Exam 3 Review Session Review of Practice Exam













- Slides: 38

Exam 3 Review Session Review of Practice Exam and Concepts

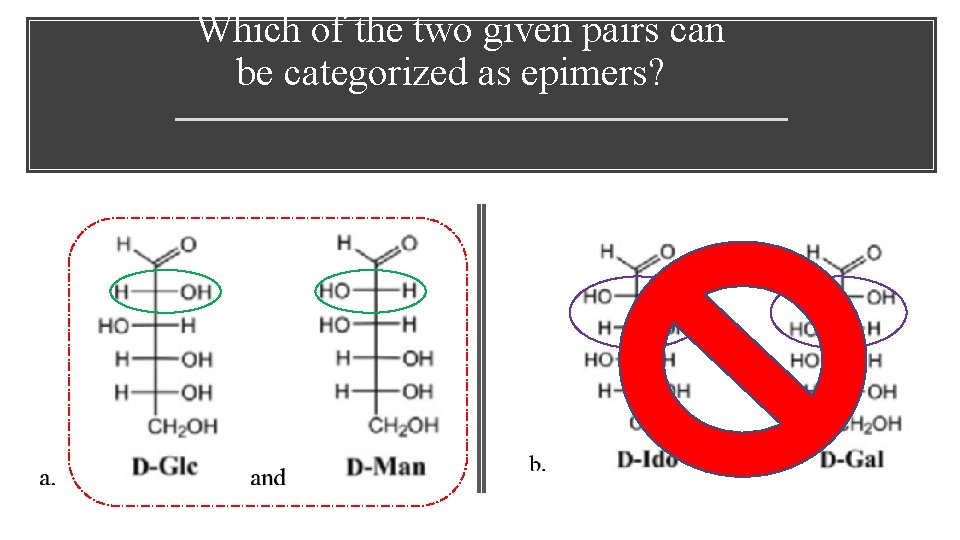
Which of the two given pairs can be categorized as epimers?

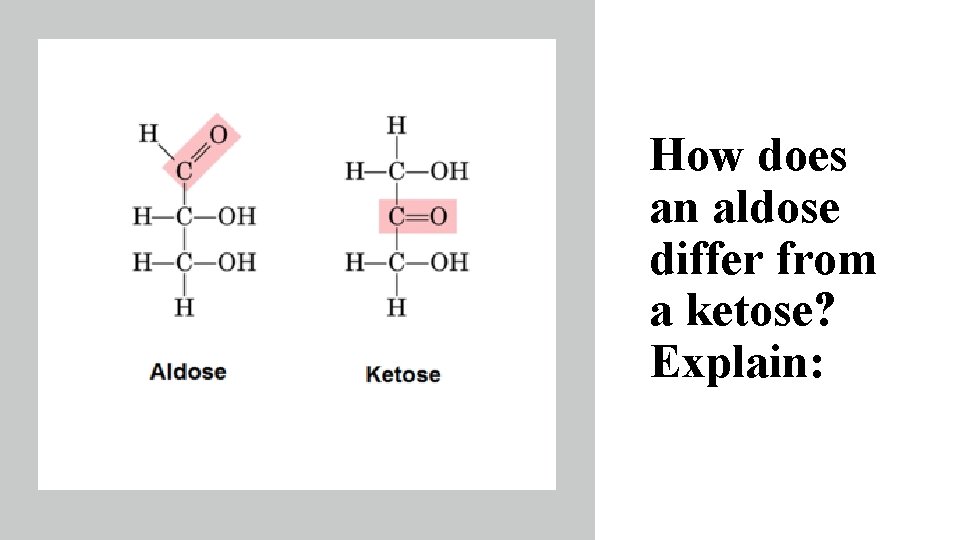
How does an aldose differ from a ketose? Explain:

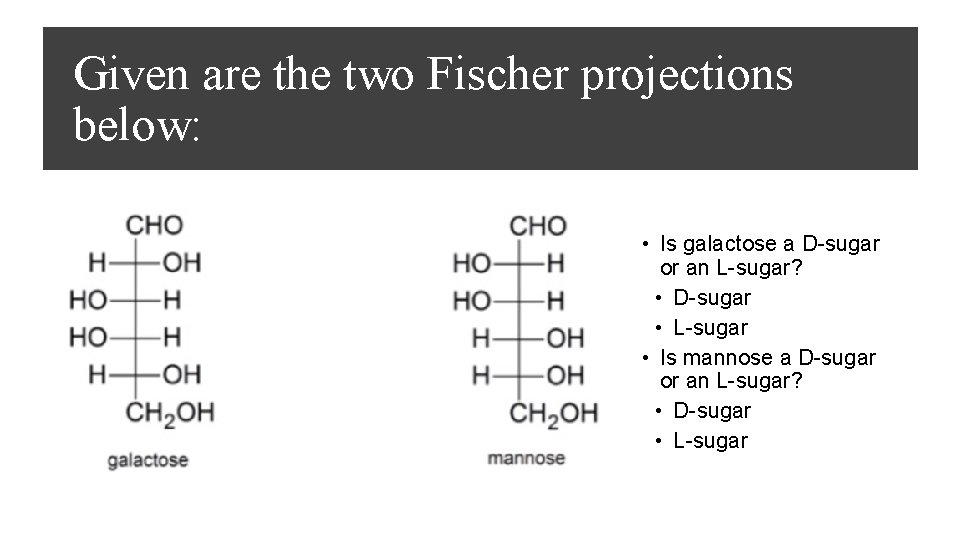
Given are the two Fischer projections below: • Is galactose a D-sugar or an L-sugar? • D-sugar • L-sugar • Is mannose a D-sugar or an L-sugar? • D-sugar • L-sugar

Given are the two Fischer projections below: • Is galactose a D-sugar or an L-sugar? • D-sugar • L-sugar • Is mannose a D-sugar or an L-sugar? • D-sugar • L-sugar


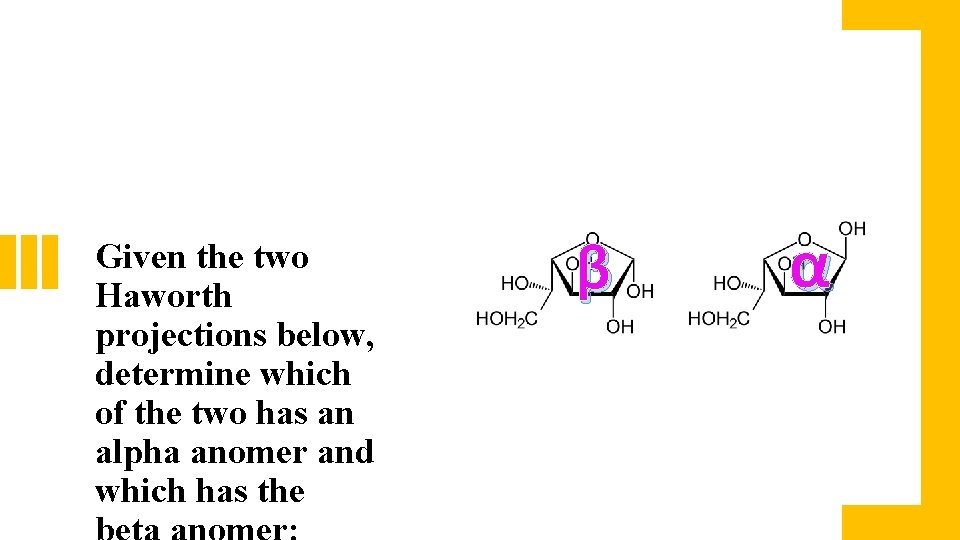
Given the two Haworth projections below, determine which of the two has an alpha anomer and which has the β α

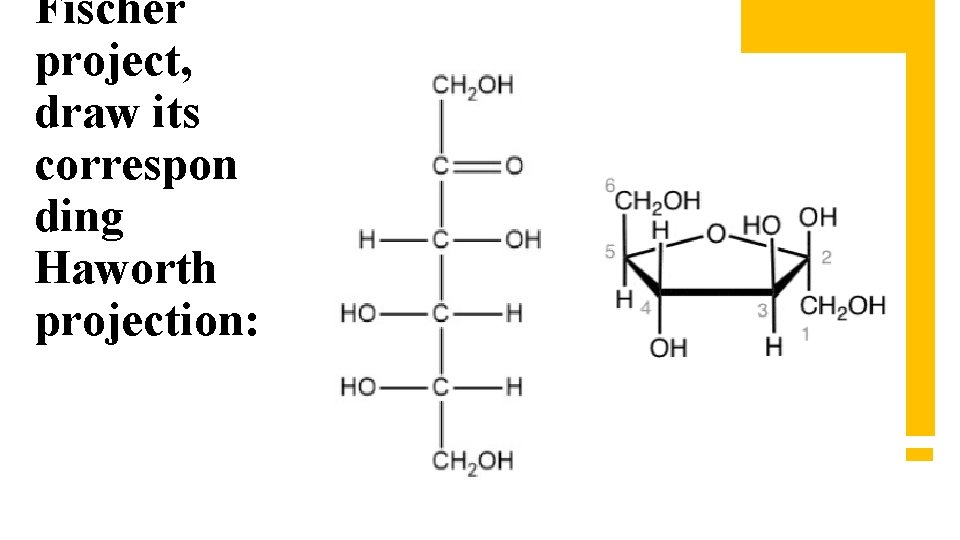
Fischer project, draw its correspon ding Haworth projection:


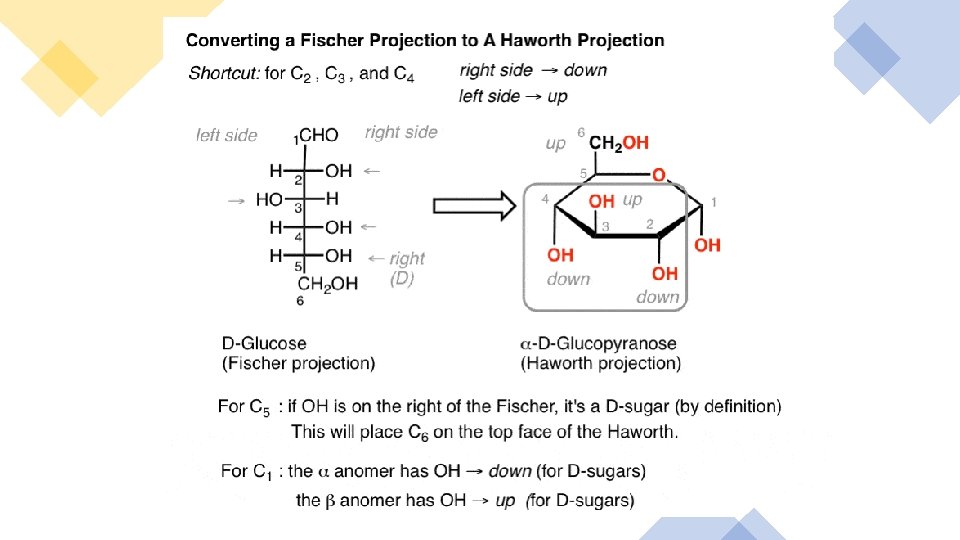
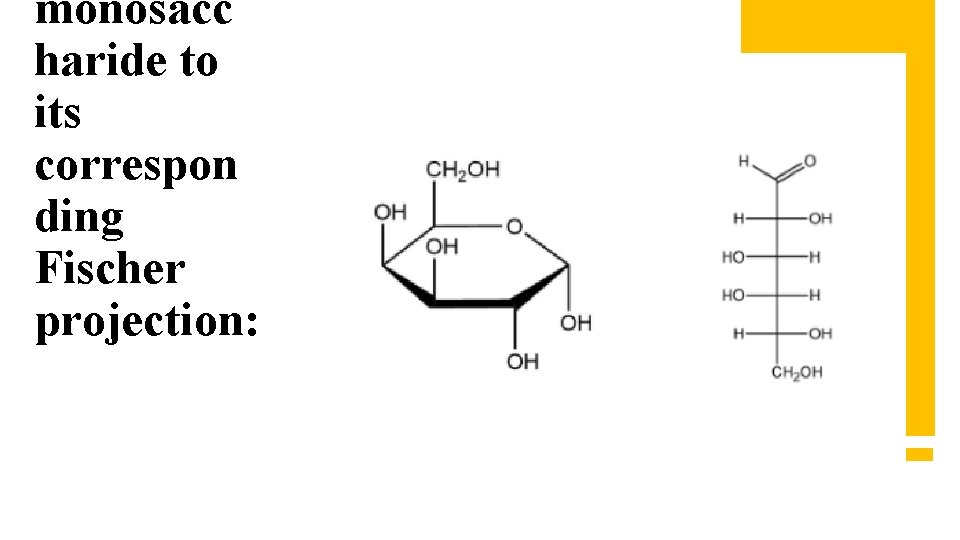
monosacc haride to its correspon ding Fischer projection:

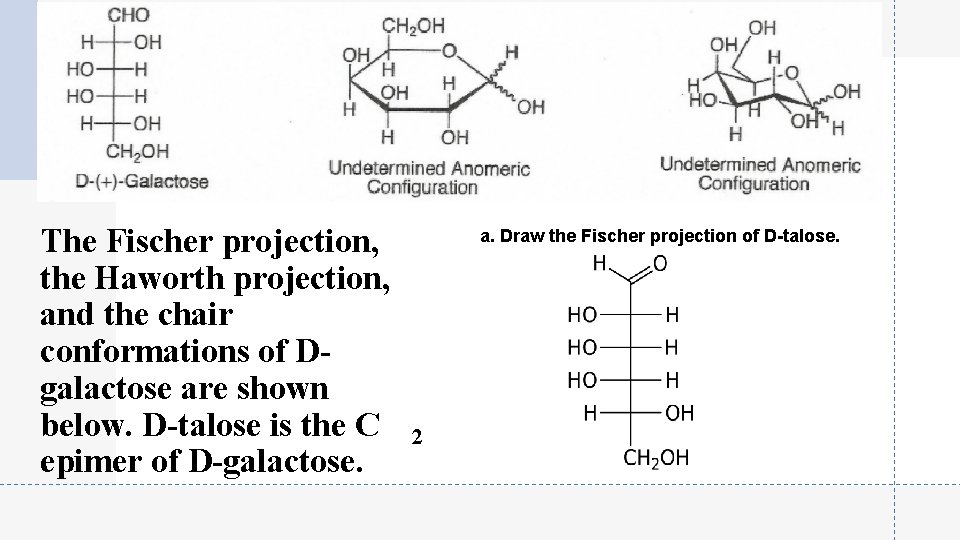
The Fischer projection, the Haworth projection, and the chair conformations of Dgalactose are shown below. D-talose is the C epimer of D-galactose. a. Draw the Fischer projection of D-talose. 2

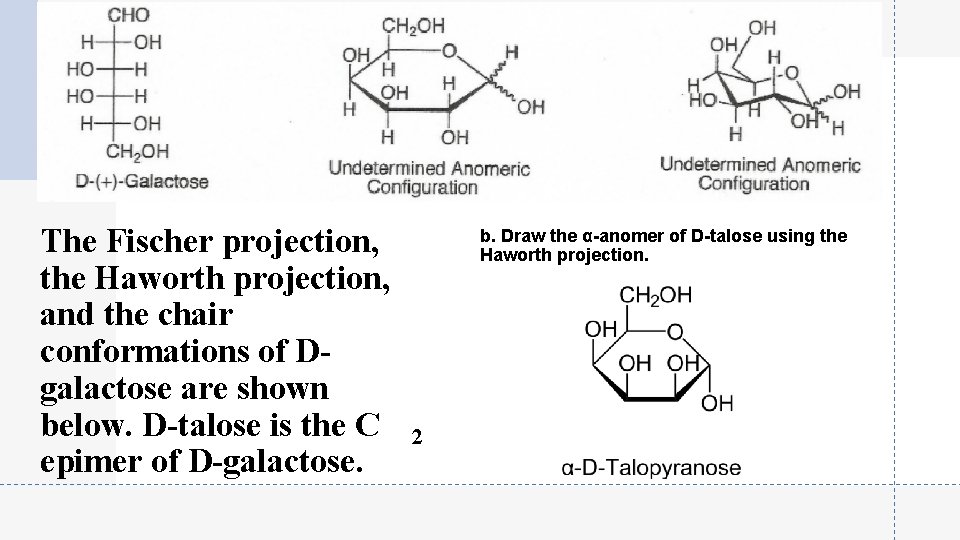
The Fischer projection, the Haworth projection, and the chair conformations of Dgalactose are shown below. D-talose is the C epimer of D-galactose. b. Draw the α-anomer of D-talose using the Haworth projection. 2

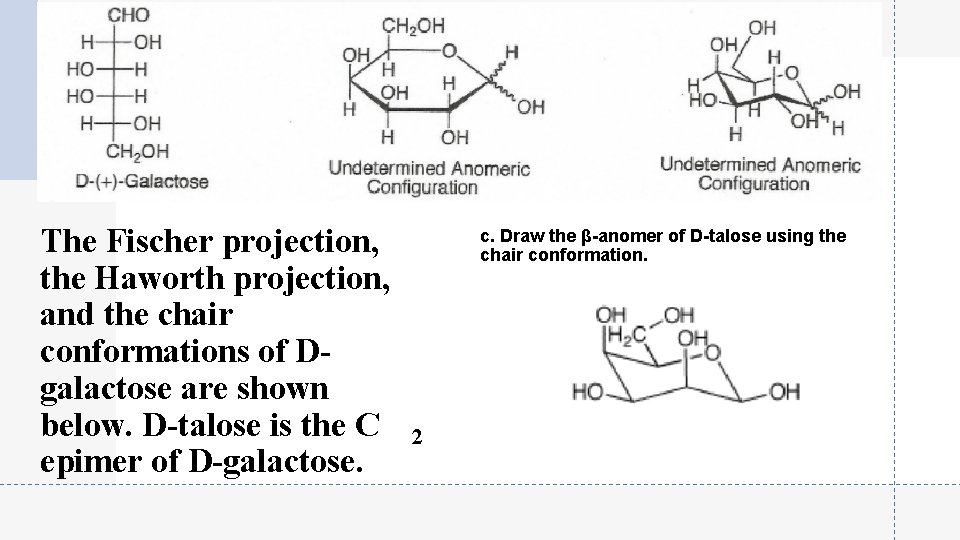
The Fischer projection, the Haworth projection, and the chair conformations of Dgalactose are shown below. D-talose is the C epimer of D-galactose. c. Draw the β-anomer of D-talose using the chair conformation. 2

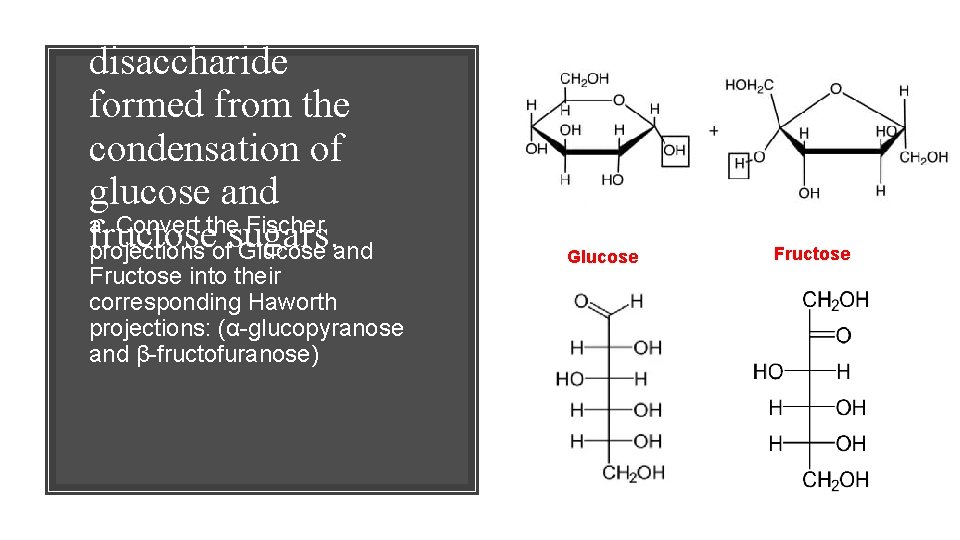
Sucrose is a disaccharide formed from the condensation of glucose and a. Convert the Fischer fructose projections ofsugars. Glucose and Fructose into their corresponding Haworth projections: (α-glucopyranose and β-fructofuranose) Glucose Fructose

Sucrose is a disaccharide formed from the condensation of glucose and b. Draw the structure of sucrose fructose sugars. that results from the condensation between the two Haworth projections drawn for glucose and fructose:

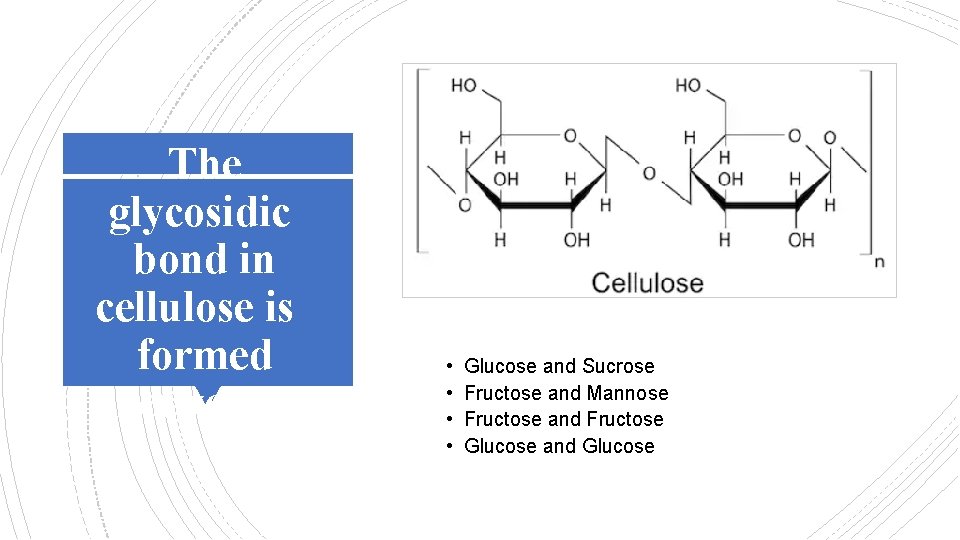
The glycosidic bond in cellulose is formed between: • • Glucose and Sucrose Fructose and Mannose Fructose and Fructose Glucose and Glucose

The glycosidic bond in cellulose is formed between: • • Glucose and Sucrose Fructose and Mannose Fructose and Fructose Glucose and Glucose

Cellulose and amylose Starch is a mixture composed of: Amylopectin and amylose Cellulose and chitin Amylopectin and keratin

Cellulose and amylose Starch is a mixture composed of: Amylopectin and amylose Cellulose and chitin Amylopectin and keratin

Oxidation of an aldose converts its aldehyde group to a carboxylic acid group. True False

Oxidation of an aldose converts its aldehyde group to a carboxylic acid group. True False

Identify and label the phosphat e, sugar, and nitrogeno us base compone nts of a nucleotide. 22

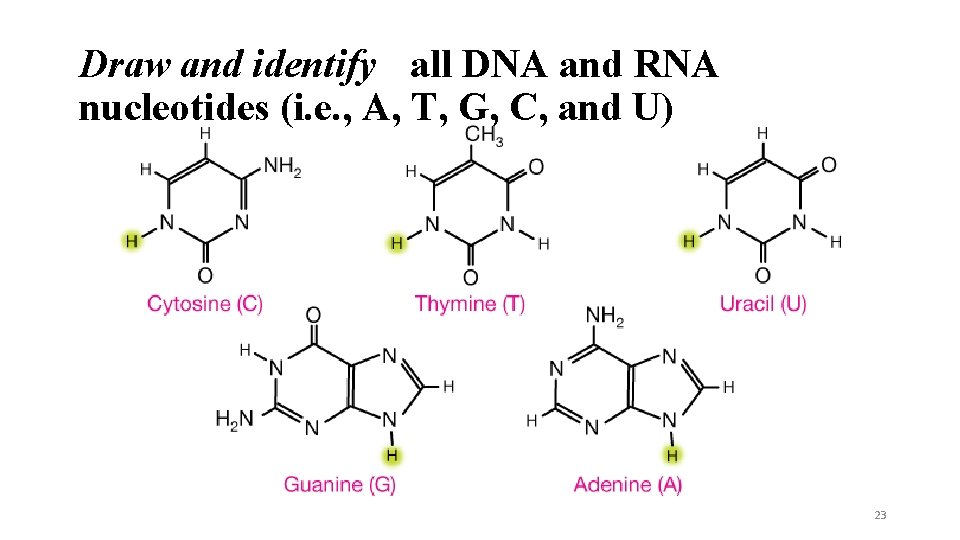
Draw and identify all DNA and RNA nucleotides (i. e. , A, T, G, C, and U) 23

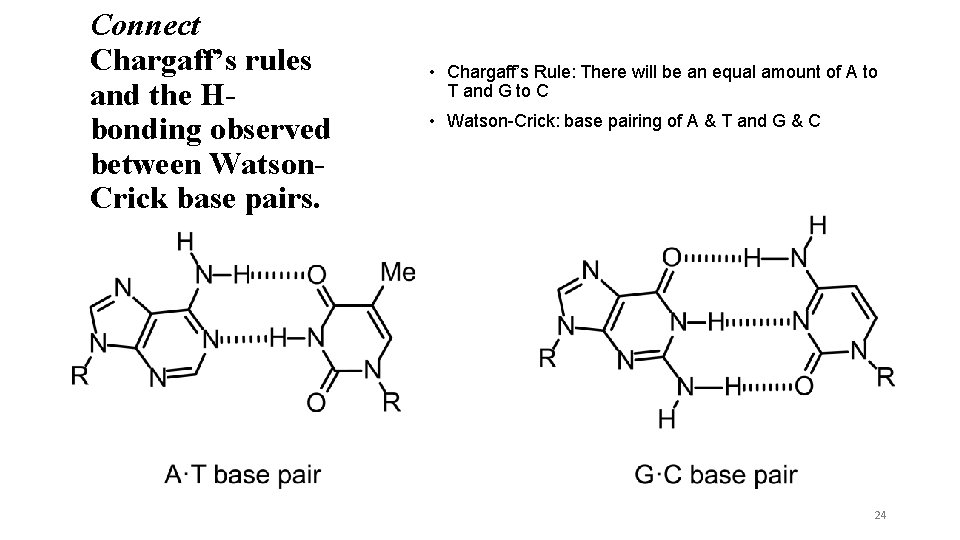
Connect Chargaff’s rules and the Hbonding observed between Watson. Crick base pairs. • Chargaff’s Rule: There will be an equal amount of A to T and G to C • Watson-Crick: base pairing of A & T and G & C 24

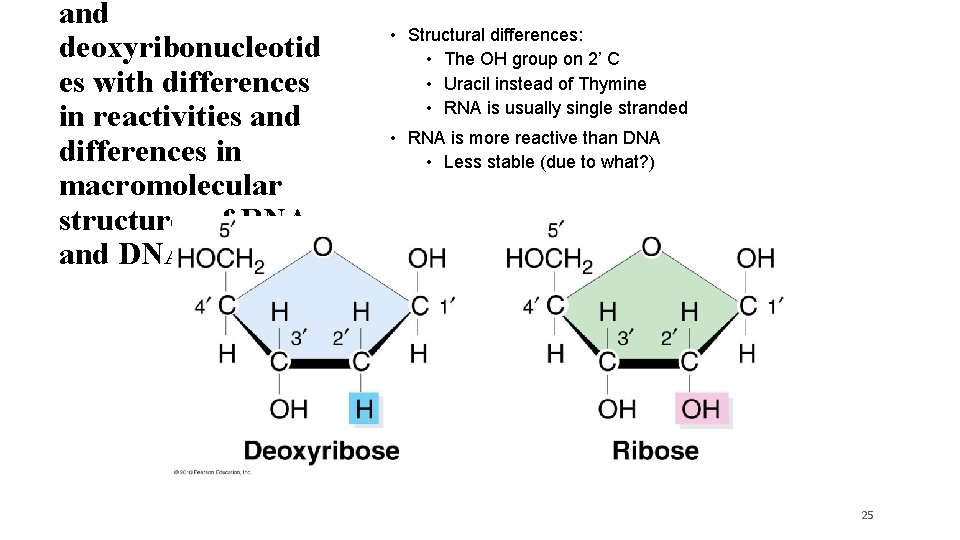
and deoxyribonucleotid es with differences in reactivities and differences in macromolecular structures of RNA and DNA. • Structural differences: • The OH group on 2’ C • Uracil instead of Thymine • RNA is usually single stranded • RNA is more reactive than DNA • Less stable (due to what? ) 25

Interpret DNA electrophoresis data used for DNA sequencing (Sanger method). 26

Design pri mers for DNA amplificatio n by PCR. • The oligonucleotide: (5’-GACTACCACG CTTCCATTAA GCTTACC) was cleaved usingthe restriction enzyme Hind. III, which has a cleavage site at A//AGCTT. a. Cleavage results in two fragments; write the sequence of the shortest fragment. b. Design a 3 nt primer 27

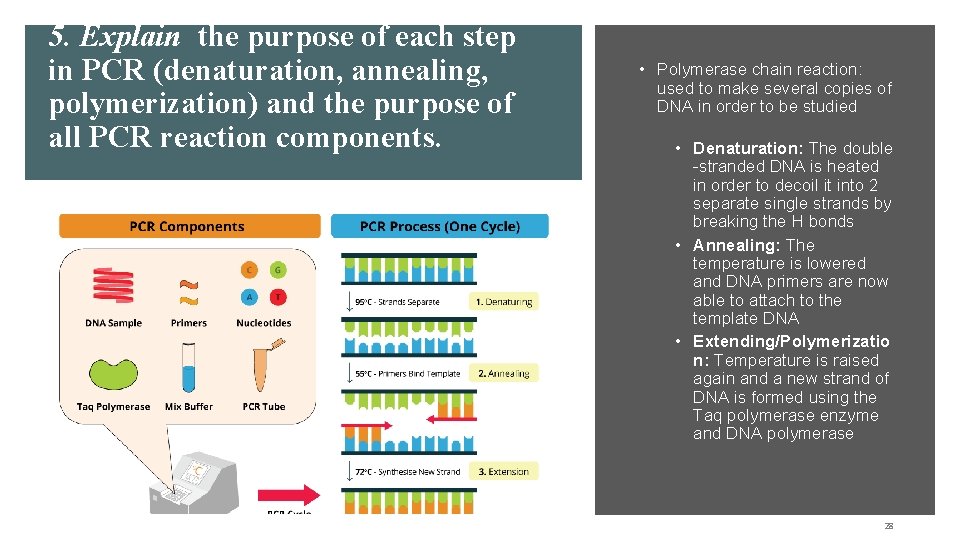
5. Explain the purpose of each step in PCR (denaturation, annealing, polymerization) and the purpose of all PCR reaction components. • Polymerase chain reaction: used to make several copies of DNA in order to be studied • Denaturation: The double -stranded DNA is heated in order to decoil it into 2 separate single strands by breaking the H bonds • Annealing: The temperature is lowered and DNA primers are now able to attach to the template DNA • Extending/Polymerizatio n: Temperature is raised again and a new strand of DNA is formed using the Taq polymerase enzyme and DNA polymerase 28

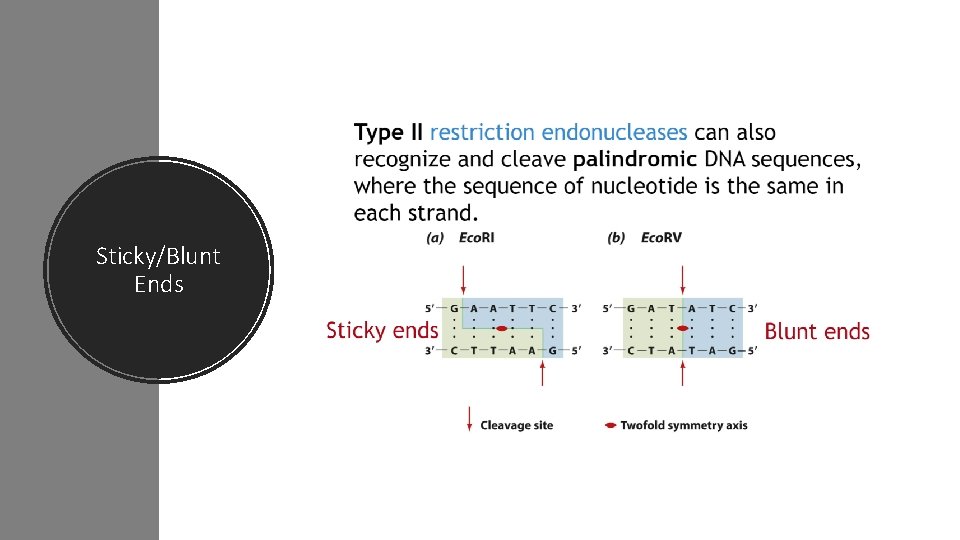
Sticky/Blunt Ends

Identify and differentiate between lipids and other membrane components 30

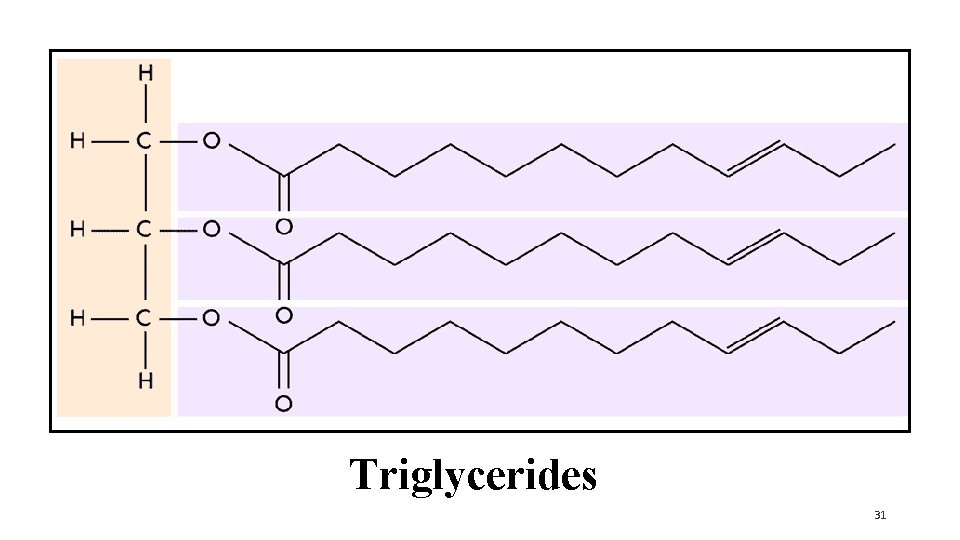
Triglycerides 31

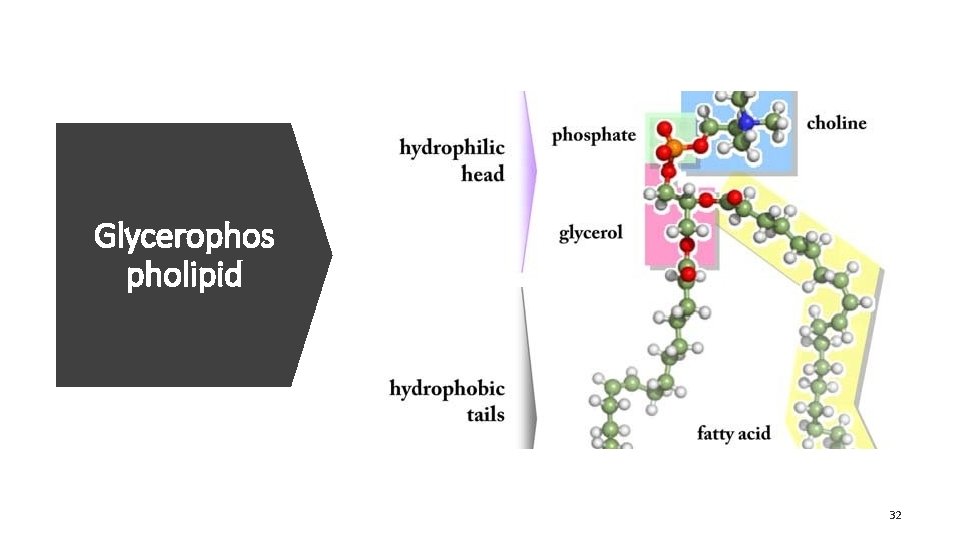
Glycerophos pholipid 32

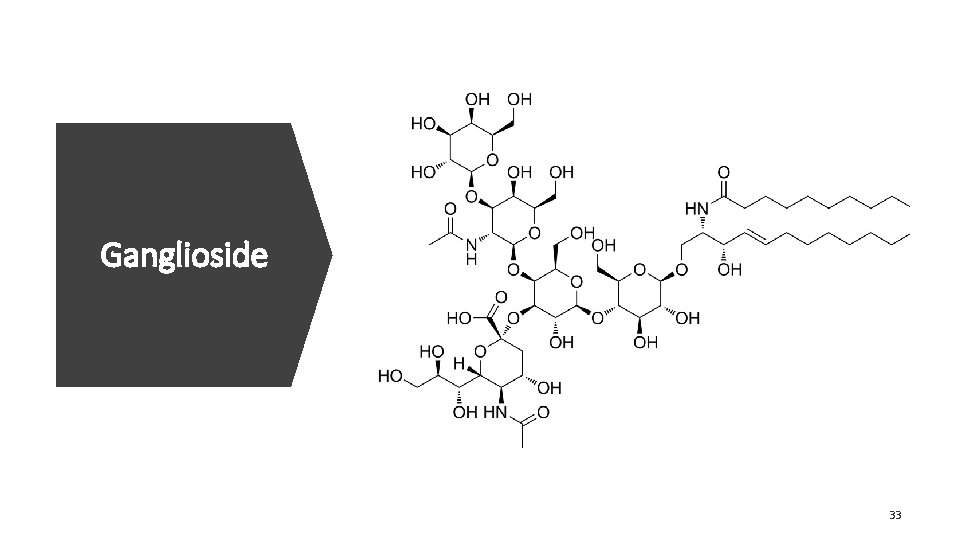
Ganglioside 33

Sphingolipid 34

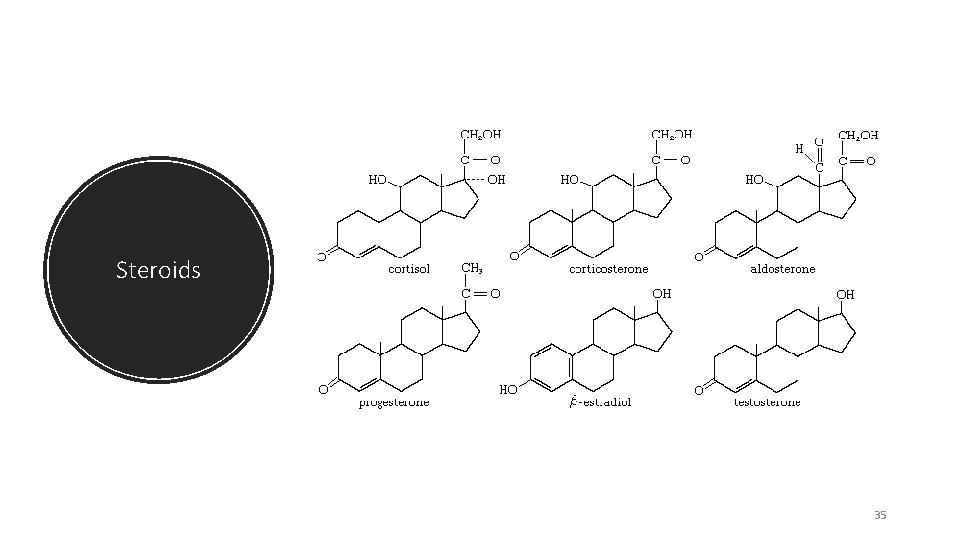
Steroids 35

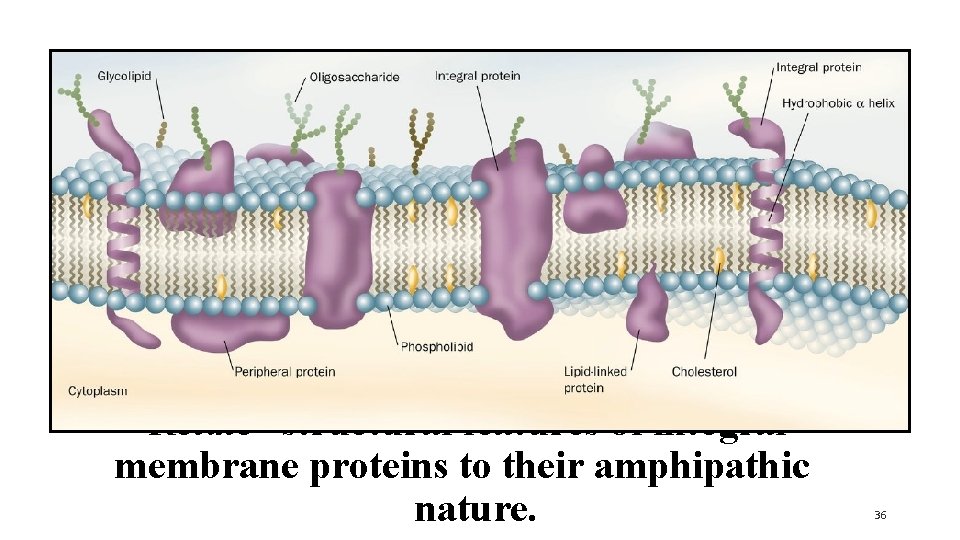
Relate structural features of integral membrane proteins to their amphipathic nature. 36

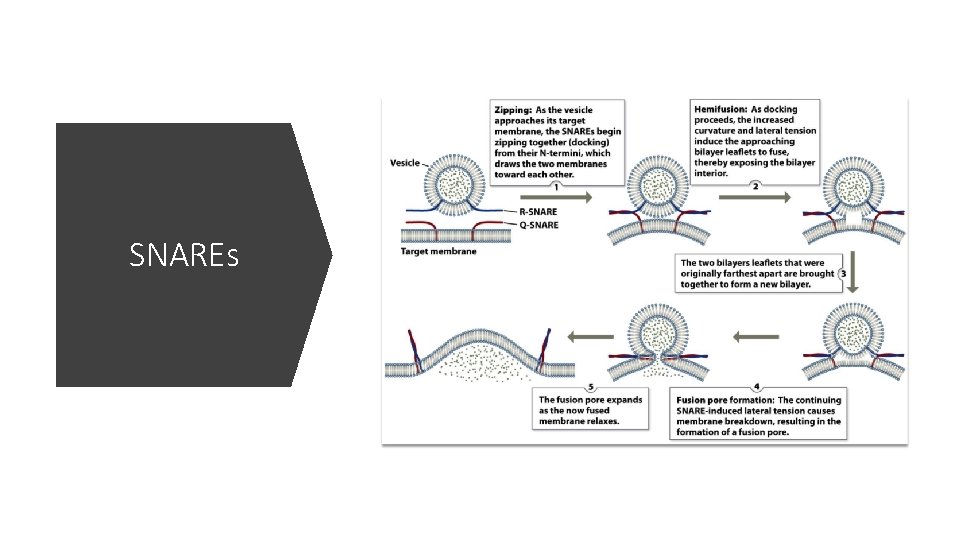
SNAREs

Thank you!
 World history semester 1 exam review
World history semester 1 exam review Ap gov final review
Ap gov final review Ece329
Ece329 Ece 310
Ece 310 Ece120 wiki
Ece120 wiki Hkn uiuc review sessions
Hkn uiuc review sessions Ece329
Ece329 Ece 391 mp3
Ece 391 mp3 Uiuc ece 313
Uiuc ece 313 Myeplg
Myeplg Apush practice exam 2017 answer key
Apush practice exam 2017 answer key Abfm exam
Abfm exam National mythology exam practice test
National mythology exam practice test Mta security fundamentals practice exam
Mta security fundamentals practice exam Hbs end of course exam review
Hbs end of course exam review Ache board of governors practice exam
Ache board of governors practice exam Her husbands wallet was full of curious items
Her husbands wallet was full of curious items Educational diagnostician practice exam
Educational diagnostician practice exam Ap stats practice exam multiple choice
Ap stats practice exam multiple choice Apes practice questions
Apes practice questions 2017 ap chemistry practice exam
2017 ap chemistry practice exam Cfei practice exam
Cfei practice exam Latin 1 final exam
Latin 1 final exam Physics 20 final exam practice
Physics 20 final exam practice Cpacc practice exam
Cpacc practice exam Statutory interpretation example answers
Statutory interpretation example answers Rac certification study material
Rac certification study material Bc-adm study guide
Bc-adm study guide Ctbs practice exam
Ctbs practice exam Latg practice exam
Latg practice exam Cahims certification cost
Cahims certification cost Flacs exam 2020
Flacs exam 2020 Board of governors exam
Board of governors exam Cfre requirements
Cfre requirements Oxfordenglishtesting answers
Oxfordenglishtesting answers Spanish 2 review
Spanish 2 review Cdt practice exam
Cdt practice exam Ap spanish literature glossary
Ap spanish literature glossary Exercise 38
Exercise 38