FILOVIRIDAE Introduction These are long threadlike viruses hence

















- Slides: 42

FILOVIRIDAE

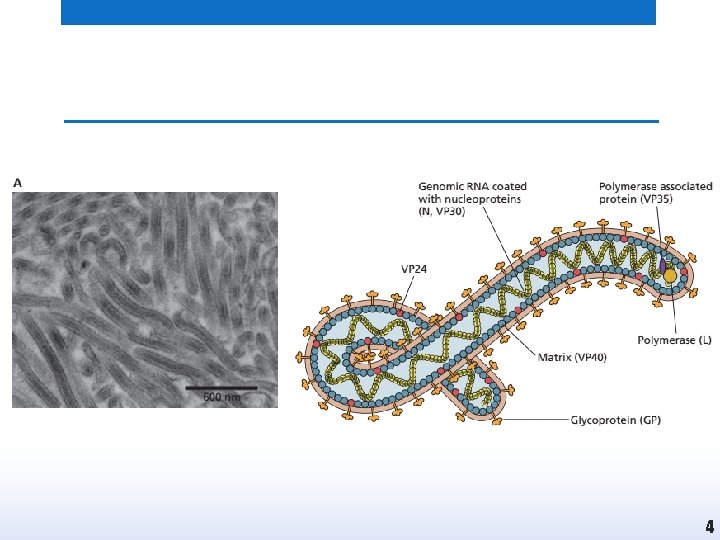
Introduction • These are long threadlike viruses, hence the name (filum means thread). • Marburg and Ebolaviruses causing hemorrhagic fever belong to the genus Filovirus. • These agents cause severe or fatal hemorrhagic fevers and are endemic in Africa. 2

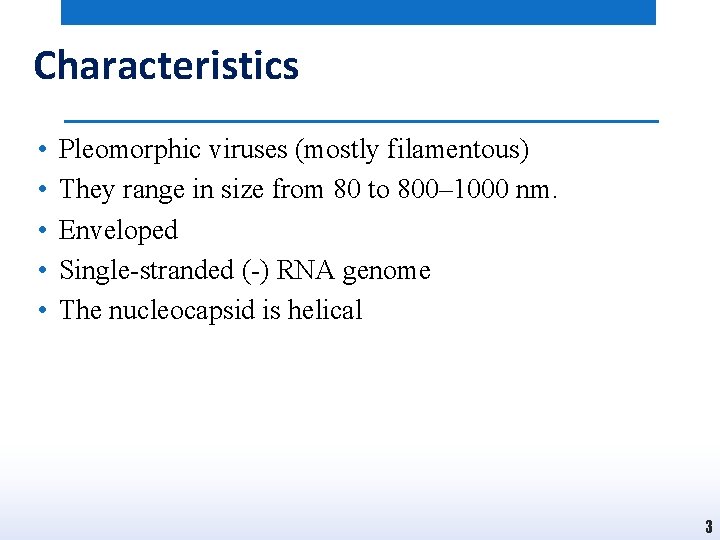
Characteristics • • • Pleomorphic viruses (mostly filamentous) They range in size from 80 to 800– 1000 nm. Enveloped Single-stranded (-) RNA genome The nucleocapsid is helical 3

4

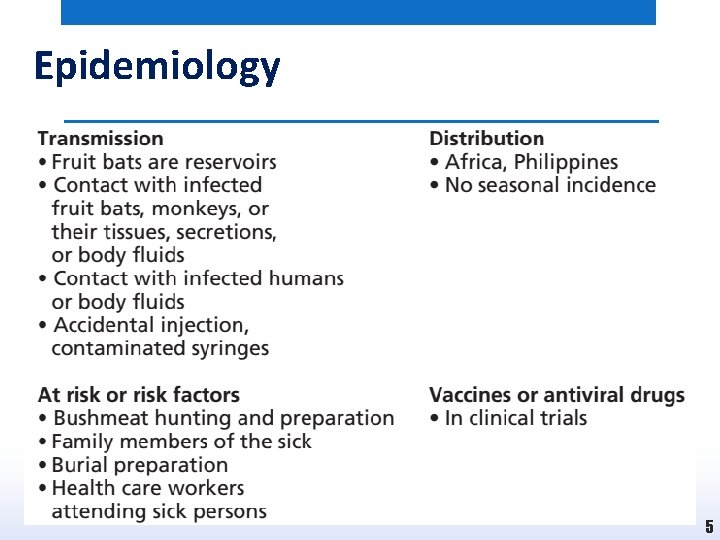
Epidemiology 5

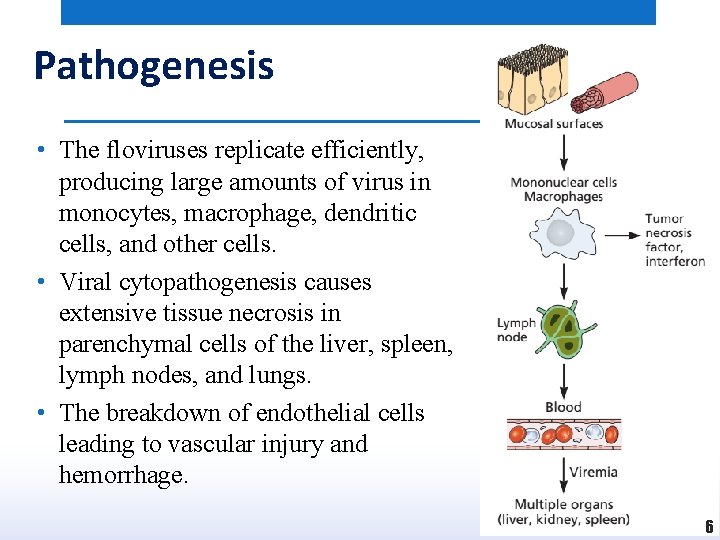
Pathogenesis • The floviruses replicate efficiently, producing large amounts of virus in monocytes, macrophage, dendritic cells, and other cells. • Viral cytopathogenesis causes extensive tissue necrosis in parenchymal cells of the liver, spleen, lymph nodes, and lungs. • The breakdown of endothelial cells leading to vascular injury and hemorrhage. 6

Marburg Virus • Marburg disease is a hemorrhagic fever that occurred simultaneously in laboratory workers in Marburg, Frankfurt (Germany) and Belgrade (Yugoslavia) in 1967. • In each case the infection arose from tissues of African green monkeys to which the laboratory workers had been exposed imported to laboratories from Uganda. 7

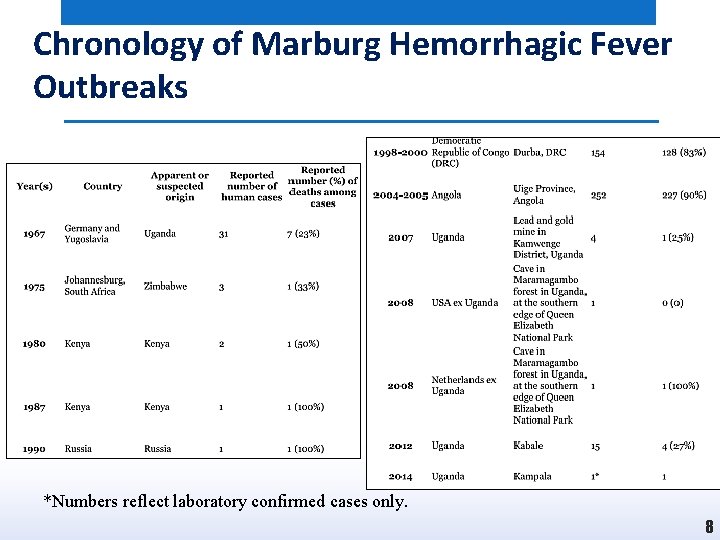
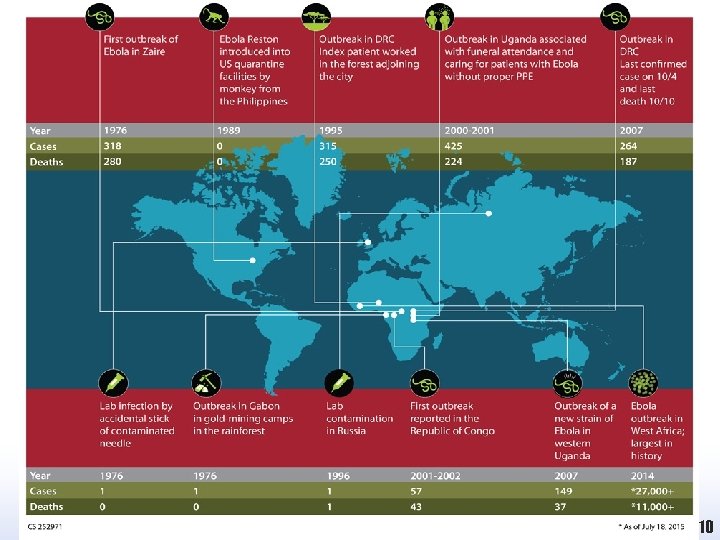
Chronology of Marburg Hemorrhagic Fever Outbreaks *Numbers reflect laboratory confirmed cases only. 8

Ebola Virus • In 1976, two severe epidemics of hemorrhagic fever occurred in Sudan and Zaire with high fatality. • The causative virus was morphologically identical to the Marburg virus but antigenically distinct. • The virus responsible was named Ebola virus after the Ebola river. • In 1979 Ebola re-emerged in Sudan, with serial person to-person spread. • Another epidemic occurred in Kikwit, Zaire, in 1995. • Three distinct strains of Ebola virus have been recognized. 1. Zaire strain (EBO- Z) 2. Sudan strain (EBO-S) 3. Reston strain (EBO-R) 9

10

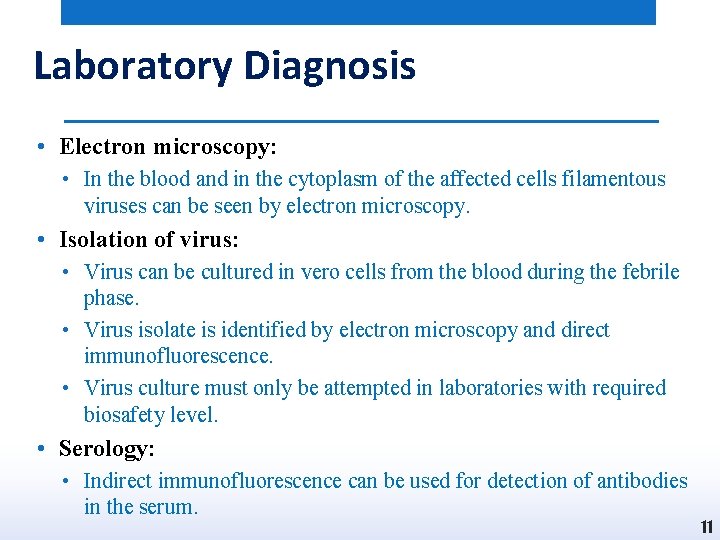
Laboratory Diagnosis • Electron microscopy: • In the blood and in the cytoplasm of the affected cells filamentous viruses can be seen by electron microscopy. • Isolation of virus: • Virus can be cultured in vero cells from the blood during the febrile phase. • Virus isolate is identified by electron microscopy and direct immunofluorescence. • Virus culture must only be attempted in laboratories with required biosafety level. • Serology: • Indirect immunofluorescence can be used for detection of antibodies in the serum. 11

Treatment, Prevention, and Control • Antibody-containing serum and interferon therapies have been tried in patients with filovirus infections. • Infected patients should be quarantined, and contaminated animals should be sacrificed. • Handling of the viruses or contaminated materials requires very stringent isolation procedures. 12

REOVIRIDAE

Introduction • Reoviridae constitutes a diverse family of viruses that infect humans, many mammals, other vertebrates (including birds), plants and insects. • The family Reoviridae derives its name from the prototype virus which was known as the Respiratory enteric orphan virus, • The term "orphan virus" refers to the fact that some of these viruses have been observed not associated with any known disease. • The family Reoviridae is divided into nine genera. Four of the genera are able to infect humans and animals: • Orthoreovirus, Rotavirus, Coltivirus, and Orbivirus. 14

15

16

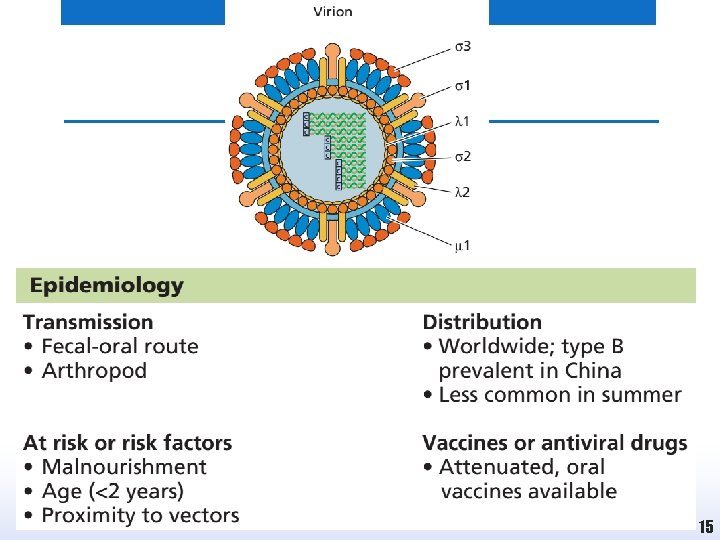
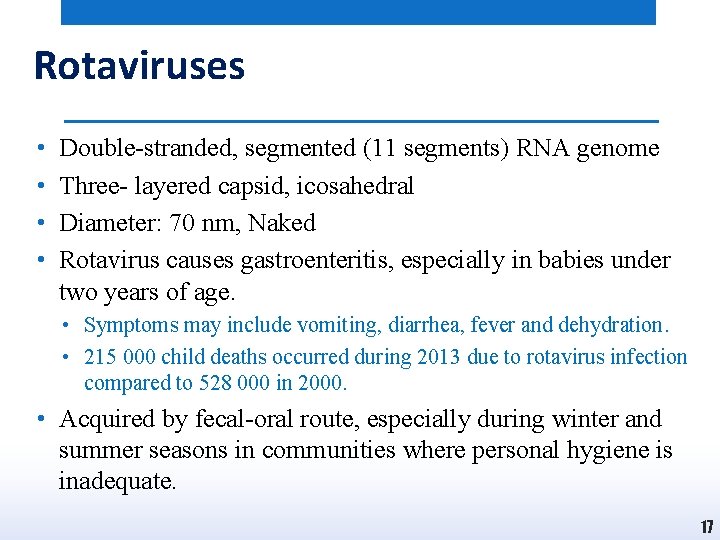
Rotaviruses • • Double-stranded, segmented (11 segments) RNA genome Three- layered capsid, icosahedral Diameter: 70 nm, Naked Rotavirus causes gastroenteritis, especially in babies under two years of age. • Symptoms may include vomiting, diarrhea, fever and dehydration. • 215 000 child deaths occurred during 2013 due to rotavirus infection compared to 528 000 in 2000. • Acquired by fecal-oral route, especially during winter and summer seasons in communities where personal hygiene is inadequate. 17

Diagnosis, Treatment & Control • Diagnosis • ELISA • Immune electron microscope • Treatment • Management consists of replacement of fluids and restoration of electrolyte balance either intravenously or orally, as feasible. • Control • In view of the fecal–oral route of transmission, wastewater treatment and sanitation are significant control measures. 18

VECTOR-BORNE INFECTIONS

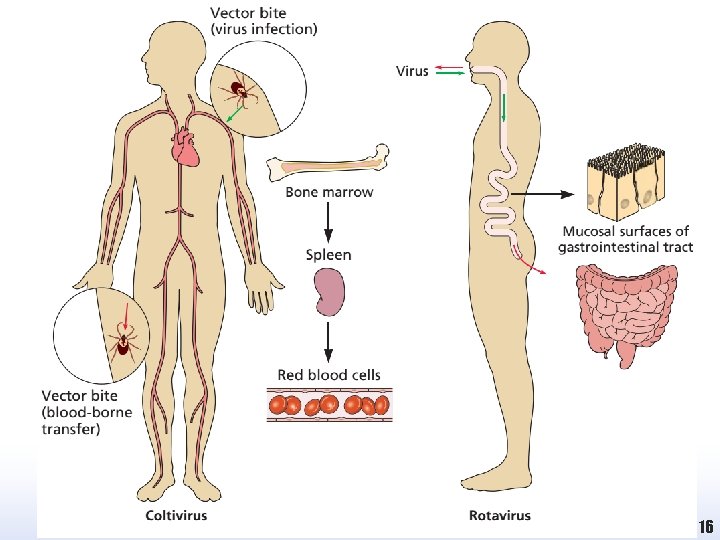
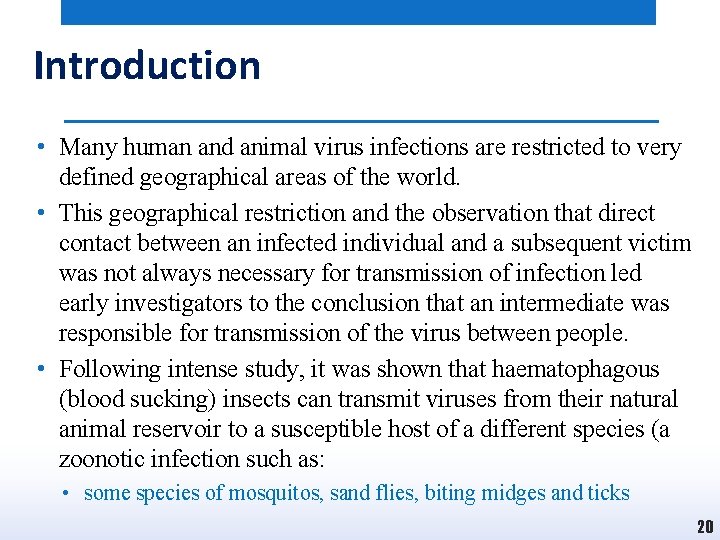
Introduction • Many human and animal virus infections are restricted to very defined geographical areas of the world. • This geographical restriction and the observation that direct contact between an infected individual and a subsequent victim was not always necessary for transmission of infection led early investigators to the conclusion that an intermediate was responsible for transmission of the virus between people. • Following intense study, it was shown that haematophagous (blood sucking) insects can transmit viruses from their natural animal reservoir to a susceptible host of a different species (a zoonotic infection such as: • some species of mosquitos, sand flies, biting midges and ticks 20

Sand Fly Biting midge Tick 21

Arboviruses and their Hosts • Arboviruses are acquired by an insect either: • directly from its mother whilst still an egg (vertical transmission) • or as an adult female when it takes a blood meal from an infected animal or person. • The viruses can only be acquired and spread if they establish a viraemic infection in which virus is found in the blood of the infected vertebrate host. • For efficient transmission the virus must be present in sufficient quantity to ensure that the insect takes up an infectious dose during the blood meal. 22

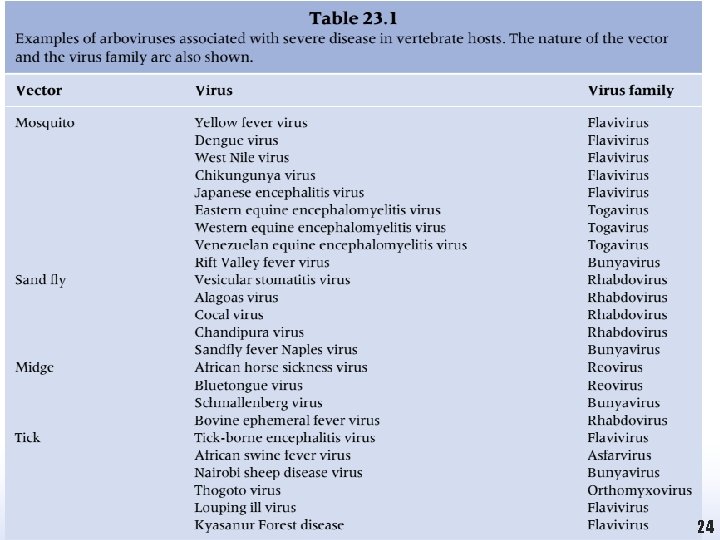
Arboviruses and their Hosts • Once the virus has been ingested, it typically replicates within the insect without causing any significant disease. • When the virus infects members of its animal reservoir it is also unlikely to cause significant disease as the virus and host will have usually established a mutually acceptable co-existence over a long period of interaction. • Some examples of arboviruses that cause severe diseases in vertebrates are shown in Table 23

24

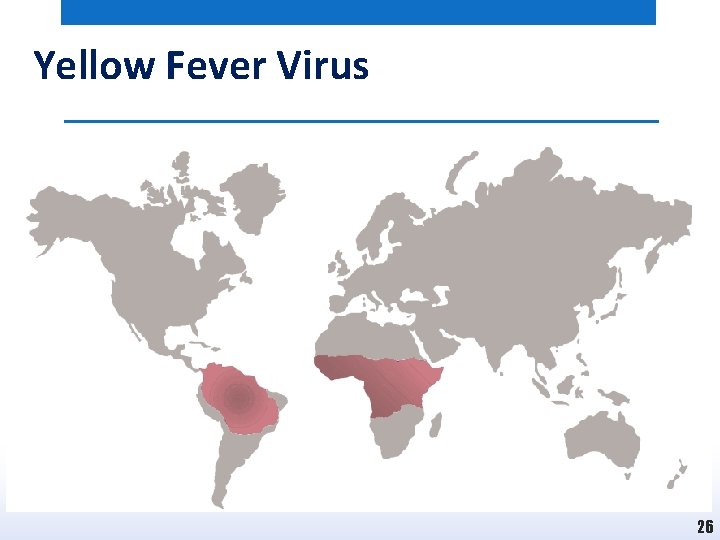
Yellow Fever Virus • Dengue virus is a member of the Flavivirus family. • As indicated in Fig. Yellow fever virus infections are restricted to regions of tropical South America and sub-Saharan Africa. • This reflects the geographical habitat of the primary mosquito vectors that transmit the virus, and in particular of Aedes aegypti. • In recent times in excess of 200, 000 cases of yellow fever with approximately 30, 000 deaths have been recorded each year. • Approximately 90% of this disease burden falls in Africa where large outbreaks occur, whilst the remainder are in South America. 25

Yellow Fever Virus 26

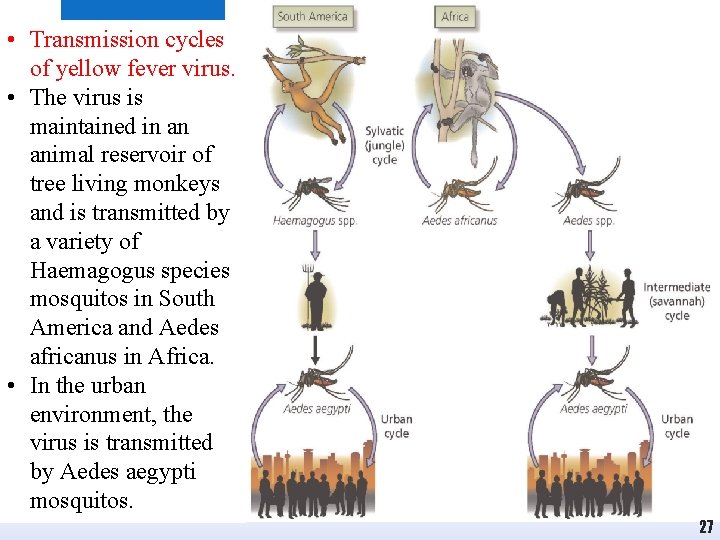
• Transmission cycles of yellow fever virus. • The virus is maintained in an animal reservoir of tree living monkeys and is transmitted by a variety of Haemagogus species mosquitos in South America and Aedes africanus in Africa. • In the urban environment, the virus is transmitted by Aedes aegypti mosquitos. 27

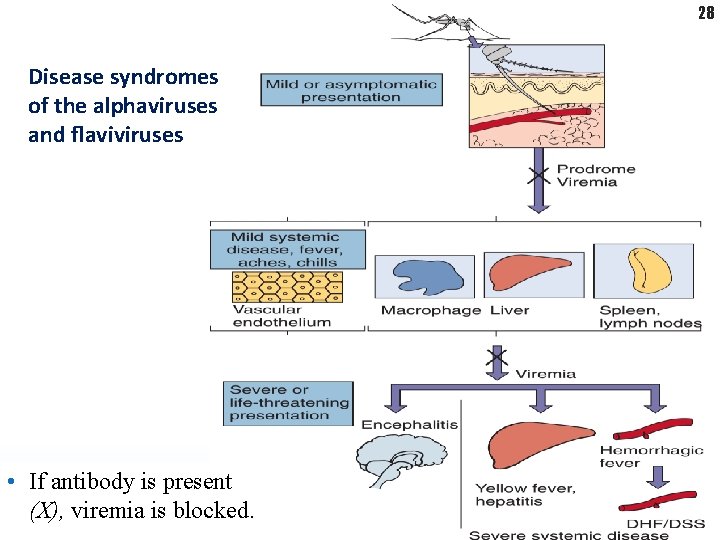
28 Disease syndromes of the alphaviruses and flaviviruses • If antibody is present (X), viremia is blocked.

Yellow Fever Virus Vaccine • Yellow fever vaccine is recommended for people aged ≥ 9 months who are traveling to or living in areas at risk for yellow fever virus transmission. • Yellow fever vaccine may be required for entry into certain countries. • Yellow fever vaccine is available only at designated vaccination centers. • For most travelers, a single dose of yellow fever vaccine provides long-lasting protection and a booster dose of the vaccine is not needed. However, some travelers may require a booster dose. • Yellow fever vaccine is a live-attenuated virus vaccine that has been available since the 1930 s. 29

Dengue virus • Dengue virus infection causes disease ranging from asymptomatic or mild to the severe dengue haemorrhagic fever and dengue shock syndrome. • Dengue virus infects monkeys which act as an animal reservoir from which the virus can be transmitted to humans. • The virus is maintained in a jungle cycle similar to that described for yellow fever virus. • Dengue virus is transmitted between humans primarily by Aedes aegypti mosquitos and, to a lesser extent, by Aedes albopictus, which occur in all tropical and sub-tropical region of the world. 30

Dengue virus • Dengue virus is endemic in South-East Asia, the Pacific, East and West Africa, the Caribbean and the Americas and is now considered to be a major international disease threat with up to 40% of the world’s population potentially at risk. • Current estimates suggest that there may be as many as 400 million Dengue virus infections every year up to 500, 000 people, predominantly children, are hospitalized with severe Dengue virus disease of whom approximately 2. 5% die. 31

Prevention and Treatment of Dengue Virus Infection • There is currently no vaccine to prevent dengue virus infection. • The presence of an animal reservoir makes elimination of dengue virus unachievable. • Consequently, the only preventative measures are aimed at reducing exposure to infected mosquitos. • Treatment of infected patients is generally supportive to reduce the impact of blood and fluid loss in the circulatory system and to reduce the impact of tissue damage. • Early treatment of patients with dengue haemorrhagic fever can reduce mortality from levels of 10% seen with untreated individuals to 1% if treated. 32

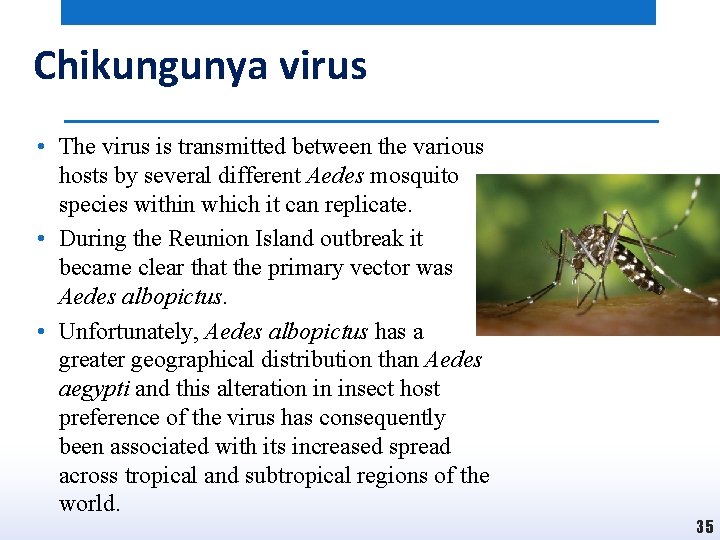
Chikungunya virus • Chikungunya virus was first identified in 1952 in Tanzania and was subsequently found to be responsible for several large outbreaks. • In 2005– 2007, an outbreak on Reunion Island in the Indian Ocean resulted in almost a third of the entire population becoming infected, and this marked a rapid increase in the incidence and geographical range of Chikungunya virus. • The virus has now been found in many parts of South-East Asia, with small numbers of cases also reported in Australia and in Italy and France in Europe. • The animal reservoir of Chikungunya virus is not clear, but there is evidence suggesting the involvement of rodents, birds and small mammals in Africa. 33

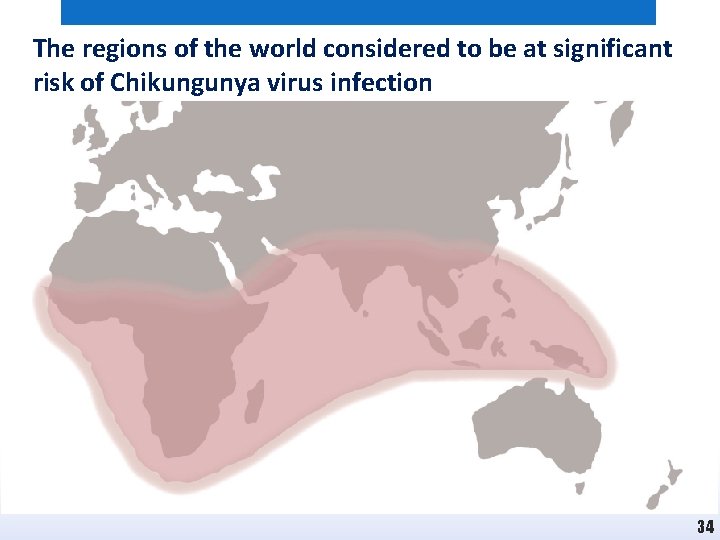
The regions of the world considered to be at significant risk of Chikungunya virus infection 34

Chikungunya virus • The virus is transmitted between the various hosts by several different Aedes mosquito species within which it can replicate. • During the Reunion Island outbreak it became clear that the primary vector was Aedes albopictus. • Unfortunately, Aedes albopictus has a greater geographical distribution than Aedes aegypti and this alteration in insect host preference of the virus has consequently been associated with its increased spread across tropical and subtropical regions of the world. 35

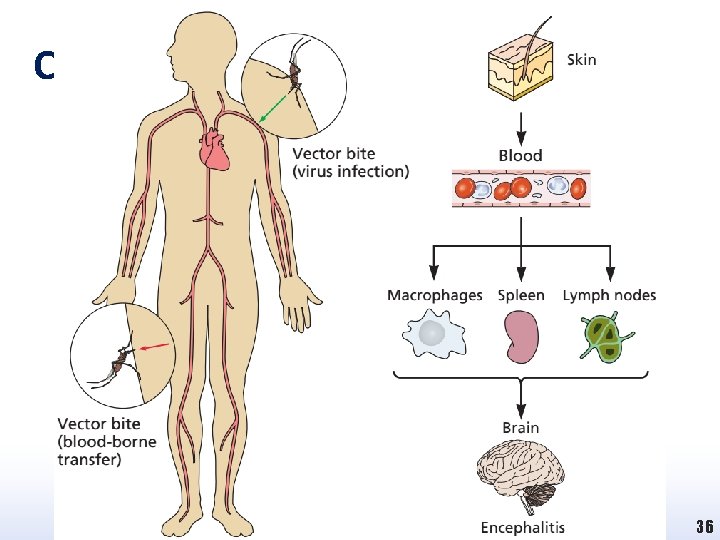
Chikungunya Virus Disease 36

Prevention and treatment of Chikungunya virus infection • No vaccine is available for Chikungunya virus and no alternative form of protective treatment has been discovered. • For this reason the major effort for protection is focused on control of the mosquito vector, as for dengue virus. • Similarly, no drugs that attack the virus have been approved for use and treatment is thus restricted to addressing the symptoms of disease. • Treatment primarily takes the form of anti-inflammatory drugs to reduce the joint pains that are characteristic of the disease. 37

West Nile virus in the USA • West Nile virus is a member of the Flavivirus family. • West Nile virus can cause a fatal neurological disease in humans. • However, the majority of infections are asymptomatic and only approximately 20% result in overt disease of which a small proportion then develop serious acute neurological complications. 38

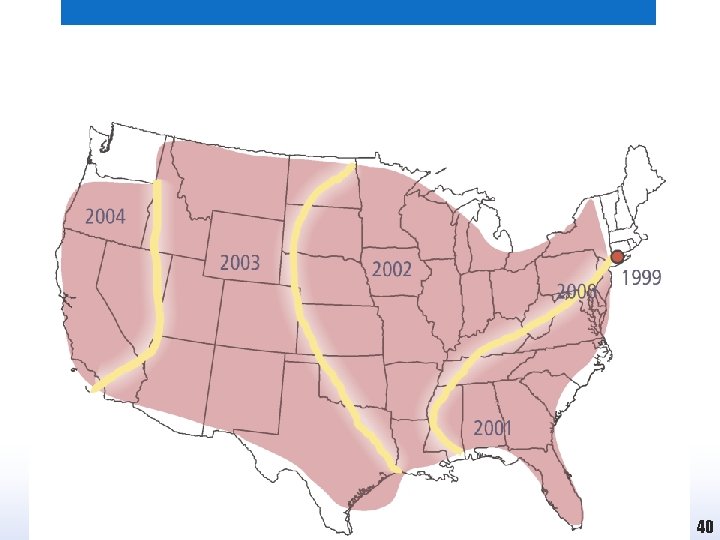
West Nile virus in the USA • West Nile virus is transmitted from one vertebrate host to another by mosquitoes within which the virus also multiplies. • The virus is able to infect a very wide range of invertebrate and vertebrate hosts, including humans and birds. • In 1999, West Nile virus was detected during an outbreak of encephalitis in New York, the first time that it had been seen in the Americas. • Following the first signs of its presence the virus spread relentlessly across the USA and southern Canada, and after five years was present from the east to the west coast of the continent. 39

40

Prevention and treatment of West Nile virus infection • While effective vaccines for prevention of West Nile virus in horses are available there is no human vaccine. • No antiviral drugs to treat West Nile virus infections are routinely available and treatment for West Nile disease is limited to supportive therapy to address the symptoms. 41

Laboratory Diagnosis • The alphaviruses and flaviviruses can be grown in both vertebrate and mosquito cell lines, but most are difficult to isolate. • Infection can be detected through the use of cytopathologic studies and immunofluorescence. • Detection and characterization can be performed by RT-PCR testing of genomic RNA or viral m. RNA in blood or other samples. • After isolation, the viral RNA can also be distinguished by the finding of RNA “fngerprints” of the genomic RNA. • A variety of serologic methods can be used to diagnose infections, including hemagglutination inhibition, enzyme-linked immunosorbent assays, and latex agglutination. • The presence of specific Ig. M or a fourfold increase in titer between acute and convalescent sera is used to indicate a recent infection. 42
 Youtube . com / watch v = roxnvcaezjs
Youtube . com / watch v = roxnvcaezjs @virux127:https://m.youtube.com/watch?v=3wxyhhyuvws
@virux127:https://m.youtube.com/watch?v=3wxyhhyuvws Antigentest åre
Antigentest åre Ebola prevention
Ebola prevention Filoviridae scientific name
Filoviridae scientific name Hence vs thus
Hence vs thus Hence vs thus
Hence vs thus What are transitional expressions
What are transitional expressions Projection of line definition
Projection of line definition Go get thee hence for i will not away
Go get thee hence for i will not away Tall+short h
Tall+short h Running running running
Running running running Threadlike fungi
Threadlike fungi Club fungi examples
Club fungi examples Multicellular fungi example
Multicellular fungi example Tiny, threadlike photosynthetic organisms
Tiny, threadlike photosynthetic organisms Spore forming protists
Spore forming protists Saclike structure that stores materials
Saclike structure that stores materials Why are viruses considered nonliving?
Why are viruses considered nonliving? Bacteriophage characteristics
Bacteriophage characteristics General characteristics of viruses
General characteristics of viruses Viruses
Viruses Lysogenic viruses do not
Lysogenic viruses do not Section 19-3 diseases caused by bacteria and viruses
Section 19-3 diseases caused by bacteria and viruses Cultivation of viruses
Cultivation of viruses Egg inoculation technique
Egg inoculation technique Egrette chapter 21
Egrette chapter 21 Nonliving particle that replicates inside a living cell
Nonliving particle that replicates inside a living cell Blood borne viruses
Blood borne viruses Chapter 7 lesson 1 what are bacteria answer key
Chapter 7 lesson 1 what are bacteria answer key Are viruses alive yes or no
Are viruses alive yes or no Importance of viruses
Importance of viruses Lytic infection
Lytic infection General characters of viruses
General characters of viruses Biosynthesis of rna viruses
Biosynthesis of rna viruses Mackay memorial hospital
Mackay memorial hospital Hepatotropic viruses
Hepatotropic viruses Hepatotropic viruses
Hepatotropic viruses Hepatotropic viruses
Hepatotropic viruses Chapter 20 viruses and prokaryotes
Chapter 20 viruses and prokaryotes Virus taxonomy
Virus taxonomy Study guide chapter 18 section 1 bacteria
Study guide chapter 18 section 1 bacteria Replication of viruses
Replication of viruses