Hepatitis A Milad Haddad MD Head of Gastroenterology










- Slides: 28

Hepatitis A Milad Haddad, MD Head of Gastroenterology Department, Ibn Alnafis Hospital

Liver Functions • Metabolic • Synthetic & storage • Excretory & detoxification • Immune & Filtering

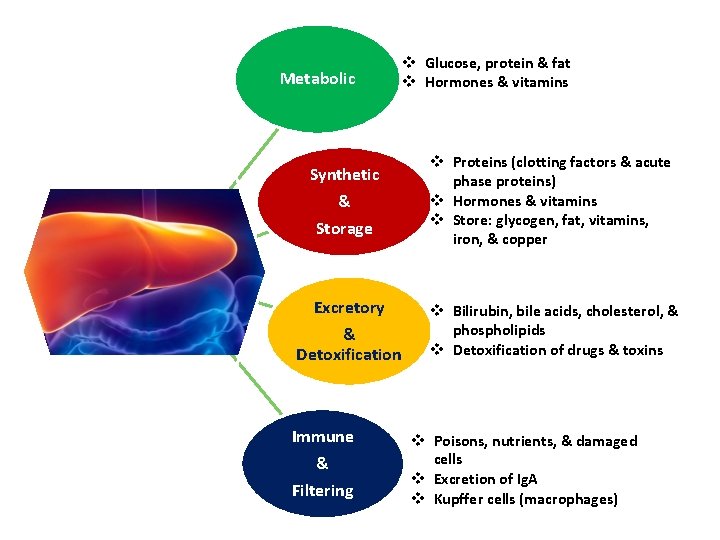
Metabolic v Glucose, protein & fat v Hormones & vitamins Synthetic & Storage v Proteins (clotting factors & acute phase proteins) v Hormones & vitamins v Store: glycogen, fat, vitamins, iron, & copper Excretory & Detoxification v Bilirubin, bile acids, cholesterol, & phospholipids v Detoxification of drugs & toxins Immune & Filtering v Poisons, nutrients, & damaged cells v Excretion of Ig. A v Kupffer cells (macrophages)

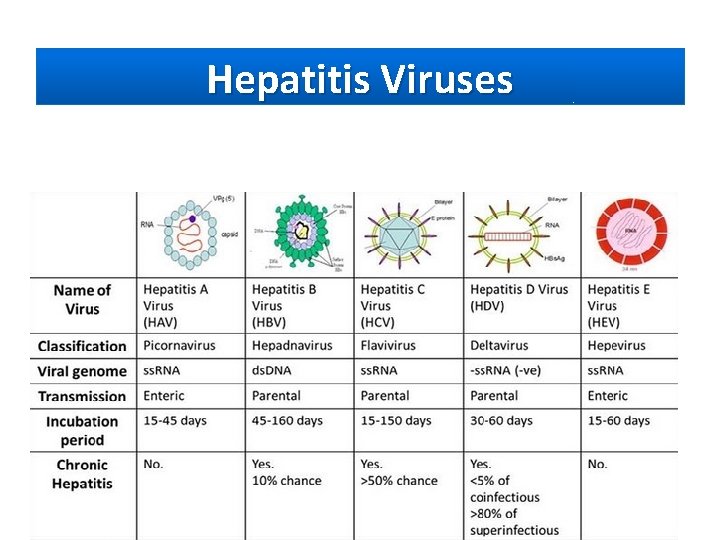
Hepatitis Viruses

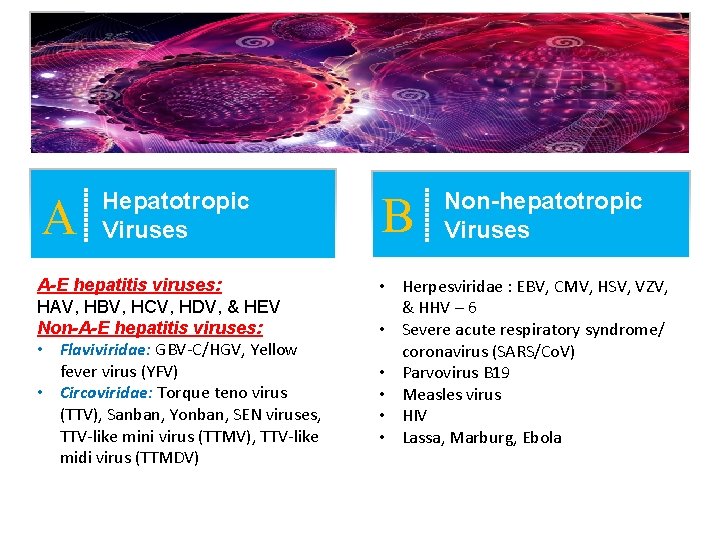
A Hepatotropic Viruses A-E hepatitis viruses: HAV, HBV, HCV, HDV, & HEV Non-A-E hepatitis viruses: • Flaviviridae: GBV-C/HGV, Yellow fever virus (YFV) • Circoviridae: Torque teno virus (TTV), Sanban, Yonban, SEN viruses, TTV-like mini virus (TTMV), TTV-like midi virus (TTMDV) B Non-hepatotropic Viruses • Herpesviridae : EBV, CMV, HSV, VZV, & HHV – 6 • Severe acute respiratory syndrome/ coronavirus (SARS/Co. V) • Parvovirus B 19 • Measles virus • HIV • Lassa, Marburg, Ebola

Hepatitis Viruses

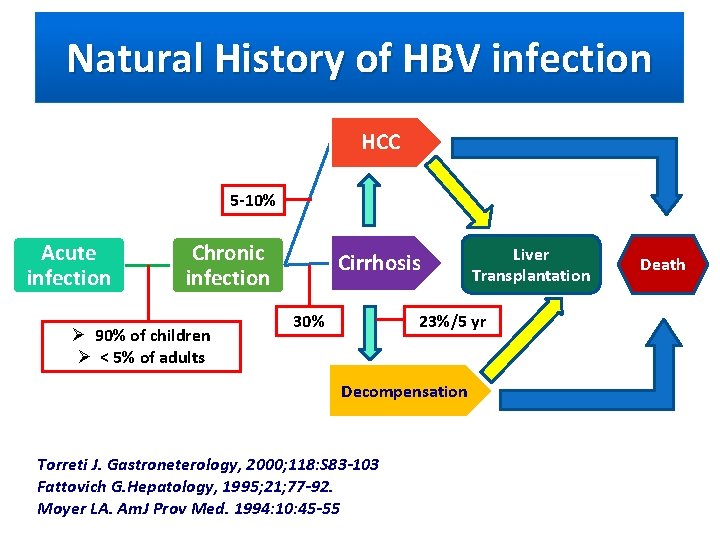
Natural History of HBV infection HCC 5 -10% Acute infection Chronic infection Ø 90% of children Ø < 5% of adults Cirrhosis Liver Transplantation 23%/5 yr 30% Decompensation Torreti J. Gastroneterology, 2000; 118: S 83 -103 Fattovich G. Hepatology, 1995; 21; 77 -92. Moyer LA. Am. J Prov Med. 1994: 10: 45 -55 Death

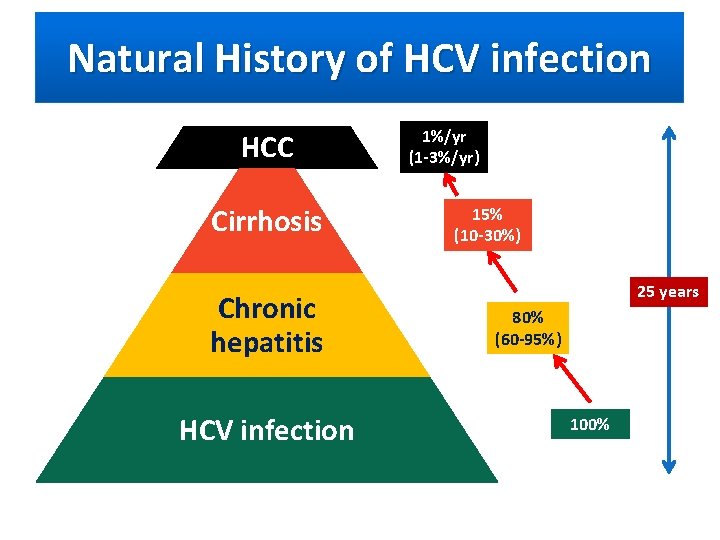
Natural History of HCV infection HCC Cirrhosis Chronic hepatitis HCV infection 1%/yr (1 -3%/yr) 15% (10 -30%) 25 years 80% (60 -95%) 100%

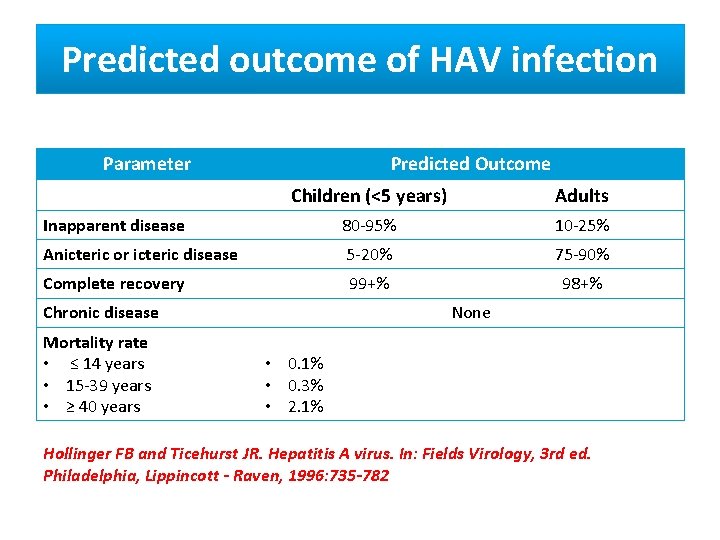
Predicted outcome of HAV infection Parameter Predicted Outcome Children (<5 years) Adults Inapparent disease 80 -95% 10 -25% Anicteric or icteric disease 5 -20% 75 -90% Complete recovery 99+% 98+% Chronic disease Mortality rate • ≤ 14 years • 15 -39 years • ≥ 40 years None • 0. 1% • 0. 3% • 2. 1% Hollinger FB and Ticehurst JR. Hepatitis A virus. In: Fields Virology, 3 rd ed. Philadelphia, Lippincott - Raven, 1996: 735 -782

Epidemiology § From 11% to 22% of patients with acute hepatitis A require hospitalization. § An average length of stay of 4. 6 days, costing on average $7926 per patient. § On average, 27 workdays are lost per adult case of hepatitis A. • Hepatitis A is the most common form of acute viral hepatitis worldwide. It is a self-limited infection


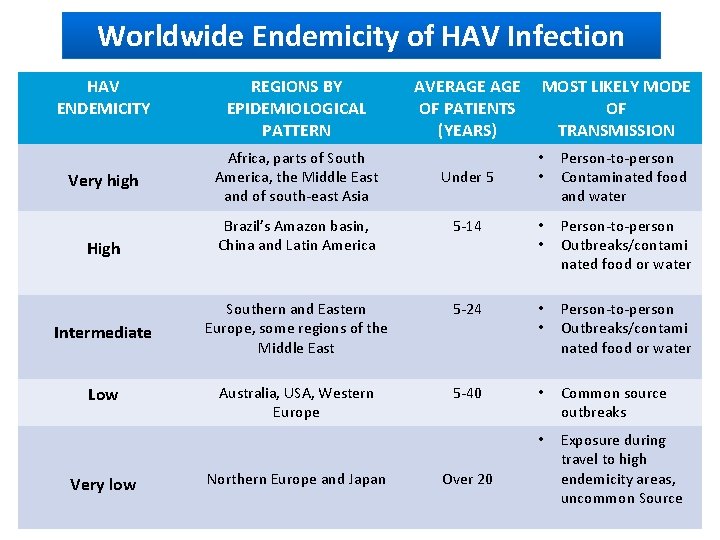
Worldwide Endemicity of HAV Infection HAV ENDEMICITY Very high High Intermediate Low Very low REGIONS BY EPIDEMIOLOGICAL PATTERN Africa, parts of South America, the Middle East and of south-east Asia AVERAGE OF PATIENTS (YEARS) Under 5 MOST LIKELY MODE OF TRANSMISSION • • Person-to-person Contaminated food and water Brazil’s Amazon basin, China and Latin America 5 -14 • • Person-to-person Outbreaks/contami nated food or water Southern and Eastern Europe, some regions of the Middle East 5 -24 • • Person-to-person Outbreaks/contami nated food or water Australia, USA, Western Europe 5 -40 • Common source outbreaks • Exposure during travel to high endemicity areas, uncommon Source Northern Europe and Japan Over 20

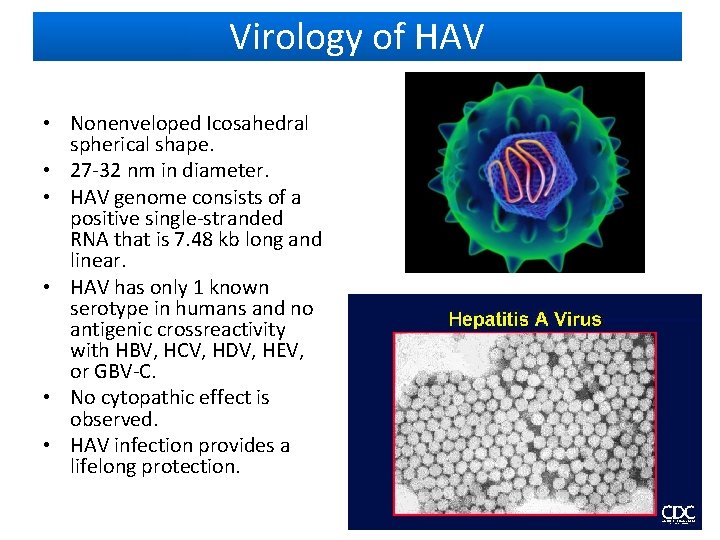
Virology of HAV • Nonenveloped Icosahedral spherical shape. • 27 -32 nm in diameter. • HAV genome consists of a positive single-stranded RNA that is 7. 48 kb long and linear. • HAV has only 1 known serotype in humans and no antigenic crossreactivity with HBV, HCV, HDV, HEV, or GBV-C. • No cytopathic effect is observed. • HAV infection provides a lifelong protection.

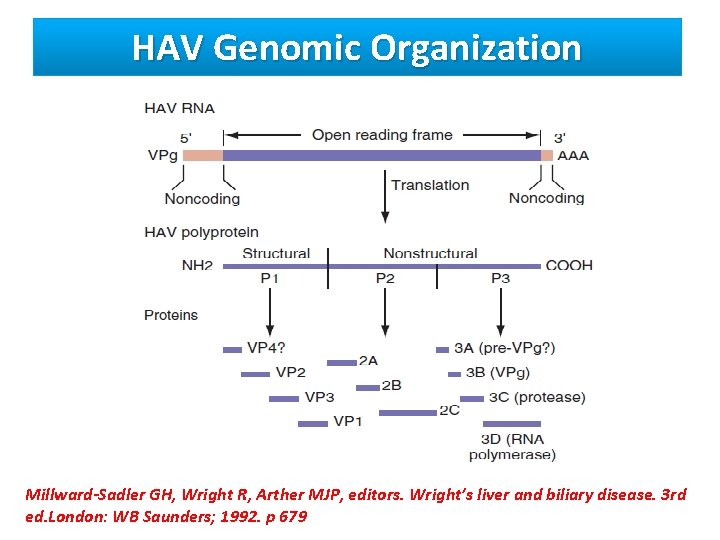
HAV Genomic Organization Millward-Sadler GH, Wright R, Arther MJP, editors. Wright’s liver and biliary disease. 3 rd ed. London: WB Saunders; 1992. p 679

Modes of Transmisiion Modes of Transmission

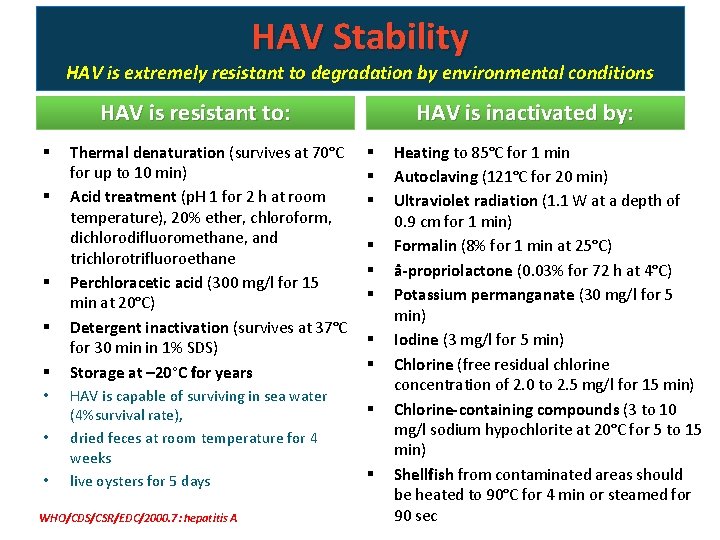
HAV Stability HAV is extremely resistant to degradation by environmental conditions HAV is resistant to: § § § • • • Thermal denaturation (survives at 70°C for up to 10 min) Acid treatment (p. H 1 for 2 h at room temperature), 20% ether, chloroform, dichlorodifluoromethane, and trichlorotrifluoroethane Perchloracetic acid (300 mg/l for 15 min at 20°C) Detergent inactivation (survives at 37°C for 30 min in 1% SDS) Storage at – 20°C for years HAV is capable of surviving in sea water (4%survival rate), dried feces at room temperature for 4 weeks live oysters for 5 days WHO/CDS/CSR/EDC/2000. 7: hepatitis A HAV is inactivated by: § § § § § Heating to 85°C for 1 min Autoclaving (121°C for 20 min) Ultraviolet radiation (1. 1 W at a depth of 0. 9 cm for 1 min) Formalin (8% for 1 min at 25°C) â-propriolactone (0. 03% for 72 h at 4°C) Potassium permanganate (30 mg/l for 5 min) Iodine (3 mg/l for 5 min) Chlorine (free residual chlorine concentration of 2. 0 to 2. 5 mg/l for 15 min) Chlorine-containing compounds (3 to 10 mg/l sodium hypochlorite at 20°C for 5 to 15 min) Shellfish from contaminated areas should be heated to 90°C for 4 min or steamed for 90 sec

Pathogenesis • • After HAV is ingested and survives gastric acid, it traverses the small intestinal mucosa, reaches the liver via the portal vein, and is taken up by hepatocytes. In hepatocytes, virus particles replicate, assemble, and are secreted into the biliary canaliculus, from which they pass into the bile duct and back to the small intestine, with eventual excretion in the feces. The enterohepatic cycles of the virus life cycle continue until neutralizing antibodies and other immune mechanisms interrupt the cycle. The lack of injury to cells in cell culture systems suggests that HAV is not cytopathic. Immunologically mediated cell damage is more likely. The emergence of anti-HAV could result in hepatic necrosis during immunologically mediated elimination of HAV. Death appears to be inevitable when necrosis involves more than 65 - 80% of the total hepatocyte fraction. In patients who survive an episode of acute fulminant hepatic failure, neither functional nor pathologic sequelae are common, despite the widespread necrosis. The damaged hepatic tissue is usually restored within 8 to 12 weeks.

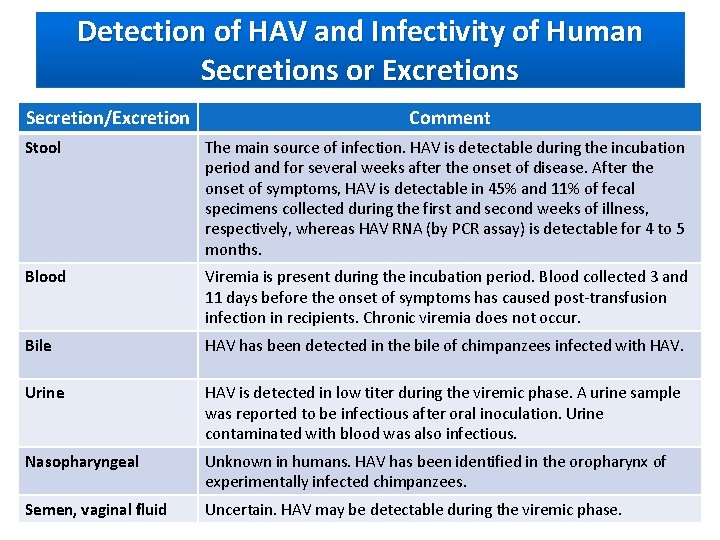
Detection of HAV and Infectivity of Human Secretions or Excretions Secretion/Excretion Comment Stool The main source of infection. HAV is detectable during the incubation period and for several weeks after the onset of disease. After the onset of symptoms, HAV is detectable in 45% and 11% of fecal specimens collected during the first and second weeks of illness, respectively, whereas HAV RNA (by PCR assay) is detectable for 4 to 5 months. Blood Viremia is present during the incubation period. Blood collected 3 and 11 days before the onset of symptoms has caused post-transfusion infection in recipients. Chronic viremia does not occur. Bile HAV has been detected in the bile of chimpanzees infected with HAV. Urine HAV is detected in low titer during the viremic phase. A urine sample was reported to be infectious after oral inoculation. Urine contaminated with blood was also infectious. Nasopharyngeal Unknown in humans. HAV has been identified in the oropharynx of experimentally infected chimpanzees. Semen, vaginal fluid Uncertain. HAV may be detectable during the viremic phase.

Person-to-person contact - Households - Sexual contact: men who have sex with men - Child day care centers Food & water contamination - Infected food handlers - Raw shellfish Blood & blood products exposure (rare) - Transfusion - IV drug abusers

Modes of Transmission route Hepatitis A Hepatitis B Hepatitis C Hepatitis D Hepatitis E Food-Borne • Ø Ø Ø • Fecal-Oral • Ø Ø Ø • Water-Borne • Ø Ø Ø • Raw-Shellfish • Ø Ø Ø Suspected Intra. Institutional • • • IV drug use ∆ • • • Ø Transfusion ∆ • • • ∆ Hemodialysis ∆ • • • Ø Sexual ∆ • ∆ Oral-Oral • ∆ Ø Ø • Household • ∆ ∆ ∆ • Perinatal ∆ • Common ∆ Infrequent Ø Never

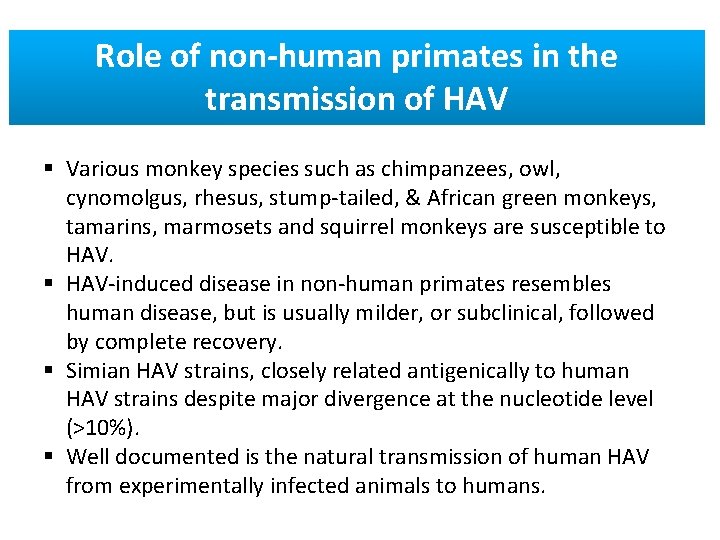
Role of non-human primates in the transmission of HAV § Various monkey species such as chimpanzees, owl, cynomolgus, rhesus, stump-tailed, & African green monkeys, tamarins, marmosets and squirrel monkeys are susceptible to HAV. § HAV-induced disease in non-human primates resembles human disease, but is usually milder, or subclinical, followed by complete recovery. § Simian HAV strains, closely related antigenically to human HAV strains despite major divergence at the nucleotide level (>10%). § Well documented is the natural transmission of human HAV from experimentally infected animals to humans.

High Risk Groups for HAV Infection § People in household/sexual contact with infected persons § Medical and paramedical personnel in hospitals § International travellers from developed countries to regions of the world where HAV is endemic (3/1000 to 20/1000 people per month’s stay abroad) § Persons living in regions with endemic hepatitis A § Persons residing in areas where extended community outbreaks exist § Preschool children attending daycare centres, their parents and siblings § Day-care centre employees § Residents and staff of closed communities (institutions) § Refugees residing in temporary camps following catastrophes § Homosexually active men § Injecting drug users using unsterilized injection needles § Persons with clotting factor disorders § Persons with chronic liver disease § Food-service establishments/food handlers § Persons working with non-human primates

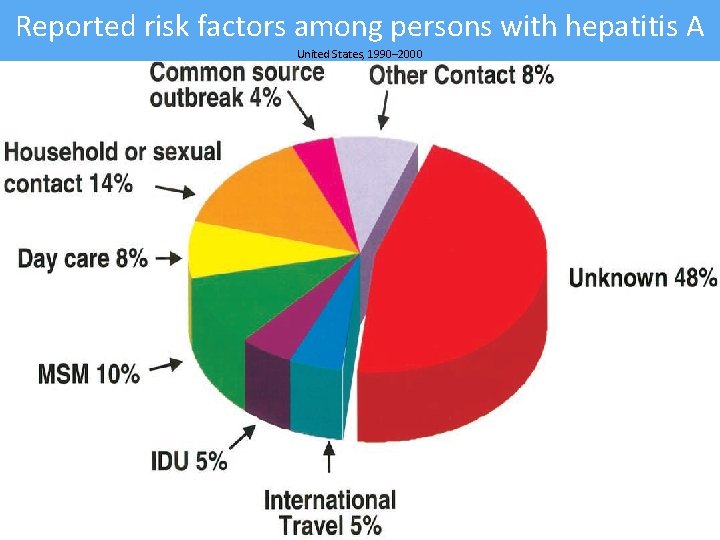
Reported risk factors among persons with hepatitis A United States, 1990– 2000

CHARACTERISTICS OF FOODBORNE TRANSMISSION Transmission due to contamination of food at the point of sale or service q The source of most reported foodborne hepatitis A outbreaks has been HAV-infected food handlers present at the point of sale (such as in a restaurant) or who prepare food for social events (such as a wedding). q A single HAV-infected food handler can transmit HAV to dozens or even hundreds of persons and cause a substantial economic burden to public health [18, 19]. q. The societal cost of a single foodborne outbreak of hepatitis A in Denver involving 43 cases was estimated to be more than $800, 000, with 190% of these costs borne by the public health department and attributed to immunoglobulin administration [18].

CHARACTERISTICS OF FOODBORNE TRANSMISSION Transmission due to contamination of food during growing, harvesting, processing, or distribution q. Hepatitis A outbreaks have been also associated with consumption of fresh produce contaminated with HAV during cultivation, harvesting, processing, or distribution q Outbreaks involving a food item that was contaminated before distribution are particularly challenging to identify and might be widely distributed geographically.

Transmission due to exposure to contaminated water. • Water treatment processes and dilution within municipal water systems are apparently sufficient to render HAV noninfectious, although no studies have demonstrated which specific treatment processes are the most effective. • Outbreaks of hepatitis A among persons who use small private or community wells or swimming pools have been reported, and contamination by adjacent septic systems has been implicated as the source of contamination [49– 53]. • hepatitis A outbreaks after flooding-related sewage contamination of potable water sources is recognized.

Guidelines for Epidemic Measures § Determination of mode of transmission, whether person-to -person or by common vector (vehicle). § Identification of the population exposed to increased risk of infection. Elimination of common sources of infection. § Improvement of sanitary and hygienic practices to eliminate faecal contamination of food and water. § Hepatitis A vaccination has been shown to be effective in controlling outbreaks of infection in communities that have high or intermediate rates of infection, provided a sufficient percent of the target population is reached. § Passive immunization provides temporary protection, but it is not effective in controlling HAV on a community level. WHO/CDS/CSR/EDC/2000. 7

Thank You
 Hepatotropic viruses
Hepatotropic viruses Seng dan fai lok
Seng dan fai lok Heent ncat
Heent ncat Galt anatomy
Galt anatomy Gastroenterology medical terminology
Gastroenterology medical terminology Ruben gonzalez pain management
Ruben gonzalez pain management Gastroenterology board review
Gastroenterology board review Gastroenterology diagnosis codes
Gastroenterology diagnosis codes Bilitec
Bilitec Mario bieringer
Mario bieringer Institute of sport exercise and health
Institute of sport exercise and health Safertec
Safertec Marwan haddad producer
Marwan haddad producer Waleed haddad
Waleed haddad Dr rami haddad
Dr rami haddad Haddad toyota
Haddad toyota Afaf haddad
Afaf haddad Lahcen haddad
Lahcen haddad Victoria haddad
Victoria haddad Duhamel haddad cirurgia
Duhamel haddad cirurgia Body parts shin
Body parts shin Pre-head head tonic syllable tail
Pre-head head tonic syllable tail The attacking firm goes head-to-head with its competitor.
The attacking firm goes head-to-head with its competitor. What is tonic syllable
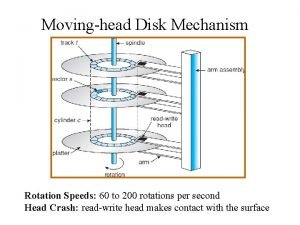
What is tonic syllable Moving head disk mechanism
Moving head disk mechanism Html teksta noformēšana
Html teksta noformēšana Flooded suction vs suction lift
Flooded suction vs suction lift Operation of moving head disk storage
Operation of moving head disk storage Biceps femoris innervation
Biceps femoris innervation