GCPs IV Study Records FDA Inspection Common Deficiencies



















- Slides: 21

GCPs IV Study Records

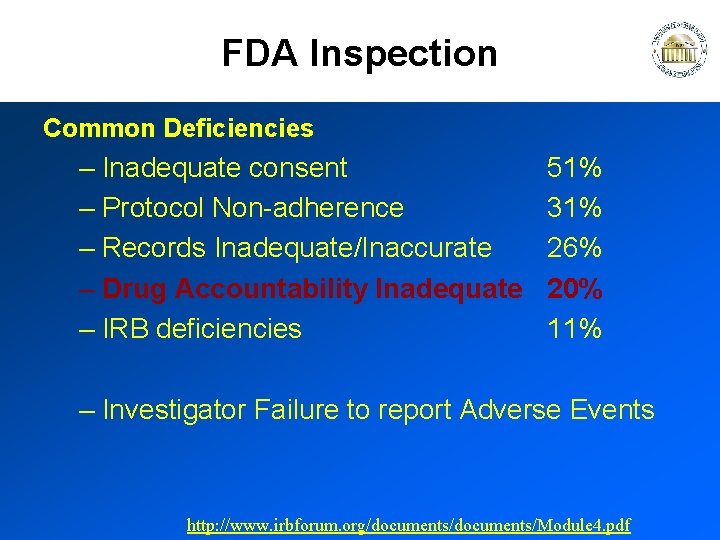
FDA Inspection Common Deficiencies – Inadequate consent – Protocol Non-adherence – Records Inadequate/Inaccurate – Drug Accountability Inadequate – IRB deficiencies 51% 31% 26% 20% 11% – Investigator Failure to report Adverse Events http: //www. irbforum. org/documents/Module 4. pdf

Source Documents What is a source document? – The first place things are written down • Traditionally Chart notes Examples – Chart Notes – Clinic Charts – Lab Reports – Phone Logs – Physician Letter – X-rays, CTs, MRI etc.

Source Documents For some studies (traditionally industry) – Pre-templated Source documents could be provided for you – This will make your life easier!!!!! – The source documents walk you through all the questions you need to ask and the procedures you need to perform!

Case Report Forms (CRF’s) What is a case report form? – Collects the trial required data

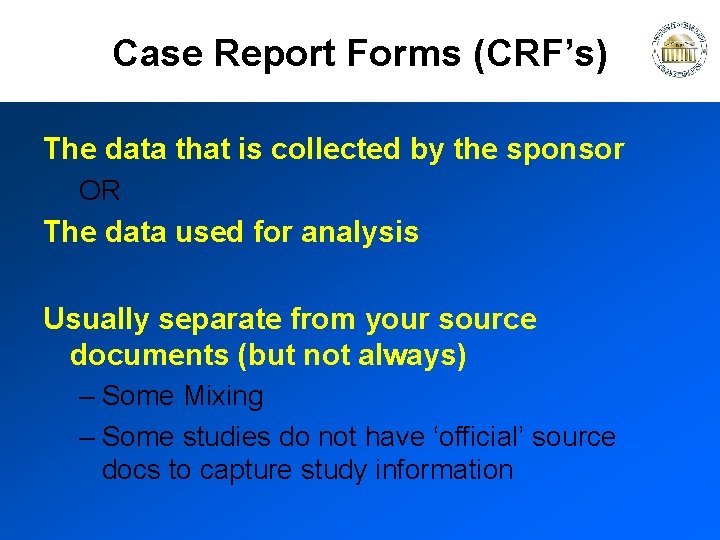
Case Report Forms (CRF’s) The data that is collected by the sponsor OR The data used for analysis Usually separate from your source documents (but not always) – Some Mixing – Some studies do not have ‘official’ source docs to capture study information

Documentation in Source Docs / CRFs Complete in black ink only Cross out error with a single black line Write correct information in as close as possible Date and initial the change Both original and new information must be legible



Source Document Helpful Hints Obtain as much information on medical history and concomitant medications as is available – if the patient does not know (ex: dosage) ask them to find out and to call you If patient has been diagnosed with depression the Investigator should indicate that subject is stable or the depression will not interfere with the study Document Birth Control as well as the discussion about proper birth control

Source Document Helpful Hints Make sure all clocks are synchronized and if possible, use 24: 00 digital clocks Include explanations of mistakes – (Ex: Patient accidentally wrote the incorrect year) Initial and Date Mistakes Document reason for late entries Document all attempts to contact subject and schedule visits

CRF Helpful Hints Remember on NCR pages – use the cardboard divider so you have clean records – It may help to put some plain white paper between the source docs and CRFs as well USE BLACK BALLPOINT INK Remember to fill out your headers Take your time when transcribing – Make sure you are using the right patient binders – Copy neatly and correctly – Correct mistakes immediately

Helpful Hints Double check everything before the patient leaves Document everything!! – Use Notes to File Re-check documents BEFORE the next patient visit – FLAG missing items – If you fix / correct / add anything make sure you initial – date – explain the addition

Definitions: Source Documents (Source Doc) Original (medical) records including hospital records, clinics visit records, lab reports, memos, subject diaries, evaluation checklists, pharmacy records, recorded data from automated instruments, radiology reports and X-rays, etc… related to the study.

Definitions: Research Record Often, a separate research record is maintained in addition to the patient medical record, however – the original is always the source doc and a site monitor will need to see the source doc. A separate research record is recommended if the medical record is stored in a remote location not under the control of research staff. SOPs regarding this are sponsor specific.

Definitions: Case Report Form (CRF) A standardized form used to abstract study data from the source doc and report it to the sponsor data management center. All data reported on the CRF must exactly match the source doc and be verifiable using the source doc alone. (ICH E 6 4. 9. 2).

Documentation Guidelines: • Every person making an entry must sign and date the document and should be on the signature/initials log. • NEVER obliterate. Correct errors with single line, initial, date. (ICH E 6 4. 9. 3) • If serial evaluations or entries in a note, write the time. • Follow-up on the status of diagnoses at the prior visit.

Study visit #3 activities / CRF data – Subject arrives, varicella vaccine is reconstituted in the pharmacy CRF: history negative for local site reactions, rash, fever post vaccine #1. CRF: PE negative for fever, skin lesions Vaccine #2 is administered Blood drawn for CTL assay to varicella antigen and CD 4 count Adherence to concomitant medications interview completed Subject is observed 2 hours in the clinic and then PE above repeated. Parent is phoned 48 hours later and queried re fever, local site reactions List the required source doc

There must be ‘source doc’ for everything – Dispensing of additional study drug, administration of study vaccine – Completion of questionnaires or instruments – Venipunctures or injection (include how tolerated) – Follow-up observations in clinic or phone calls – Patient compliance with protocol requirements (for example – fasting or not taking other meds) – The absence of complications or events specifically asked for on the CRF – Required laboratory tests obtained (or missed) Develop checklists or use a copy of the CRF (signed and dated) as ‘source doc’ if needed.

FDA Inspection Common Deficiencies – Inadequate consent – Protocol Non-adherence – Records Inadequate/Inaccurate – Drug Accountability Inadequate – IRB deficiencies 51% 31% 26% 20% 11% – Investigator Failure to report Adverse Events http: //www. irbforum. org/documents/Module 4. pdf

Study Drug Accountability Drug/Device Dispensing Accountability – Maintain a dispensing log (if a medication/vaccine is reconstituted, log must note time and date reconstituted) – Shipping receipt – Prescription or order for dispensing drug on file – Storage requirement compliance documented – NEVER administer study med without a physician order or prescription. Maintain record of physician orders to administer.
 Gcps gmail
Gcps gmail Bimo audit checklist
Bimo audit checklist Fda bimo inspection checklist
Fda bimo inspection checklist Exaggerated physical deficiencies
Exaggerated physical deficiencies Abnormal development adler
Abnormal development adler Fda early feasibility study
Fda early feasibility study A large study used records from canada's
A large study used records from canada's Common factors of 48 and 60
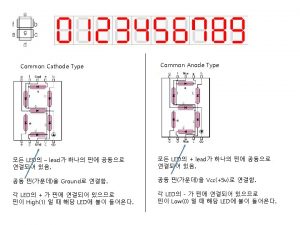
Common factors of 48 and 60 Common anode and common cathode
Common anode and common cathode Factor tree of 48
Factor tree of 48 How to find lowest common factor
How to find lowest common factor Common factors of 12 and 42
Common factors of 12 and 42 Highest common factors and lowest common multiples
Highest common factors and lowest common multiples Changfu wu fda
Changfu wu fda Nicole gillette fda
Nicole gillette fda Leonard sacks fda
Leonard sacks fda Fda mystudies
Fda mystudies Dq design qualification
Dq design qualification Sulmetin
Sulmetin Fda vs brown and williamson
Fda vs brown and williamson Klasyfikacja fda leków stosowanych w ciąży przykłady
Klasyfikacja fda leków stosowanych w ciąży przykłady Institut za kardiovaskularne bolesti sremska kamenica
Institut za kardiovaskularne bolesti sremska kamenica