Fundamentals of Materials Science Chapter 2 Fundamentals of






![[krai'tiəriən] (批评、判断等的)标准, 准则,尺度 2. Criterion for choice of unit cell n n n Symmetry [krai'tiəriən] (批评、判断等的)标准, 准则,尺度 2. Criterion for choice of unit cell n n n Symmetry](https://slidetodoc.com/presentation_image/4918a51501d8e065b289b667f2416456/image-35.jpg)
- Slides: 40

材料科学基础 Fundamentals of Materials Science Chapter 2 Fundamentals of Crystallology

Chapter 2 Basic Crystallology § 2. 1 Crystal Character & Space Lattice

Ⅰ. Crystals versus non-crystals 1. Substance states



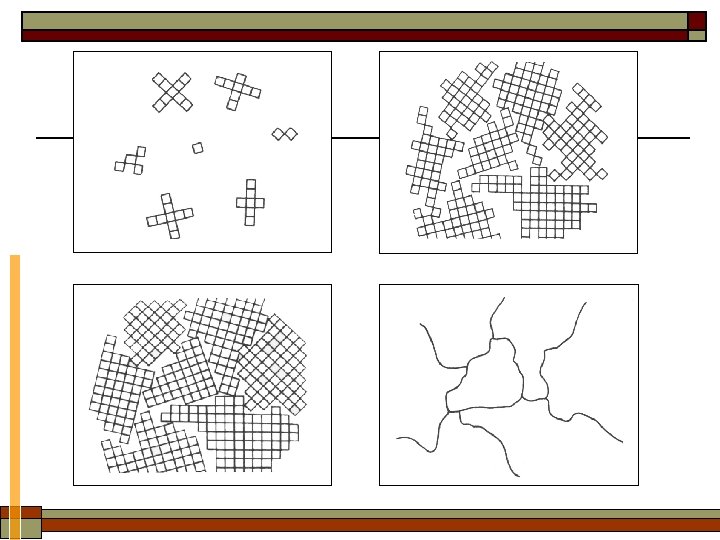
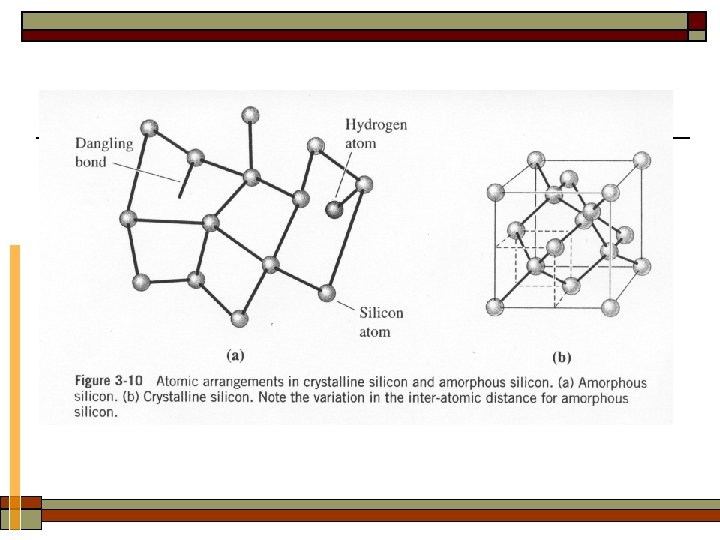
2. Classification of materials based on structure Regularity in atom arrangement —— periodic or not (amorphous) Ø Crystal: The materials atoms are arranged in a periodic fashion. ü Single crystal: in the form of one crystal grains ü Polycrystal: grain boundaries Amorphous: The material’s atoms do not have a long-range order (0. 1~ 1 nm).



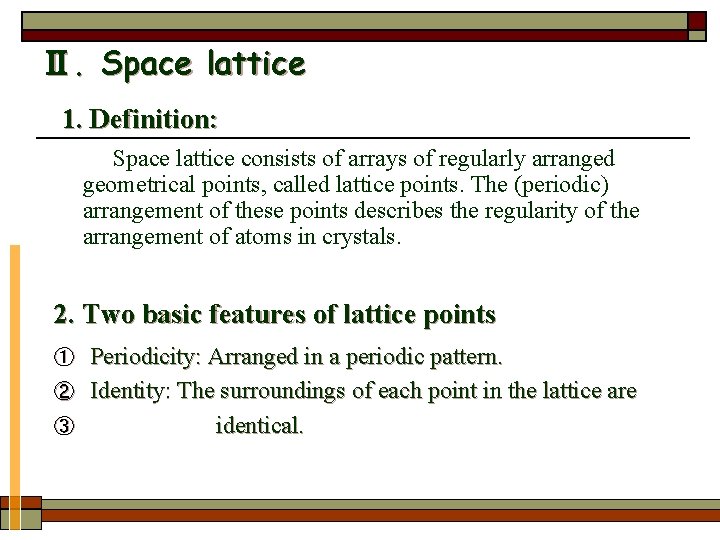
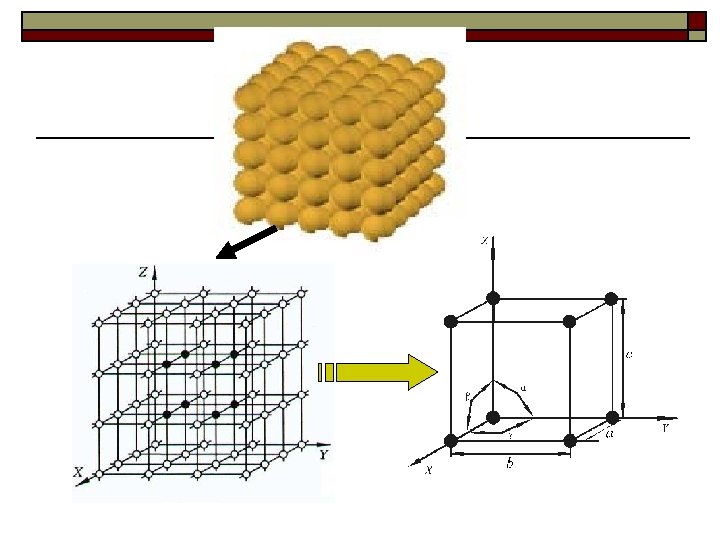
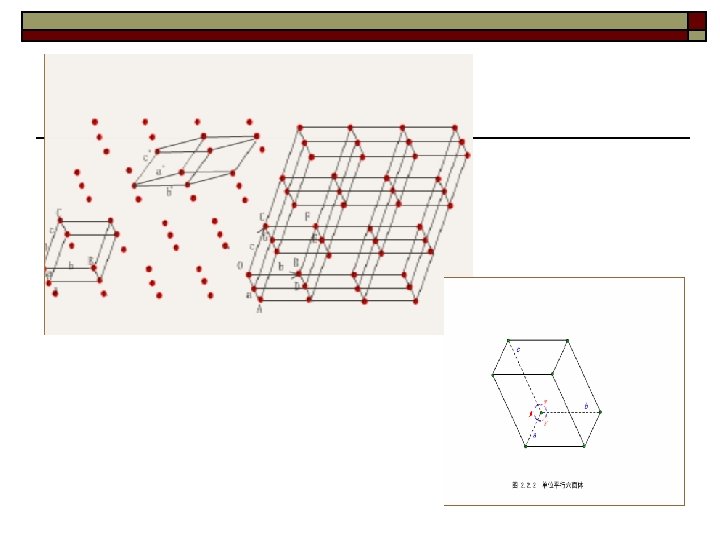
Ⅱ. Space lattice 1. Definition: Space lattice consists of arrays of regularly arranged geometrical points, called lattice points. The (periodic) arrangement of these points describes the regularity of the arrangement of atoms in crystals. 2. Two basic features of lattice points Periodicity: Arranged in a periodic pattern. ② Identity: The surroundings of each point in the lattice are ③ identical. ①


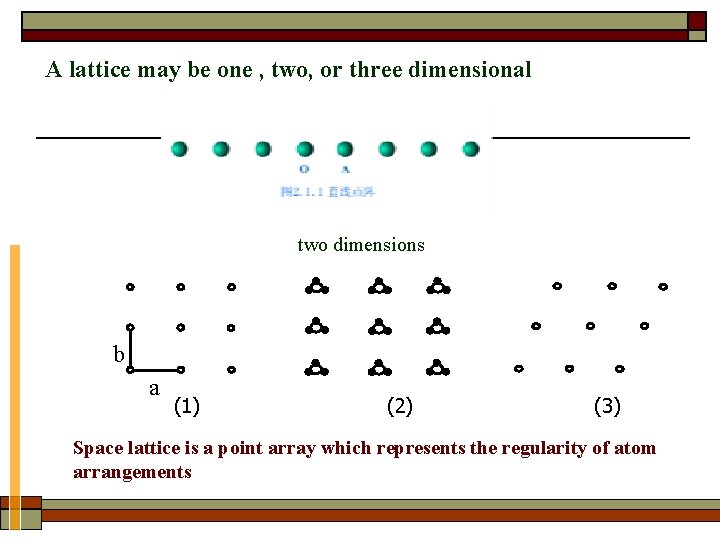
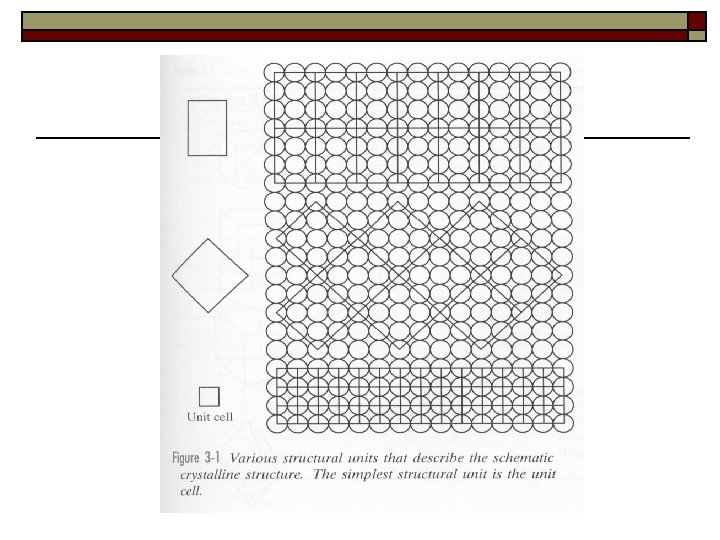
A lattice may be one , two, or three dimensional two dimensions b a (1) (2) (3) Space lattice is a point array which represents the regularity of atom arrangements

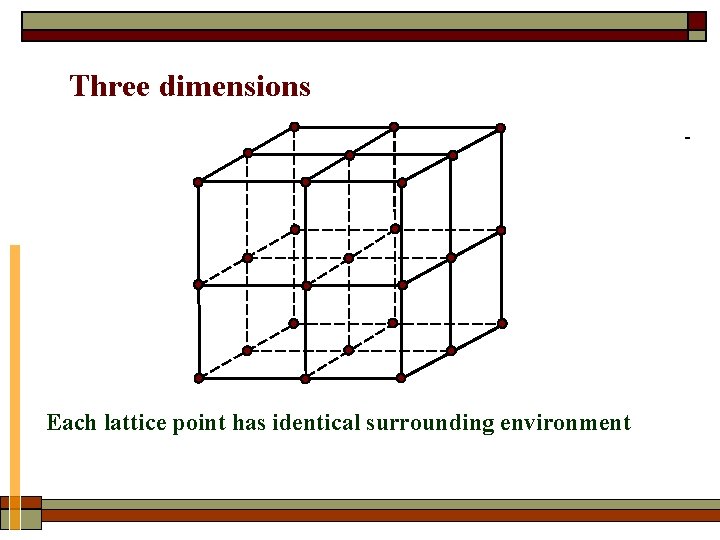
Three dimensions Each lattice point has identical surrounding environment


Ⅲ. Unit cell and lattice constants 1. Unit cell is the smallest unit of the lattice. The whole lattice can be obtained by infinitive repetition of the unit cell along it’s three edges. 2. The space lattice is characterized by the size and shape of the unit cell.



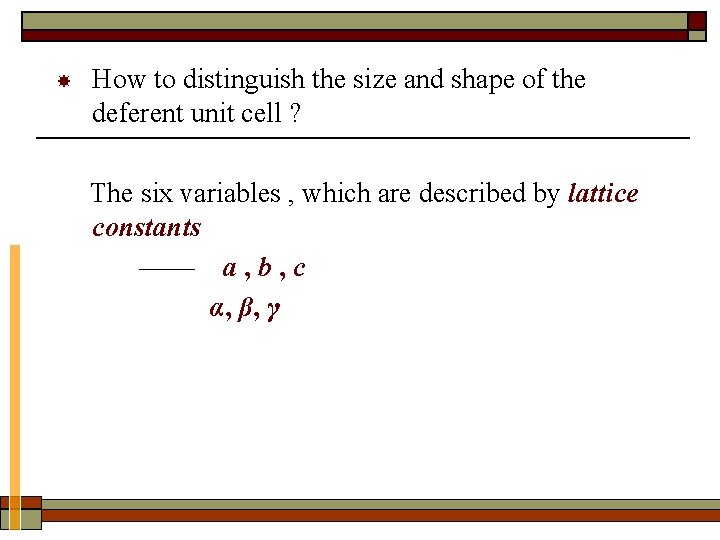
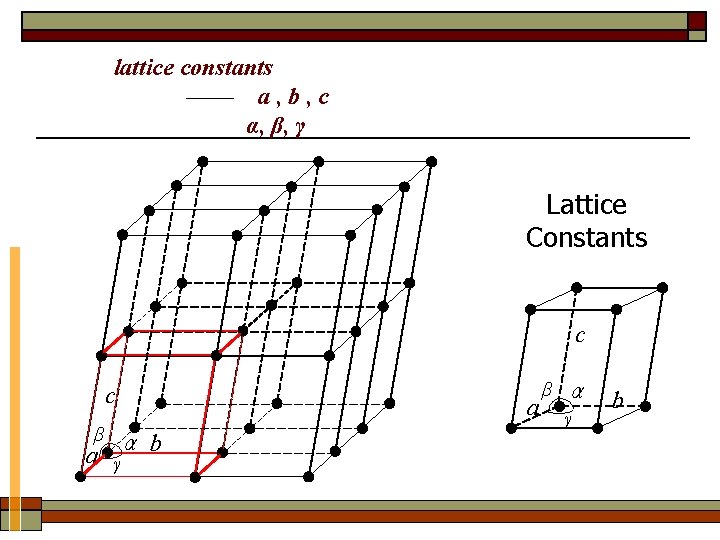
How to distinguish the size and shape of the deferent unit cell ? The six variables , which are described by lattice constants —— a , b , c α, β, γ

lattice constants —— a , b , c α, β, γ Lattice Constants c β α a γ b b

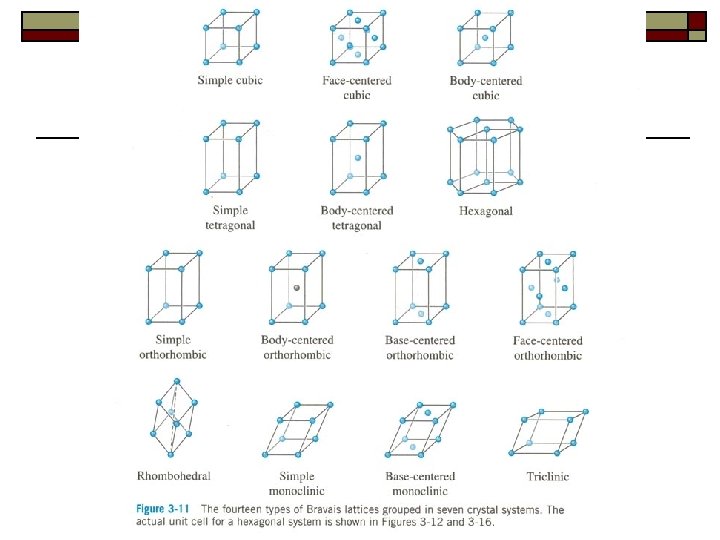
§ 2. 2 Crystal System & Lattice Types If a rotation around an axis passing through the crystal by an angle of 360 o/n can bring the crystal into coincidence with itself, the crystal is said to have a n-fold rotation symmetry. And axis is said to be n-fold rotation axis. We identify 14 types of unit cells, or Bravais lattices, grouped in seven crystal systems.

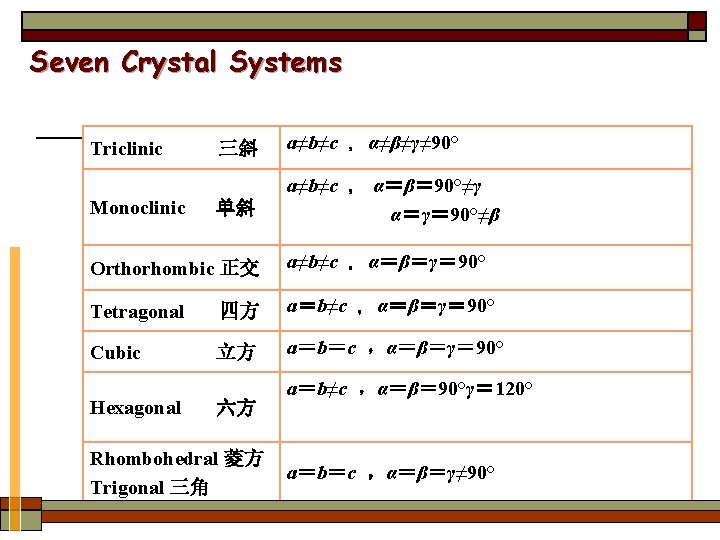
u Ⅰ. Seven crystal systems All possible structures reduce to a small number of basic unit cell geometries. ① There are only seven, unique unit cell shapes that can be stacked together to fill three-dimensional lattices. ② We must consider how atoms can be stacked together within a given unit cell.

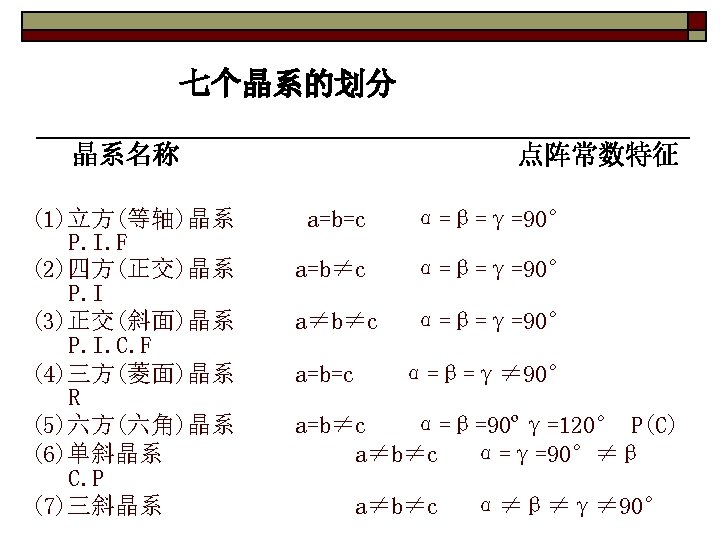
Seven Crystal Systems Triclinic Monoclinic 三斜 a≠b≠c ,α≠β≠γ≠ 90° 单斜 a≠b≠c , α=β= 90°≠γ α=γ= 90°≠β Orthorhombic 正交 a≠b≠c ,α=β=γ= 90° Tetragonal 四方 a=b≠c ,α=β=γ= 90° Cubic 立方 a=b=c ,α=β=γ= 90° Hexagonal 六方 Rhombohedral 菱方 Trigonal 三角 a=b≠c ,α=β= 90°γ= 120° a=b=c ,α=β=γ≠ 90°


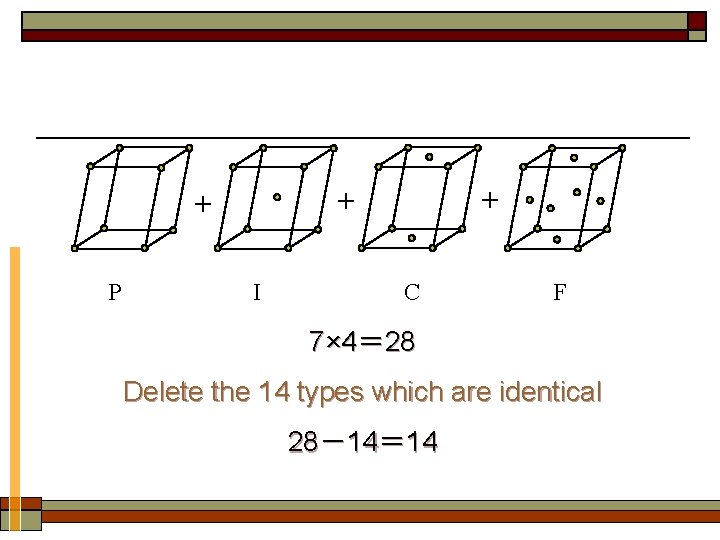
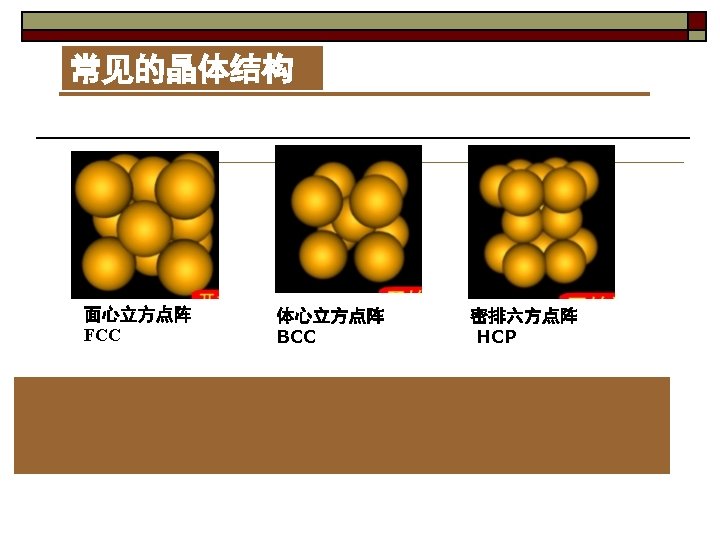
Ⅱ. 14 types of Bravais lattices 1. Derivation of Bravais lattices can be derived by adding points to the center of the body and/or external faces and deleting those lattices which are identical.

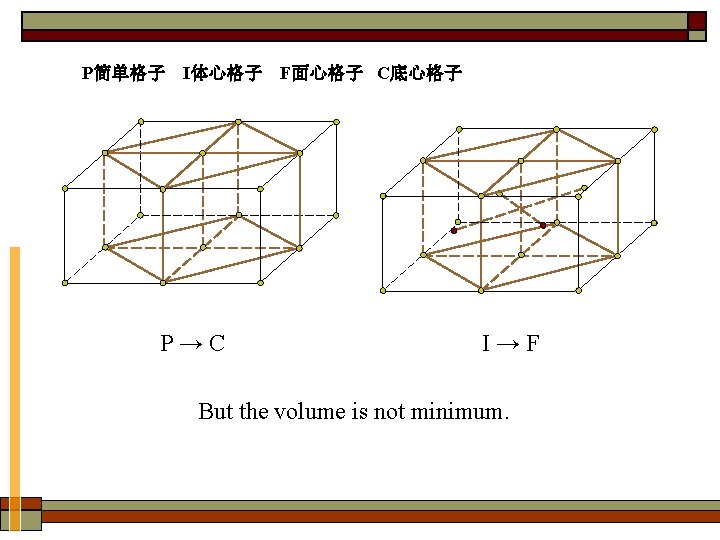
P + + + I C F 7× 4= 28 Delete the 14 types which are identical 28-14= 14

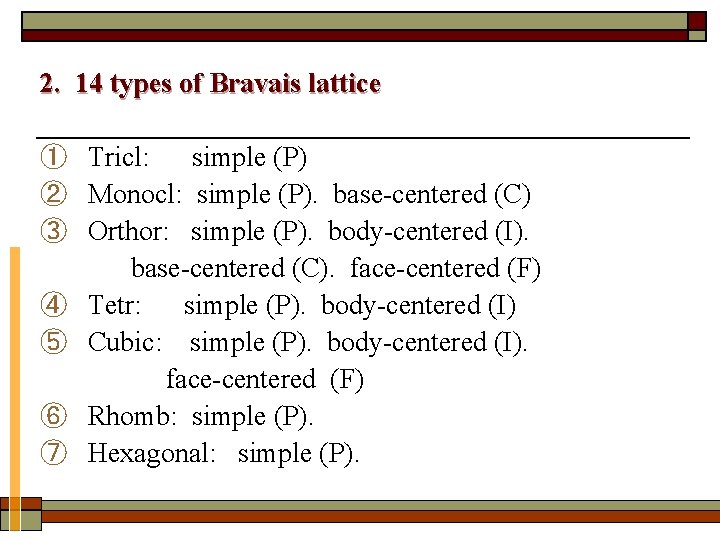
2. 14 types of Bravais lattice ① Tricl: simple (P) ② Monocl: simple (P). base-centered (C) ③ Orthor: simple (P). body-centered (I). base-centered (C). face-centered (F) ④ Tetr: simple (P). body-centered (I) ⑤ Cubic: simple (P). body-centered (I). face-centered (F) ⑥ Rhomb: simple (P). ⑦ Hexagonal: simple (P).



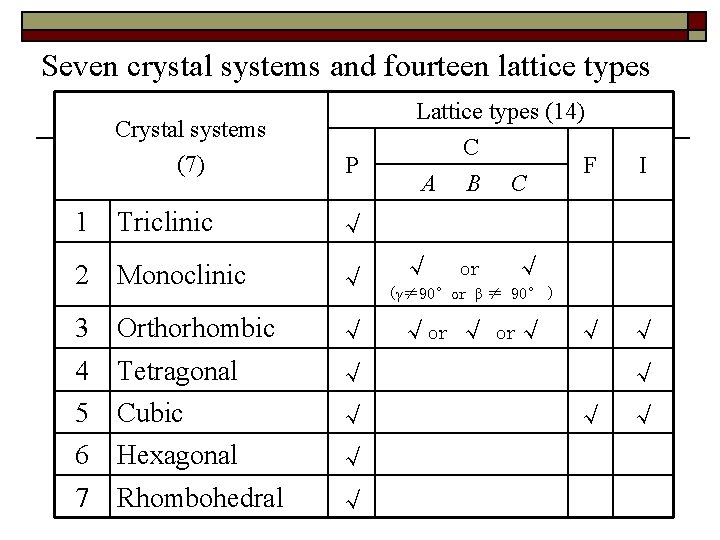
Seven crystal systems and fourteen lattice types Crystal systems (7) P Lattice types (14) C F A B C I 1 Triclinic √ 2 Monoclinic √ 3 Orthorhombic √ 4 Tetragonal √ √ 5 Cubic √ √ √ 6 Hexagonal √ 7 Rhombohedral √ √ or √ (γ≠ 90°or β ≠ 90° ) √or √ or√ √ √

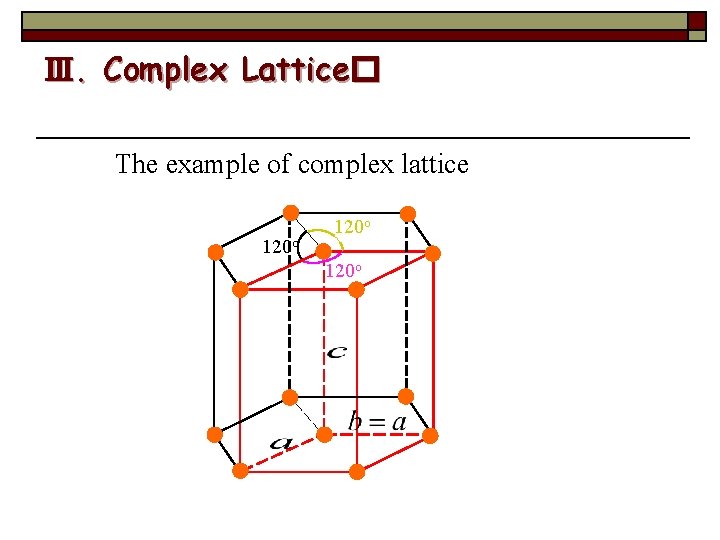
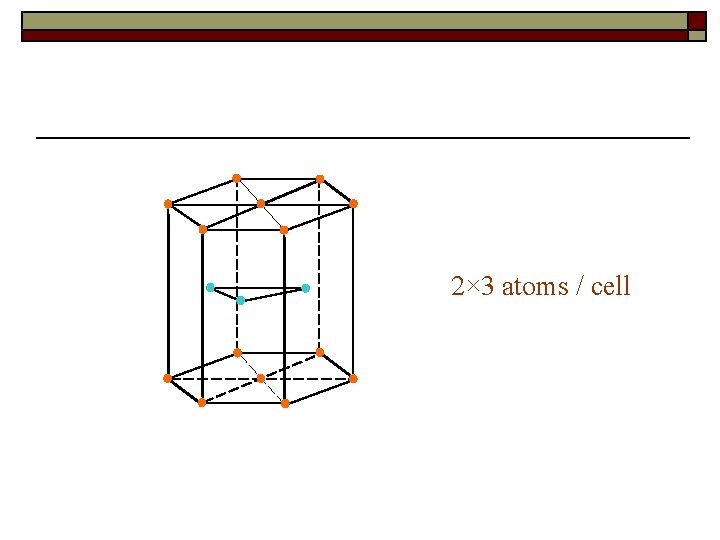
Ⅲ. Complex Lattice� The example of complex lattice 120 o

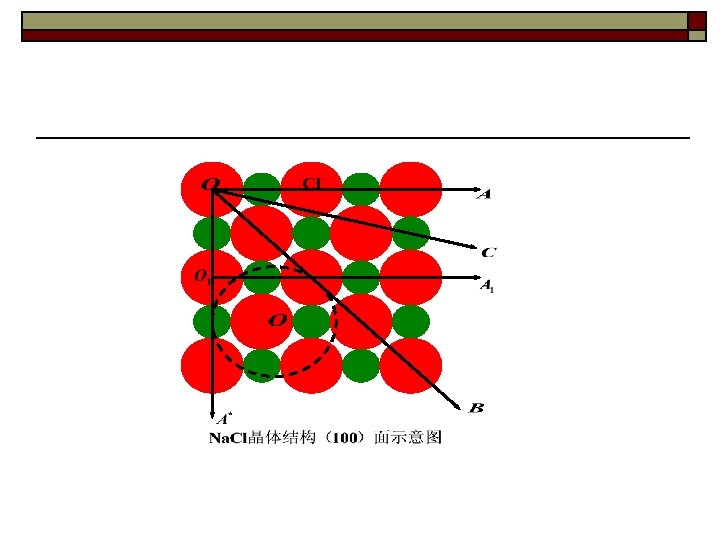
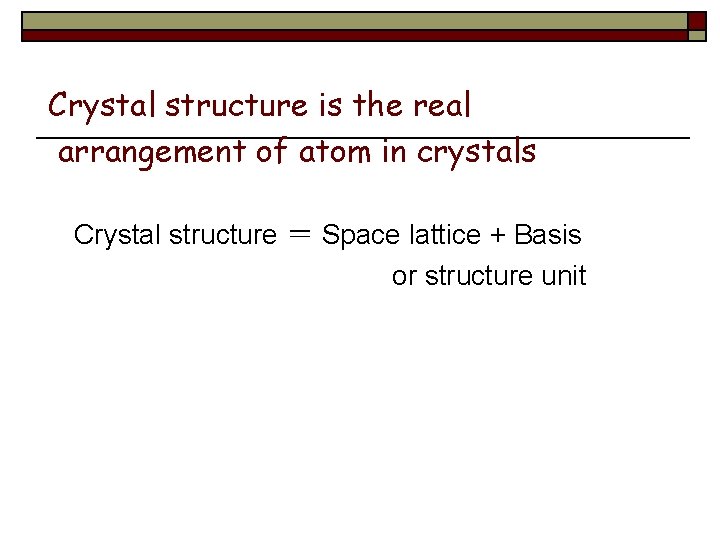
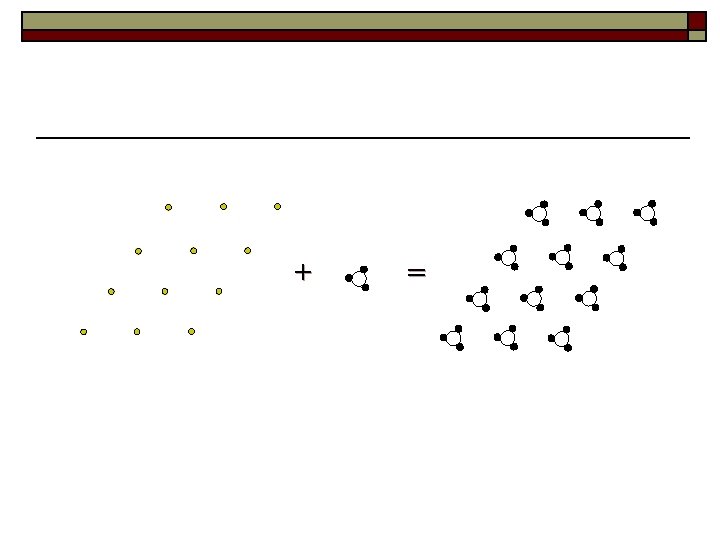
Crystal structure is the real arrangement of atom in crystals Crystal structure = Space lattice + Basis or structure unit


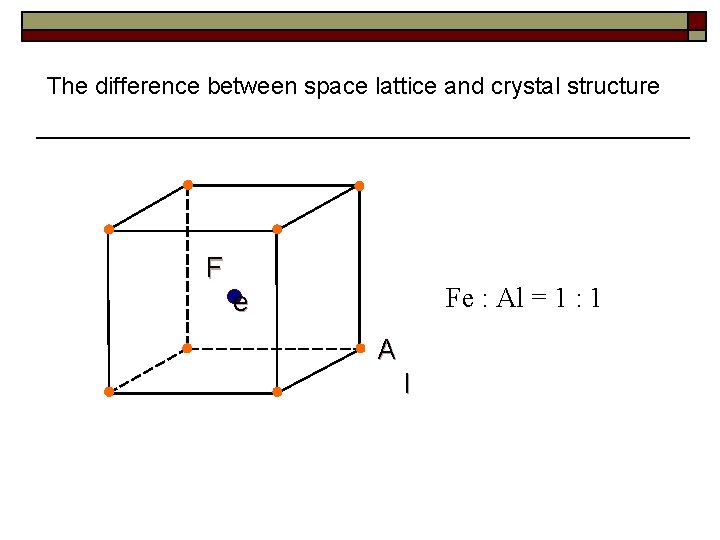
The difference between space lattice and crystal structure F Fe : Al = 1 : 1 e A l

2× 3 atoms / cell

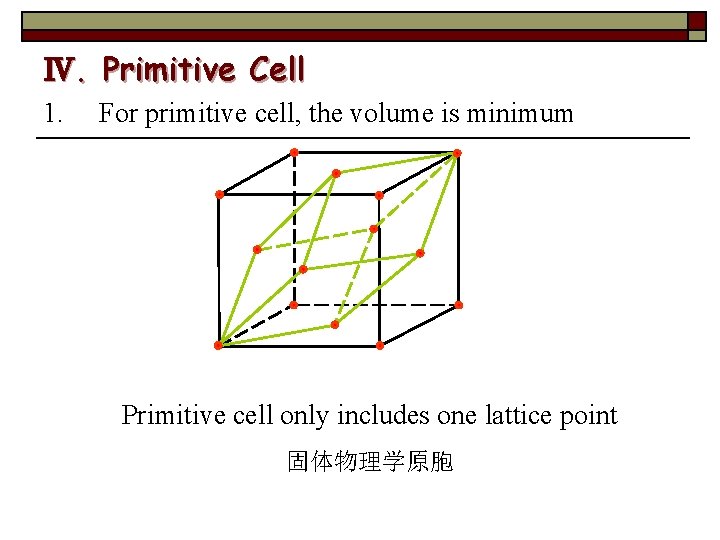
Ⅳ. Primitive Cell 1. For primitive cell, the volume is minimum Primitive cell only includes one lattice point 固体物理学原胞
![kraitiəriən 批评判断等的标准 准则尺度 2 Criterion for choice of unit cell n n n Symmetry [krai'tiəriən] (批评、判断等的)标准, 准则,尺度 2. Criterion for choice of unit cell n n n Symmetry](https://slidetodoc.com/presentation_image/4918a51501d8e065b289b667f2416456/image-35.jpg)
[krai'tiəriən] (批评、判断等的)标准, 准则,尺度 2. Criterion for choice of unit cell n n n Symmetry As many right angle as possible The size of unit cell should be as small as possible

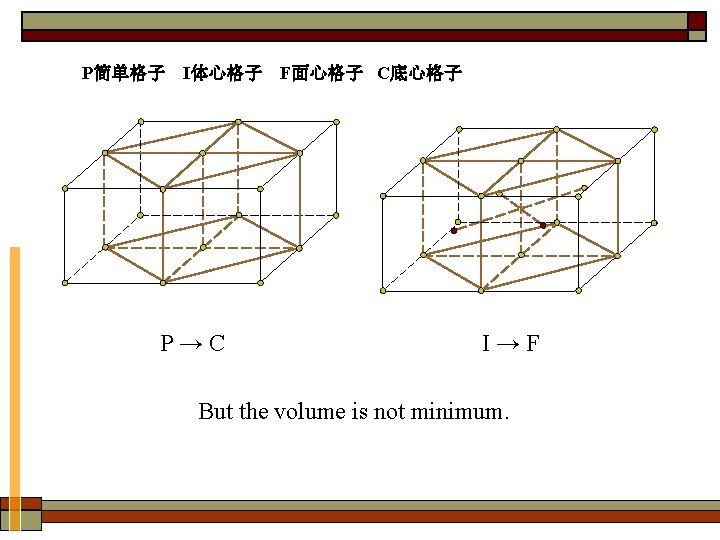
P简单格子 I体心格子 F面心格子 C底心格子 P→C I→F But the volume is not minimum.

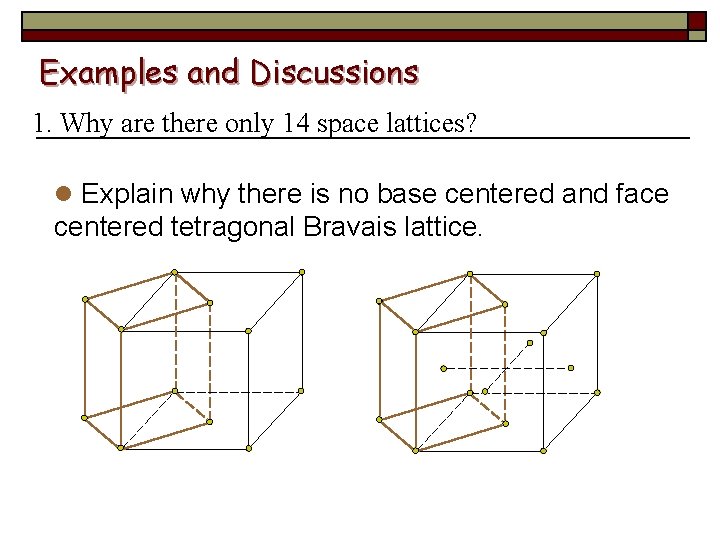
Examples and Discussions 1. Why are there only 14 space lattices? l Explain why there is no base centered and face centered tetragonal Bravais lattice.

P简单格子 I体心格子 F面心格子 C底心格子 P→C I→F But the volume is not minimum.

Exercise 1. Determine the number of lattice points per cell in the cubic crystal systems. If there is only one atom located at each lattice point, calculate the number of atoms per unit cell. 2. Determine the relationship between the atomic radius and the lattice parameter in SC, BCC, and FCC structures when one atom is located at each lattice point. 3. Determine the density of BCC iron, which has a lattice parameter of 0. 2866 nm.

4. Prove that the A-face-centered hexagonal lattice is not a new type of lattice in addition to the 14 space lattices. 5. Draw a primitive cell for BCC lattice. Thanks for your attention !
 Favourite subject science
Favourite subject science Chapter 6 fingerprints
Chapter 6 fingerprints Introduction to materials science for engineers chapter 10
Introduction to materials science for engineers chapter 10 Introduction to materials science for engineers chapter 10
Introduction to materials science for engineers chapter 10 Natural materials and man made materials
Natural materials and man made materials What are the useful and harmful materials
What are the useful and harmful materials Man made materials
Man made materials Adopting materials
Adopting materials Direct materials budget with multiple materials
Direct materials budget with multiple materials The fundamentals of political science research 2nd edition
The fundamentals of political science research 2nd edition Tea science teks
Tea science teks Material science oxford
Material science oxford Grade 7 ns term 2
Grade 7 ns term 2 Technology grade 7 term 3 notes
Technology grade 7 term 3 notes Materials: engineering, science, processing and design
Materials: engineering, science, processing and design Materials science
Materials science Tu bergakademie freiberg computational materials science
Tu bergakademie freiberg computational materials science Materials science tetrahedron
Materials science tetrahedron Dmse iitd
Dmse iitd Natural science grade 6 term 2
Natural science grade 6 term 2 Materials science
Materials science Materials science quiz
Materials science quiz Biological materials science
Biological materials science Chemistry central science 14th edition
Chemistry central science 14th edition Natural vs social science
Natural vs social science Main branches of science
Main branches of science Natural science vs physical science
Natural science vs physical science Applied science vs pure science
Applied science vs pure science Rapid change
Rapid change K5 think central
K5 think central Why environmental science is an interdisciplinary science
Why environmental science is an interdisciplinary science Julie lundquist
Julie lundquist Soft science definition
Soft science definition Fundamentals chapter 1
Fundamentals chapter 1 Fundamentals of electric circuits chapter 4 solutions
Fundamentals of electric circuits chapter 4 solutions Fundamentals of corporate finance chapter 6 solutions
Fundamentals of corporate finance chapter 6 solutions Digital fundamentals chapter 4
Digital fundamentals chapter 4 Conceptual physics magnetism
Conceptual physics magnetism Tire wheel and wheel bearing fundamentals
Tire wheel and wheel bearing fundamentals Chapter 1 health and wellness fundamentals
Chapter 1 health and wellness fundamentals 9
9