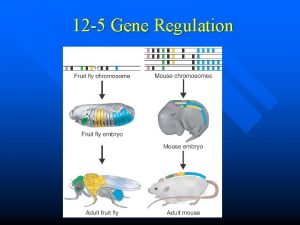
Gene regulation Genetically related genotypes with striking phenotypic





- Slides: 42

Gene regulation Genetically related genotypes with striking phenotypic differences, but similar allelic architecture. Within a genotype – striking phenotypic differences between growth stages and/or between tissues.

Gene regulation Promoters - Efficiency, constitutive, tissue-specific, inducible: • Ca. MV 35 S, Glutelin GT 1, Cis-Jasmone Transcription factors - Facilitate, enhance, repress: • Nud, Vrs 1 m. RNA stability - minutes to months: • 5’cap, 3’tail Chromatin remodeling: • Accessibility of DNA to transcription machinery. RNAi: • hn. RNA, lnc. RNA, mi. RNA, pu. RNA, sh. RNA, sno. RNA, si. RNA, ti. RNA, …. Translational and post-translational modification of proteins: • Protein synthesis rate, transport, stability, activity

Gene regulation Focus on mi. RNA, si. RNA

RNAi Regulation of m. RNA via: • m. RNA cleavage: RISC pairs with target, Slicer enzyme cuts m. RNA, m. RNA pieces degrade. • Translation inhibition: mi. RNA inhibits translation by binding with target m. RNA. • Transcriptional silencing: si. RNA silences transcription through chromatin alteration. • m. RNA degradation: Slicer-independent si. RNA + protein.

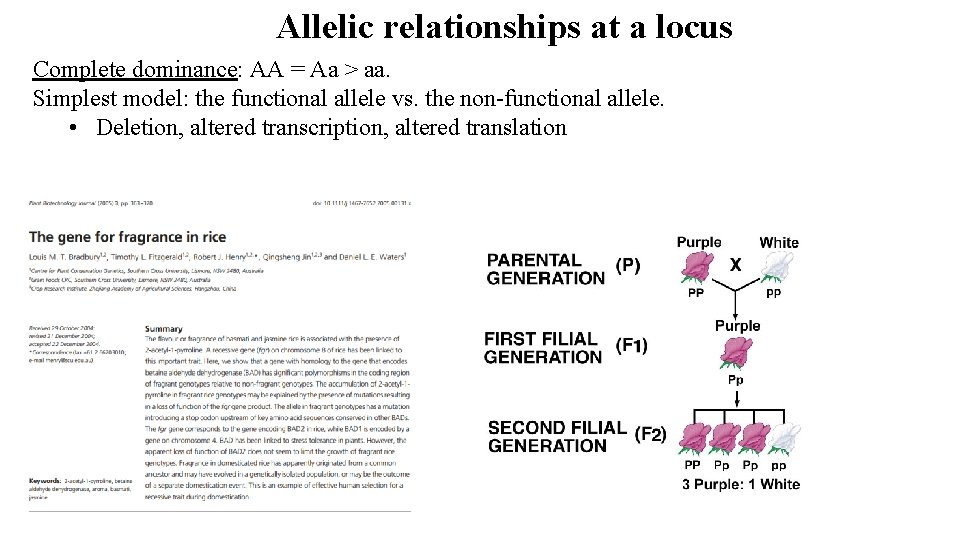
Allelic relationships at a locus Complete dominance: AA = Aa > aa. Simplest model: the functional allele vs. the non-functional allele. • Deletion, altered transcription, altered translation

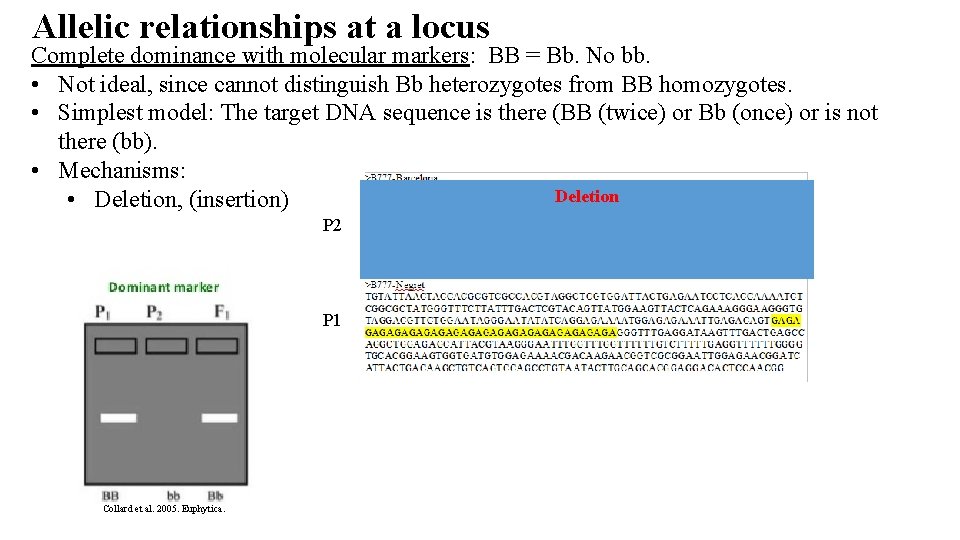
Allelic relationships at a locus Complete dominance with molecular markers: BB = Bb. No bb. • Not ideal, since cannot distinguish Bb heterozygotes from BB homozygotes. • Simplest model: The target DNA sequence is there (BB (twice) or Bb (once) or is not there (bb). • Mechanisms: Deletion • Deletion, (insertion) P 2 P 1 Collard et al. 2005. Euphytica.

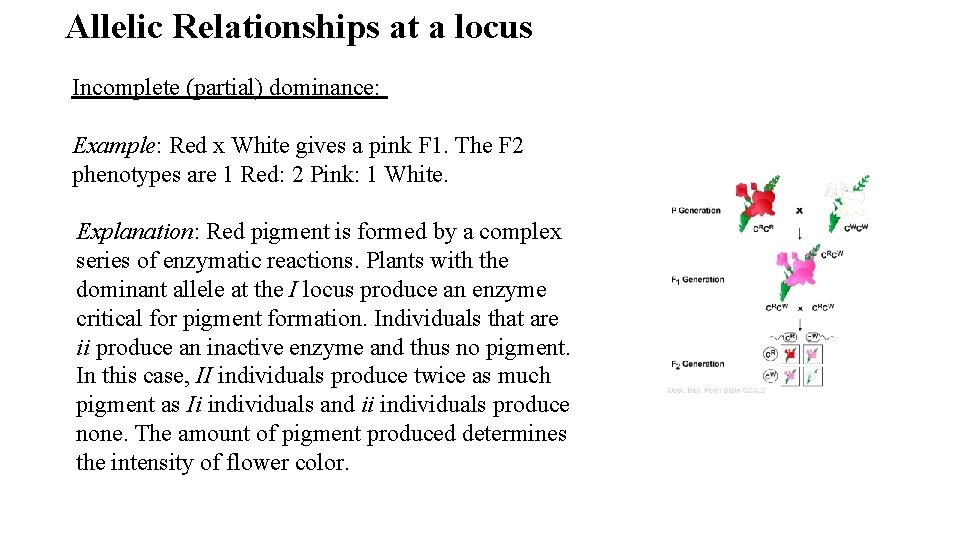
Allelic Relationships at a locus Incomplete (partial) dominance: Example: Red x White gives a pink F 1. The F 2 phenotypes are 1 Red: 2 Pink: 1 White. Explanation: Red pigment is formed by a complex series of enzymatic reactions. Plants with the dominant allele at the I locus produce an enzyme critical for pigment formation. Individuals that are ii produce an inactive enzyme and thus no pigment. In this case, II individuals produce twice as much pigment as Ii individuals and ii individuals produce none. The amount of pigment produced determines the intensity of flower color.

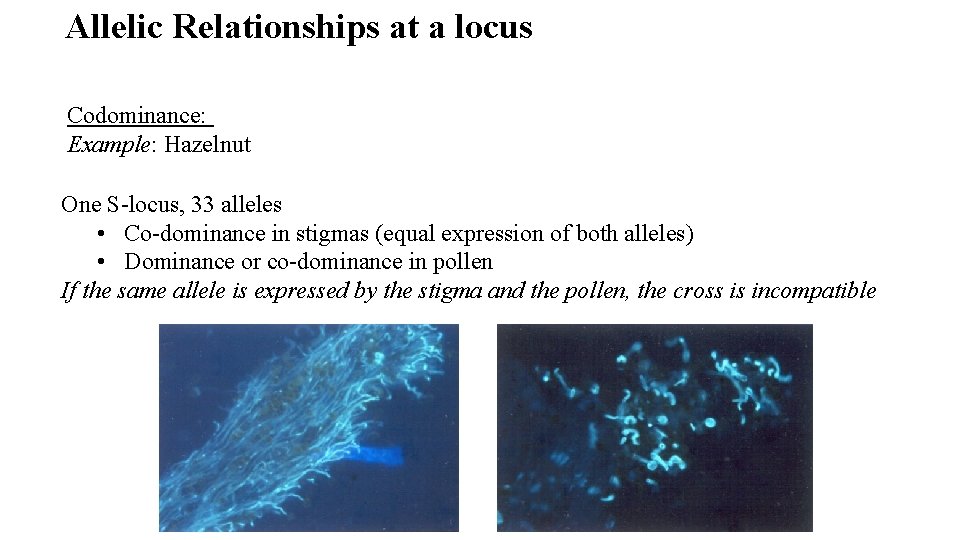
Allelic Relationships at a locus Codominance: Example: Hazelnut One S-locus, 33 alleles • Co-dominance in stigmas (equal expression of both alleles) • Dominance or co-dominance in pollen If the same allele is expressed by the stigma and the pollen, the cross is incompatible

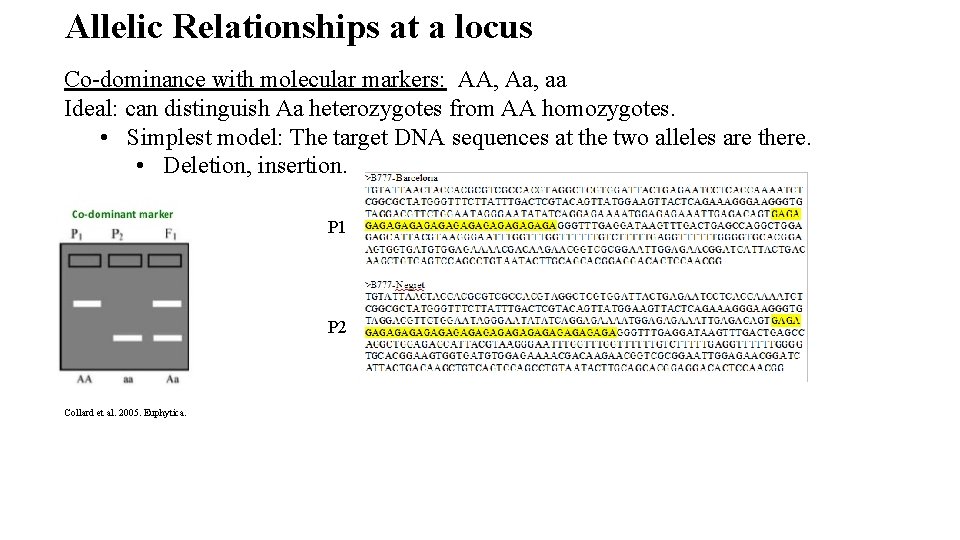
Allelic Relationships at a locus Co-dominance with molecular markers: AA, Aa, aa Ideal: can distinguish Aa heterozygotes from AA homozygotes. • Simplest model: The target DNA sequences at the two alleles are there. • Deletion, insertion. P 1 P 2 Collard et al. 2005. Euphytica.

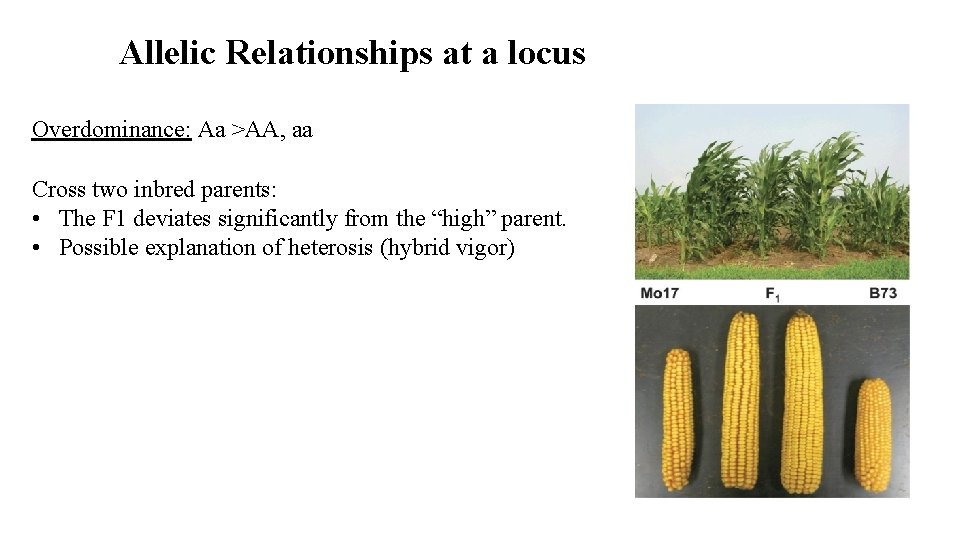
Allelic Relationships at a locus Overdominance: Aa >AA, aa Cross two inbred parents: • The F 1 deviates significantly from the “high” parent. • Possible explanation of heterosis (hybrid vigor)

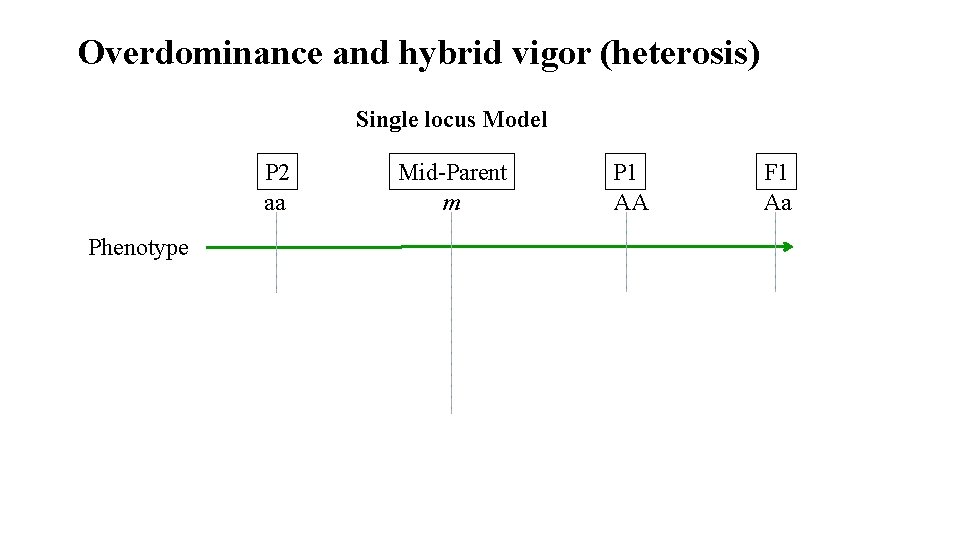
Overdominance and hybrid vigor (heterosis) Single locus Model P 2 aa Phenotype Mid-Parent m P 1 AA F 1 Aa

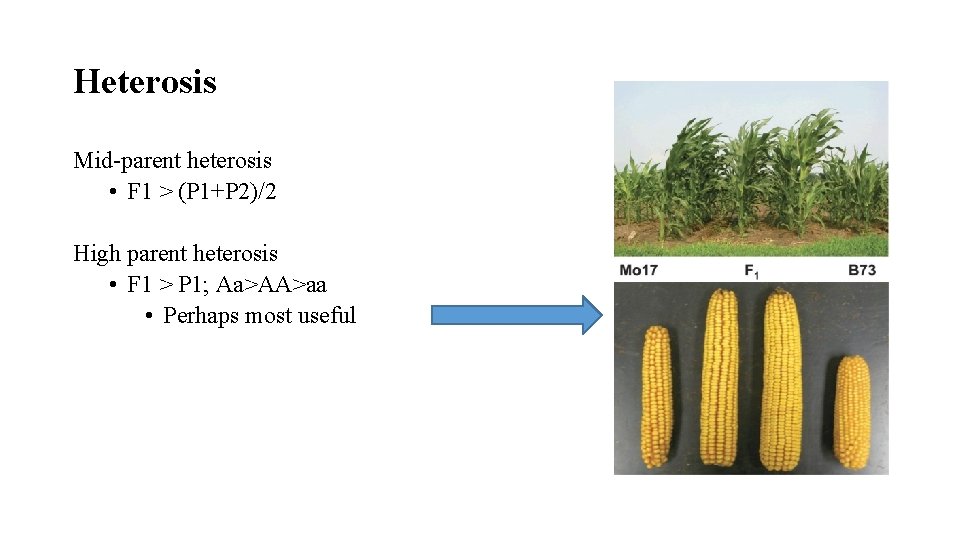
Heterosis Mid-parent heterosis • F 1 > (P 1+P 2)/2 High parent heterosis • F 1 > P 1; Aa>AA>aa • Perhaps most useful

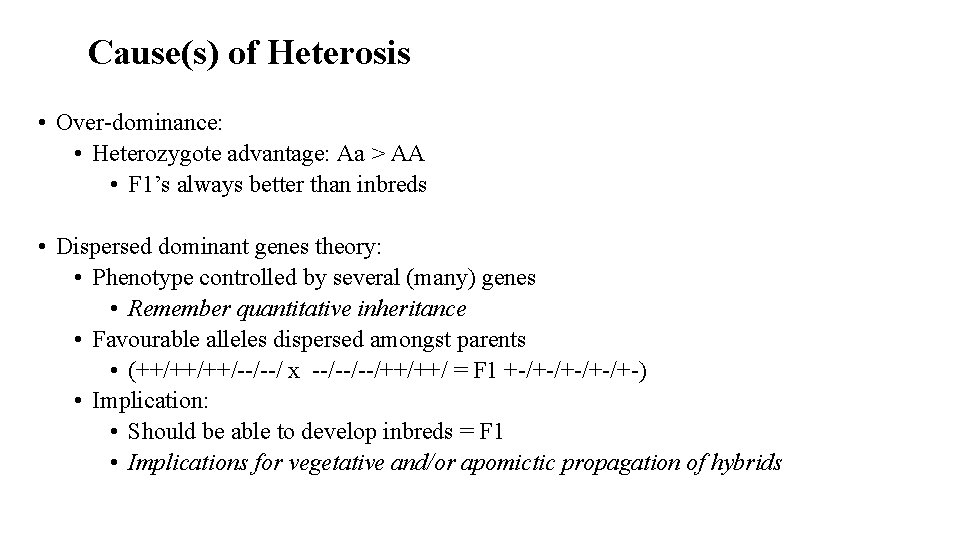
Cause(s) of Heterosis • Over-dominance: • Heterozygote advantage: Aa > AA • F 1’s always better than inbreds • Dispersed dominant genes theory: • Phenotype controlled by several (many) genes • Remember quantitative inheritance • Favourable alleles dispersed amongst parents • (++/++/++/--/--/ x --/--/--/++/++/ = F 1 +-/+-/+-) • Implication: • Should be able to develop inbreds = F 1 • Implications for vegetative and/or apomictic propagation of hybrids

The molecular basis of heterosis involves Structural variation: • SNPs and INDELs • SV (structural variation) • CNV (copy number variation) • PAV (presence/absence variation) Differences in expression level: • Parents – differential expression of most genes • F 1 • mid-parent level of gene expression • Non-additive expression Epigenetics: At the time of writing, “potential and possibilities”

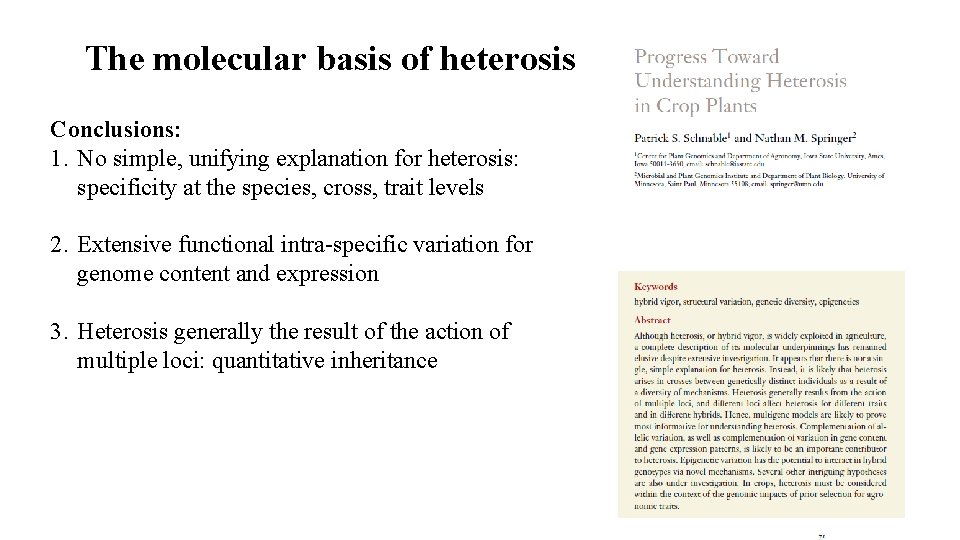
The molecular basis of heterosis Conclusions: 1. No simple, unifying explanation for heterosis: specificity at the species, cross, trait levels 2. Extensive functional intra-specific variation for genome content and expression 3. Heterosis generally the result of the action of multiple loci: quantitative inheritance

Allelic Variation - revisited 1. Many alleles are possible in a population, but in a diploid individual, there are only two alleles possible at a locus. a. Remember polyploids. 2. Mutation is the source of new alleles. a. Remember transgenics and edits. 3. There are many levels of allelic variation: a. DNA sequence changes with/without changes in phenotype. b. Differences in phenotype due to effects at the transcriptional, translational, and/or posttranslational levels. i. Remember epigenetics.

Intra-locus interactions Epistasis: Interaction(s) between alleles at different loci Remember: Gene interactions are the rules rather than the exceptions. Example: Duplicate recessive epistasis: Cyanide production in white clover.

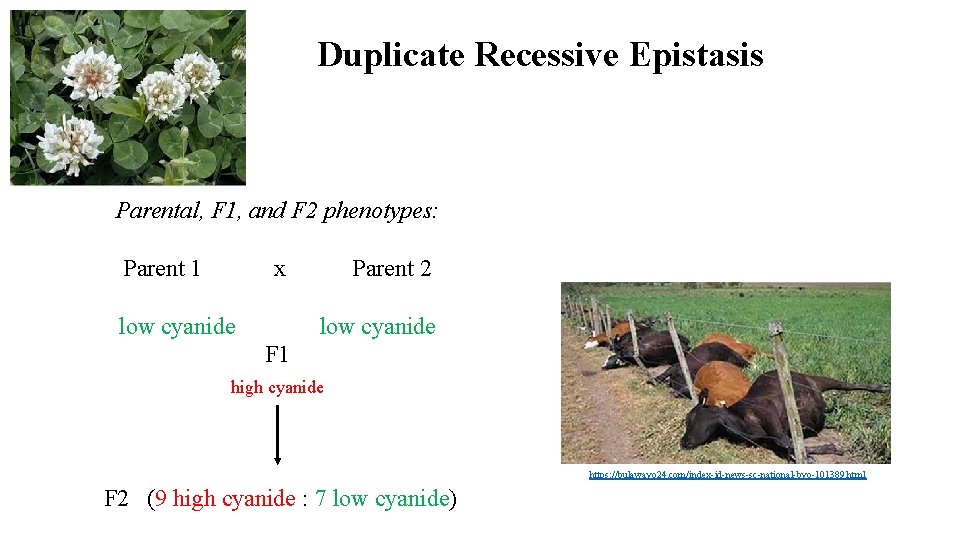
Duplicate Recessive Epistasis Parental, F 1, and F 2 phenotypes: Parent 1 x low cyanide Parent 2 low cyanide F 1 high cyanide https: //bulawayo 24. com/index-id-news-sc-national-byo-101389. html F 2 (9 high cyanide : 7 low cyanide)

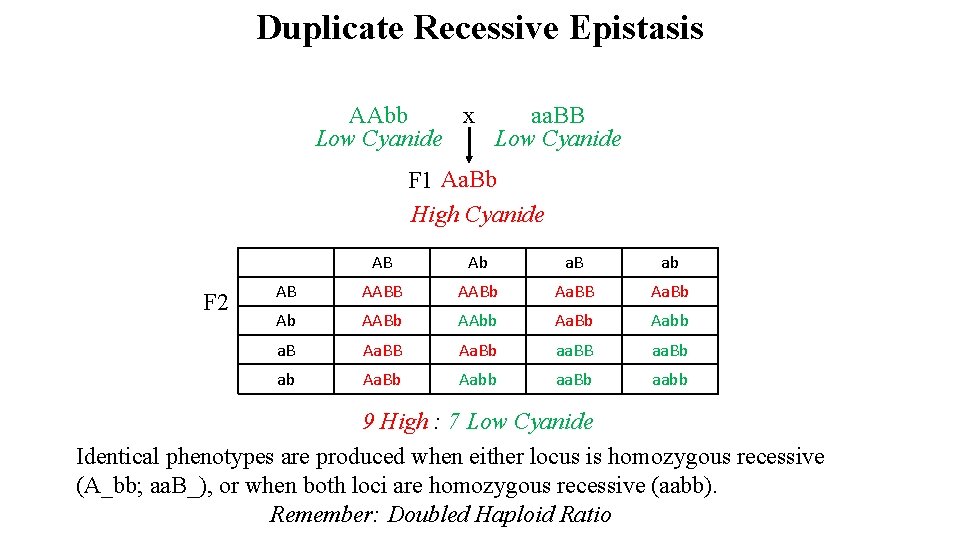
Duplicate Recessive Epistasis AAbb x aa. BB Low Cyanide F 1 Aa. Bb High Cyanide F 2 AB Ab a. B ab AB AABb Aa. BB Aa. Bb Ab AABb AAbb Aa. Bb Aabb a. B Aa. Bb aa. BB aa. Bb ab Aa. Bb Aabb aa. Bb aabb 9 High : 7 Low Cyanide Identical phenotypes are produced when either locus is homozygous recessive (A_bb; aa. B_), or when both loci are homozygous recessive (aabb). Remember: Doubled Haploid Ratio

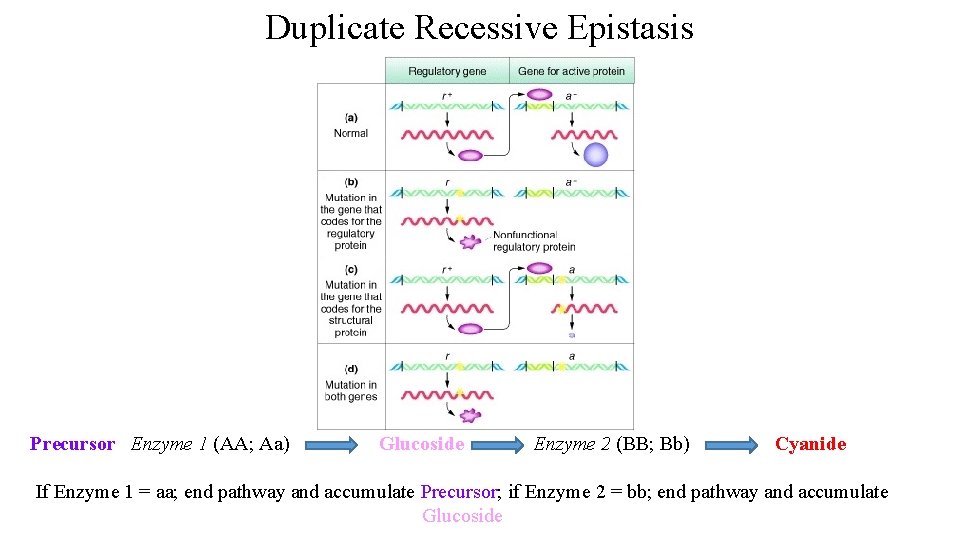
Duplicate Recessive Epistasis Precursor Enzyme 1 (AA; Aa) Glucoside Enzyme 2 (BB; Bb) Cyanide If Enzyme 1 = aa; end pathway and accumulate Precursor; if Enzyme 2 = bb; end pathway and accumulate Glucoside

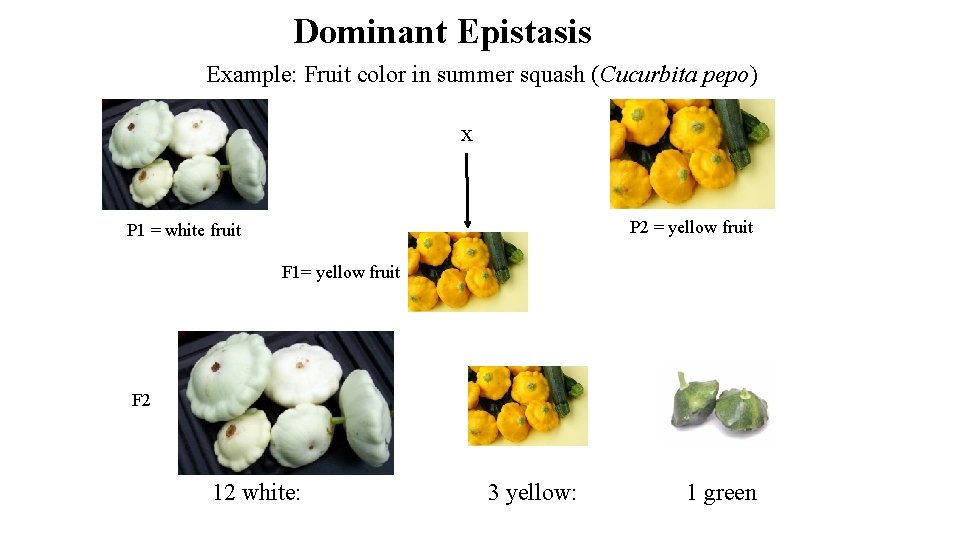
Dominant Epistasis Example: Fruit color in summer squash (Cucurbita pepo) x P 2 = yellow fruit P 1 = white fruit F 1= yellow fruit F 2 12 white: 3 yellow: 1 green

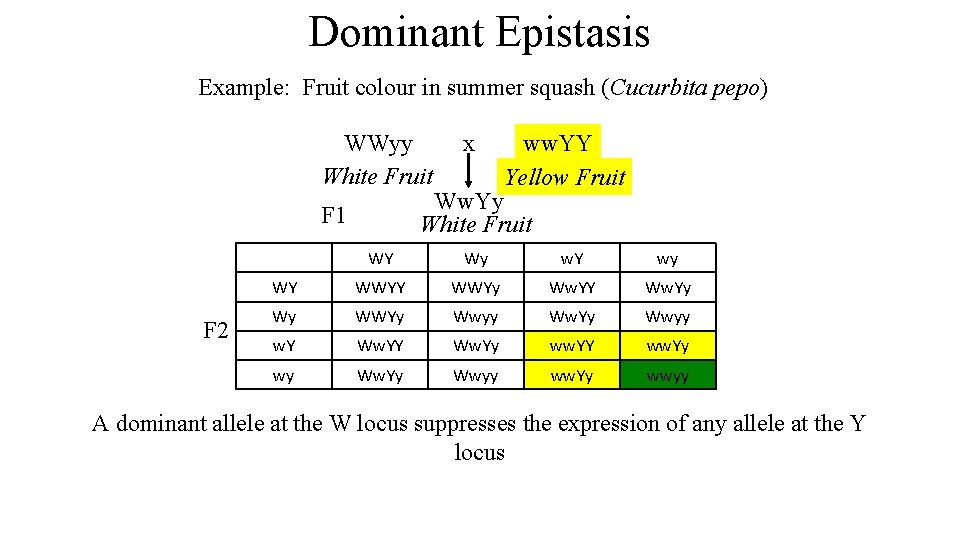
Dominant Epistasis Example: Fruit colour in summer squash (Cucurbita pepo) WWyy White Fruit ww. YY Yellow Fruit Ww. Yy White Fruit F 1 F 2 x WY Wy w. Y wy WY WWYy Ww. YY Ww. Yy Wy WWYy Wwyy Ww. Yy Wwyy w. Y Ww. Yy ww. YY ww. Yy wy Ww. Yy Wwyy ww. Yy wwyy A dominant allele at the W locus suppresses the expression of any allele at the Y locus

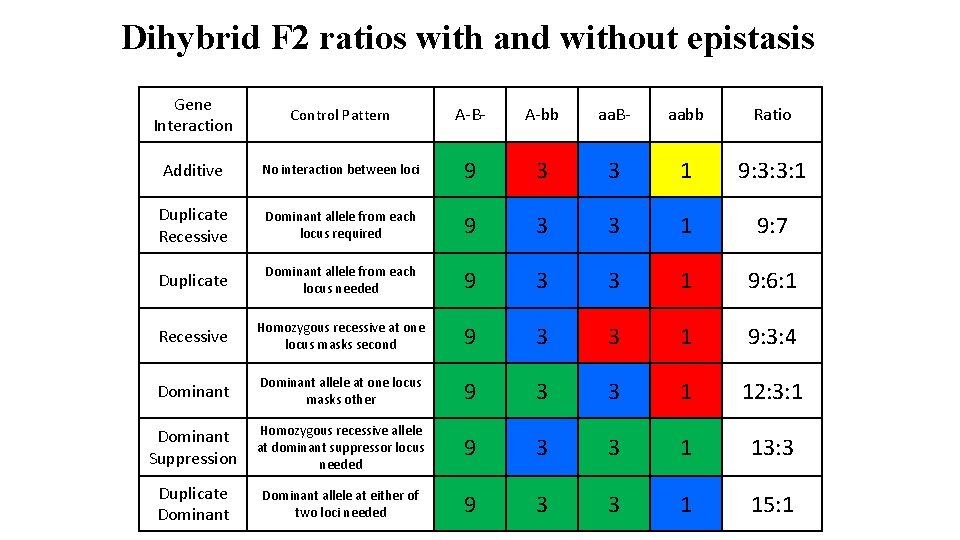
Dihybrid F 2 ratios with and without epistasis Gene Interaction Control Pattern A-B- A-bb aa. B- aabb Ratio Additive No interaction between loci 9 3 3 1 9: 3: 3: 1 Duplicate Recessive Dominant allele from each locus required 9 3 3 1 9: 7 Duplicate Dominant allele from each locus needed 9 3 3 1 9: 6: 1 Recessive Homozygous recessive at one locus masks second 9 3 3 1 9: 3: 4 Dominant allele at one locus masks other 9 3 3 1 12: 3: 1 Dominant Suppression Homozygous recessive allele at dominant suppressor locus needed 9 3 3 1 13: 3 Duplicate Dominant allele at either of two loci needed 9 3 3 1 15: 1

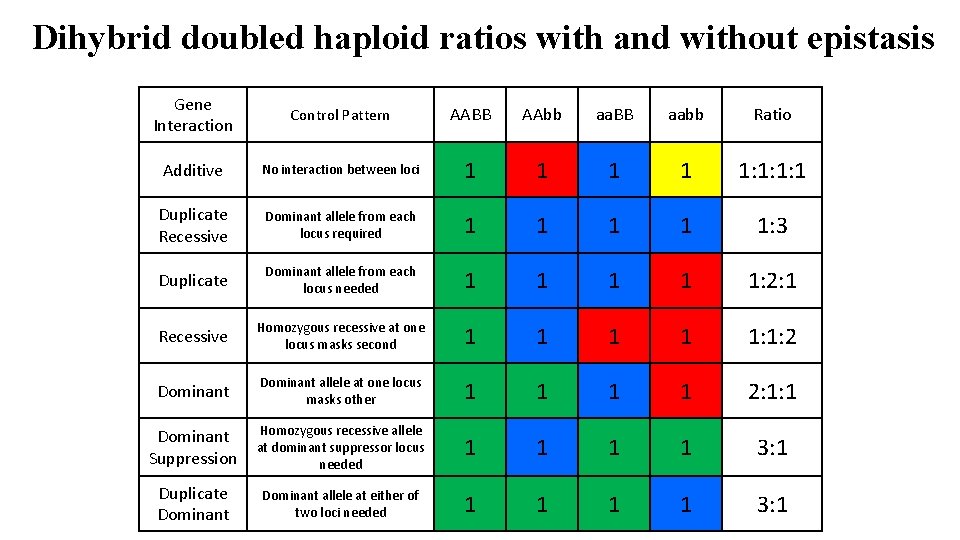
Dihybrid doubled haploid ratios with and without epistasis Gene Interaction Control Pattern AABB AAbb aa. BB aabb Ratio Additive No interaction between loci 1 1 1: 1: 1: 1 Duplicate Recessive Dominant allele from each locus required 1 1 1: 3 Duplicate Dominant allele from each locus needed 1 1 1: 2: 1 Recessive Homozygous recessive at one locus masks second 1 1 1: 1: 2 Dominant allele at one locus masks other 1 1 2: 1: 1 Dominant Suppression Homozygous recessive allele at dominant suppressor locus needed 1 1 3: 1 Duplicate Dominant allele at either of two loci needed 1 1 3: 1

Vernalization sensitivity and cold tolerance in barley Epistasis, near-isogenic lines, genotyping, sequencing, phenotyping, epigenetics, and climate change

The phenotype: Vernalization requirement/sensitivity In winter growth habit genotypes, exposure to low temperatures necessary for a timely transition from the vegetative to the reproductive growth stage. Why of interest? • Flowering biology = productivity (yield) • Correlated with low temperature tolerance • Low temperature tolerance require for winter survival • Many regions have winter precipitation patterns • Fall-planted, low temperature-tolerant cereal crops - a tool for dealing with climate change

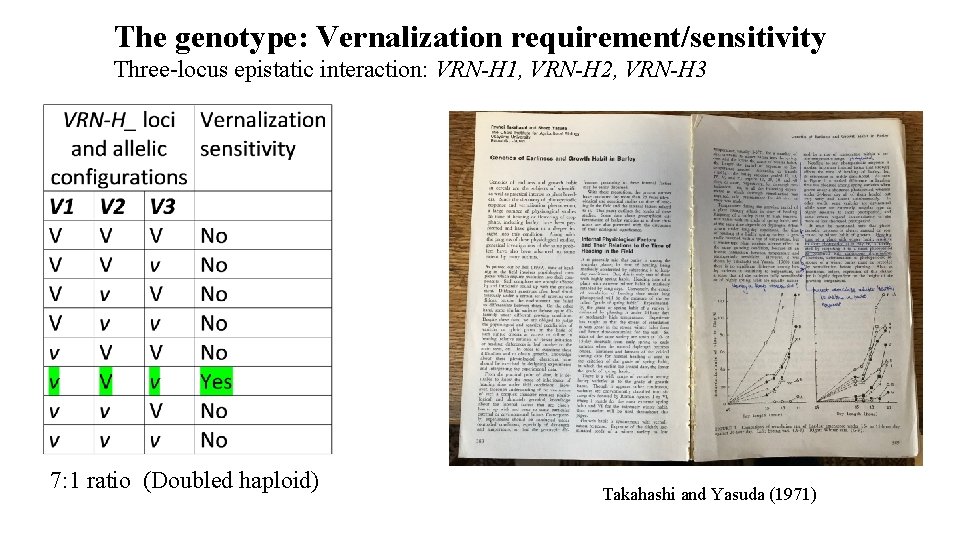
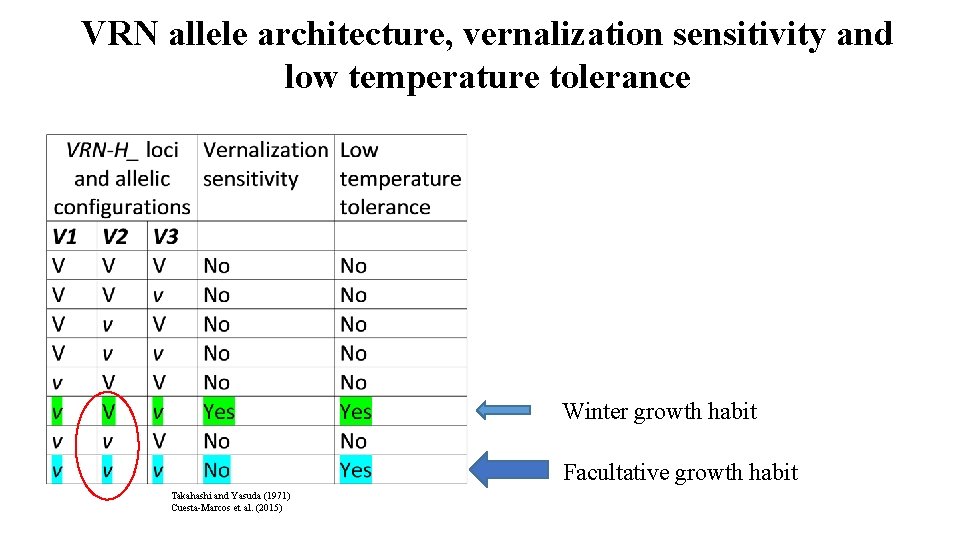
The genotype: Vernalization requirement/sensitivity Three-locus epistatic interaction: VRN-H 1, VRN-H 2, VRN-H 3 7: 1 ratio (Doubled haploid) Takahashi and Yasuda (1971)

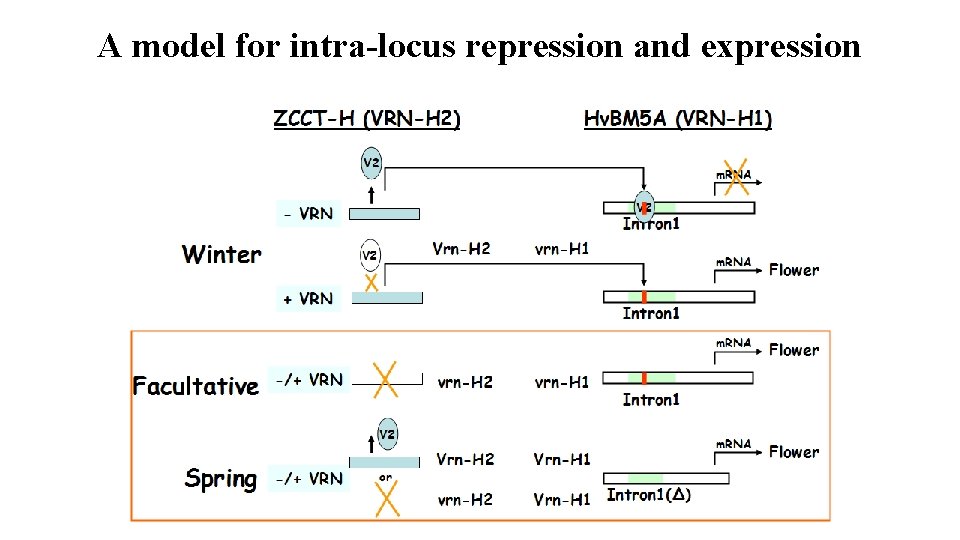
A model for intra-locus repression and expression

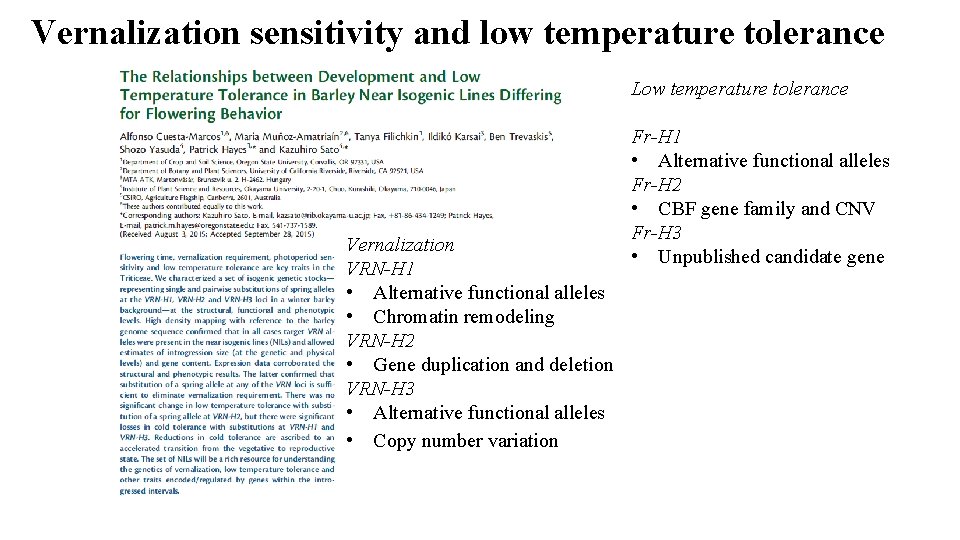
Vernalization sensitivity and low temperature tolerance Low temperature tolerance Vernalization VRN-H 1 • Alternative functional alleles • Chromatin remodeling VRN-H 2 • Gene duplication and deletion VRN-H 3 • Alternative functional alleles • Copy number variation Fr-H 1 • Alternative functional alleles Fr-H 2 • CBF gene family and CNV Fr-H 3 • Unpublished candidate gene

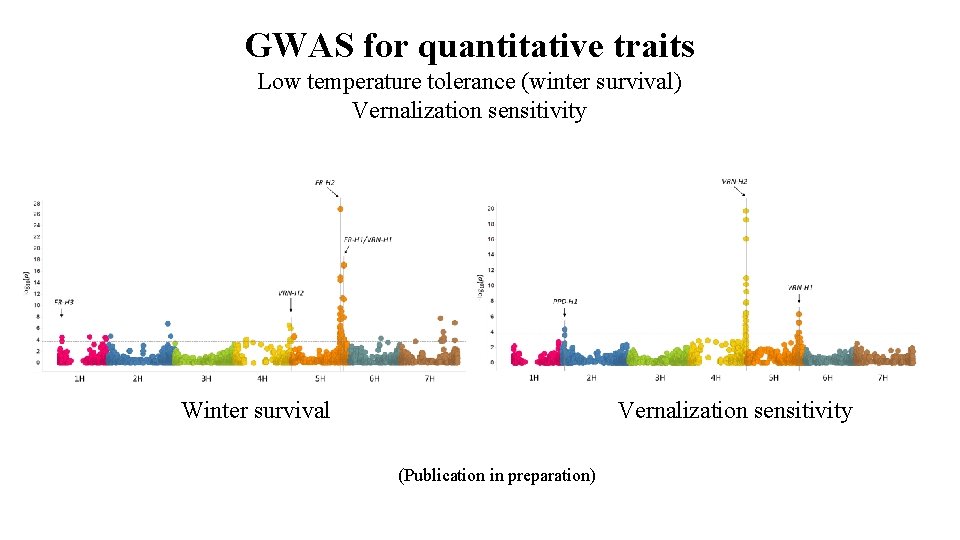
GWAS for quantitative traits Low temperature tolerance (winter survival) Vernalization sensitivity Winter survival Vernalization sensitivity (Publication in preparation)

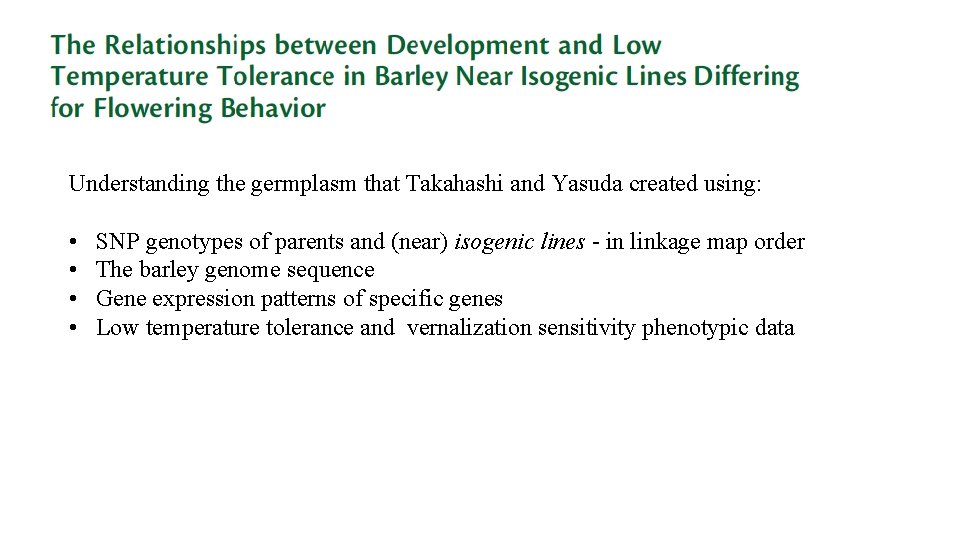
Understanding the germplasm that Takahashi and Yasuda created using: • • SNP genotypes of parents and (near) isogenic lines - in linkage map order The barley genome sequence Gene expression patterns of specific genes Low temperature tolerance and vernalization sensitivity phenotypic data

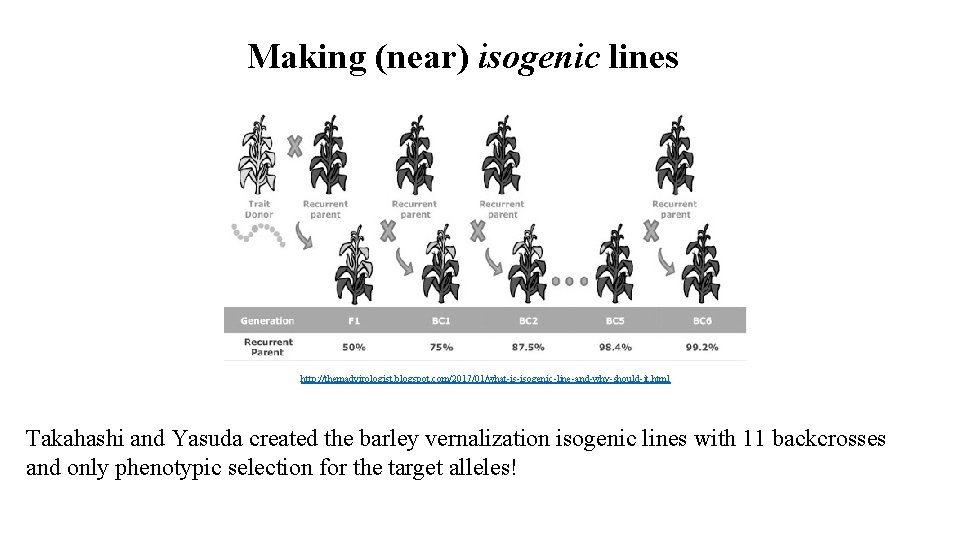
Making (near) isogenic lines http: //themadvirologist. blogspot. com/2017/01/what-is-isogenic-line-and-why-should-it. html Takahashi and Yasuda created the barley vernalization isogenic lines with 11 backcrosses and only phenotypic selection for the target alleles!

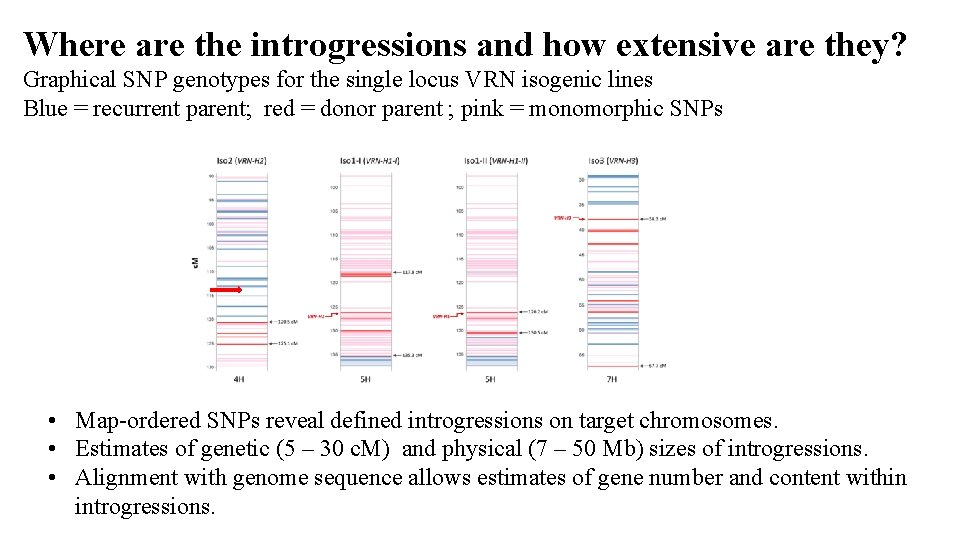
Where are the introgressions and how extensive are they? Graphical SNP genotypes for the single locus VRN isogenic lines Blue = recurrent parent; red = donor parent ; pink = monomorphic SNPs • Map-ordered SNPs reveal defined introgressions on target chromosomes. • Estimates of genetic (5 – 30 c. M) and physical (7 – 50 Mb) sizes of introgressions. • Alignment with genome sequence allows estimates of gene number and content within introgressions.

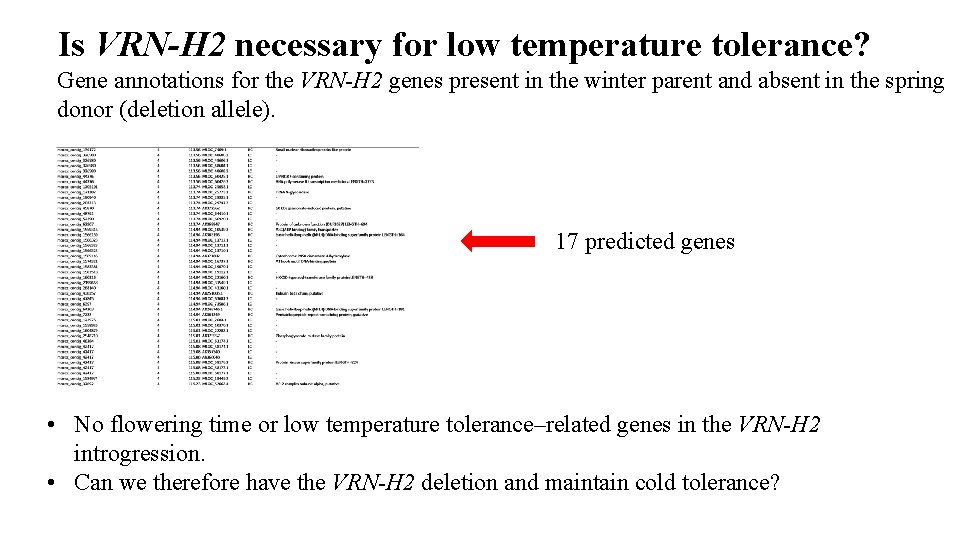
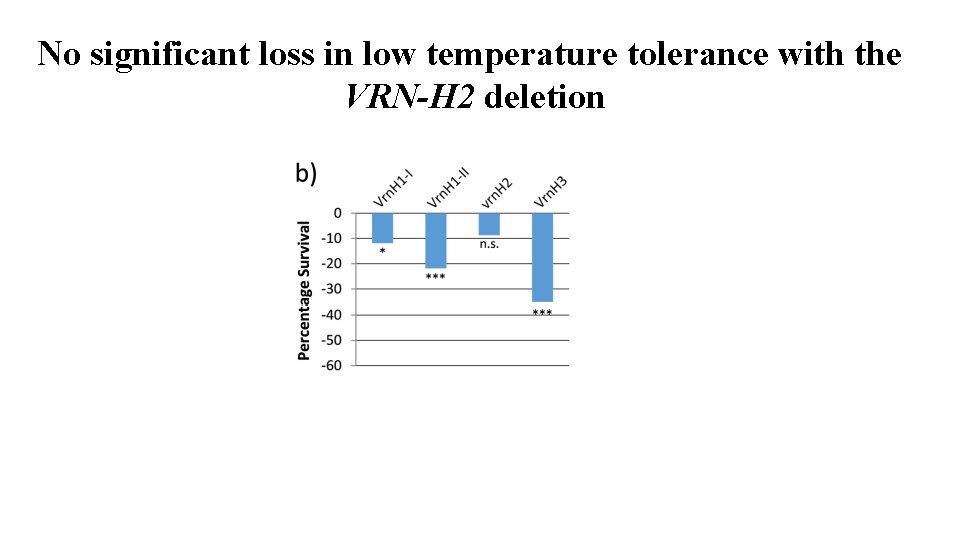
Is VRN-H 2 necessary for low temperature tolerance? Gene annotations for the VRN-H 2 genes present in the winter parent and absent in the spring donor (deletion allele). 17 predicted genes • No flowering time or low temperature tolerance–related genes in the VRN-H 2 introgression. • Can we therefore have the VRN-H 2 deletion and maintain cold tolerance?

No significant loss in low temperature tolerance with the VRN-H 2 deletion

VRN allele architecture, vernalization sensitivity and low temperature tolerance Winter growth habit Facultative growth habit Takahashi and Yasuda (1971) Cuesta-Marcos et al. (2015)

The facultative option Hard-wired for low temperature tolerance and short-day sensitivity No vernalization sensitivity The option to fall-plant and/or spring-plant the same variety • Reduces risk • Maximizes opportunities • Streamlines seed production and end-use

Facultative growth habit – ready for THE CHANGE? • “Just say no” to vernalization sensitivity with the “right” VRN-H 2 allele • A complete deletion • “Just say yes” to short day photoperiod sensitivity with the “right” photoperiod sensitivity allele (PPD-H 2) • “Ensure” a winter haplotype at all low temperature tolerance loci • Fr-H 1, FR-H 2, and FR-H 3 plus…. a continual process of discovery • Remember: Transgenics? Gene editing?

The facultative option Hard-wired for low temperature tolerance and short-day sensitivity No vernalization sensitivity The option to fall-plant and/or spring-plant the same variety • Take precautionary measures to maximize genetic diversity, or else…. the green bridge brings on Learn the lessons of the T cytoplasm, the Cavendish banana, …. . , ……….

The genetic status (degree of homozygosity) of the parents will determine which generation is appropriate for genetic analysis and the interpretation of the data (e. g. comparison of observed vs. expected phenotypes or genotypes).

The degree of homozygosity of the parents will likely be a function of their mating biology, e. g. cross vs. self-pollinated.

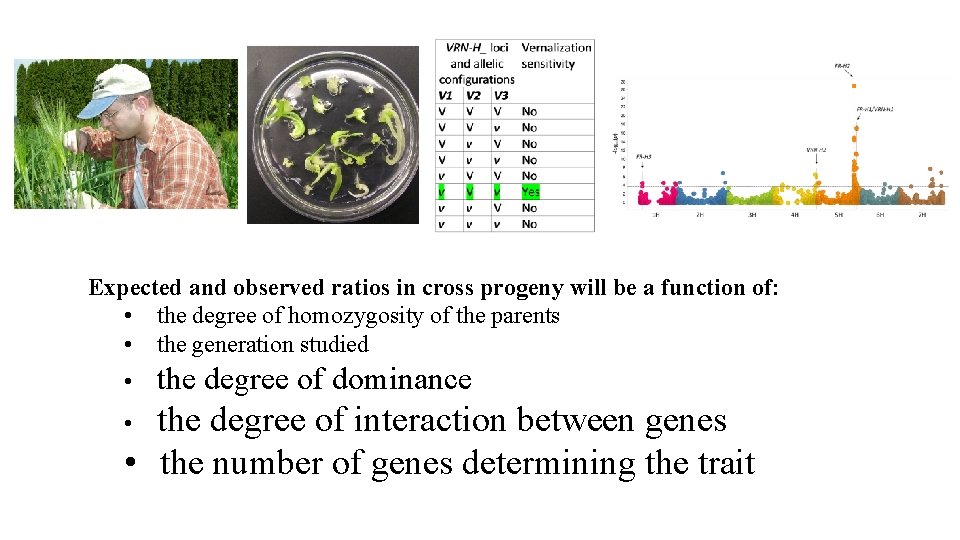
Expected and observed ratios in cross progeny will be a function of: • the degree of homozygosity of the parents • the generation studied • the degree of dominance the degree of interaction between genes • the number of genes determining the trait •
 What is gene regulation
What is gene regulation Negative control
Negative control Prokaryotes vs eukaryotes gene regulation
Prokaryotes vs eukaryotes gene regulation Chapter 18 regulation of gene expression
Chapter 18 regulation of gene expression Eukaryotic promoters are usually found just
Eukaryotic promoters are usually found just Gene regulation
Gene regulation Chapter 18 regulation of gene expression
Chapter 18 regulation of gene expression Section 12-1 dna
Section 12-1 dna Regulation of gene expression in bacteria
Regulation of gene expression in bacteria Differential gene regulation
Differential gene regulation Chapter 12 section 4 gene regulation and mutations
Chapter 12 section 4 gene regulation and mutations Section 4 gene regulation and mutation
Section 4 gene regulation and mutation Chapter 18 regulation of gene expression
Chapter 18 regulation of gene expression Chapter 18 regulation of gene expression
Chapter 18 regulation of gene expression Regulation of gene expression
Regulation of gene expression Gene structure prokaryotes vs eukaryotes
Gene structure prokaryotes vs eukaryotes Gene by gene test results
Gene by gene test results Chapter 17: from gene to protein
Chapter 17: from gene to protein Migraine management guidelines
Migraine management guidelines Firefly gene in tobacco plant
Firefly gene in tobacco plant Genetically modified crops
Genetically modified crops Rdna technology applications
Rdna technology applications Genetically modified organisms
Genetically modified organisms Gmos advantages and disadvantages
Gmos advantages and disadvantages Genetically modified crops have
Genetically modified crops have Genetically modified food
Genetically modified food Genetically modified organisms
Genetically modified organisms Are fingerprints genetically inherited
Are fingerprints genetically inherited Diverse offspring
Diverse offspring The creation of genetically identical offspring
The creation of genetically identical offspring Chapter 22 genetics and genetically linked diseases
Chapter 22 genetics and genetically linked diseases Genetically modified crops have
Genetically modified crops have The integration of eye, hand, and foot movements
The integration of eye, hand, and foot movements Example of skill-related fitness
Example of skill-related fitness Played by being struck or shaken
Played by being struck or shaken Perhap the most striking
Perhap the most striking Jenis permainan invasi
Jenis permainan invasi Welding stringer beads
Welding stringer beads What does percy find striking about annabeth's appearance
What does percy find striking about annabeth's appearance Kata khusus
Kata khusus Bright colors in striking patterns warn others
Bright colors in striking patterns warn others How to foil in biology
How to foil in biology Multiple alleles genotypes
Multiple alleles genotypes