 24 Hour Contact Protocol emergency questions PI on call 800 -915 -7320 ext 1 Eligibility questions PI on call 800 -915 -7320 ext 1 Gluco. Stabilizer emergencies PI on call Randomization Catherine Dillon Karen Briggs Safety emergencies Thomas Bleck, MD (Back up Independent MSM: Shyam Prabhakaran, MD, MS) 800 -915 -7320 ext 1 843 -792 -3980 843 -876 -1919 MUSC Emergency Randomization # 800 -915 -7320 ext 2

Study Contacts – Routine Issues Category Contact Name Business Hours Number Web. DCU support and passwords Catherine Dillon/Karen Briggs 843 -792 -3980 843 -876 -1919 Routine safety questions/SAE Catherine Dillon/Karen Briggs 843 -792 -3980 843 -876 -1919 CRF questions Karen Briggs 843 -792 -3980 Protocol, Pharmacy and Laptops Amy Fansler 434 -982 -6027 Contracts and invoicing Valerie Stevenson (NETT sites)/Amy Fansler (ancillary sites) 734 -232 -2131 434 -982 -6027 Regulatory questions Arthi Ramakrishnan 734 -936 -2454

This concludes the SHINE Protocol Training. Please click on the link below and complete the training certificate. Email this certificate to your Study Coordinator. SHINE Protocol Training Certificate