Science Midyear Exam Review Science Midyear Exam Review
















- Slides: 24

Science Midyear Exam Review

Science Midyear Exam Review Term One 1. The Atom

Science Midyear Exam Review Term One 2. History of the Atom Not on Midyear

Science Midyear Exam Review Term One 3. Periodic Table

3. Periodic Table - A row (period) tells us how many shells an element has. - A colum (group) tells us how many valence electrons an element has. Group 1: Alkali Metals Group 2: Alkaline Earth Metals Group 7: Halogens Group 8: Noble Gases Metals are to the left of the staircase, non-metals to the right and metalloids along the staircase.

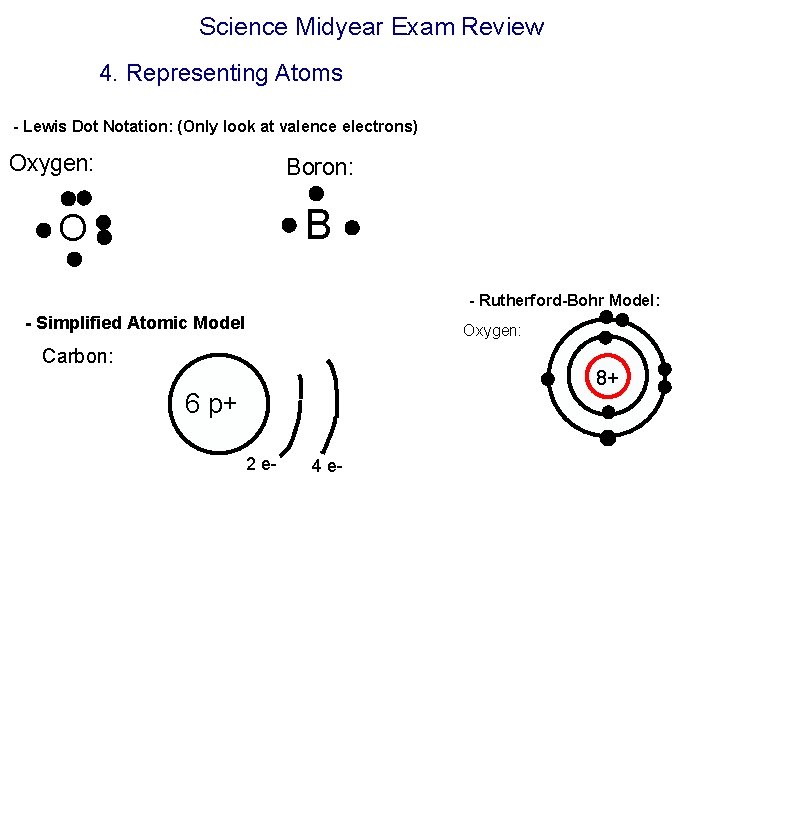
Science Midyear Exam Review 4. Representing Atoms - Lewis Dot Notation: (Only look at valence electrons) Oxygen: Boron: B O - Rutherford-Bohr Model: - Simplified Atomic Model Oxygen: Carbon: 8+ 6 p+ 2 e- 4 e-

Science Midyear Exam Review 3 -4 2 4. 5. Ions P An ION is an atom that has become electrically charged by losing or gaining one or more electrons. How do we know whether an atom will gain or lose electrons? They want to become like the noble gases, they want to become stable and have full valence electron shells. Ion form: Lithium: Charge= +1 3 p+ 2 e- 1 e- Oxygen: Charge= -2 8 p+ 2 e- 2 e 6 e-

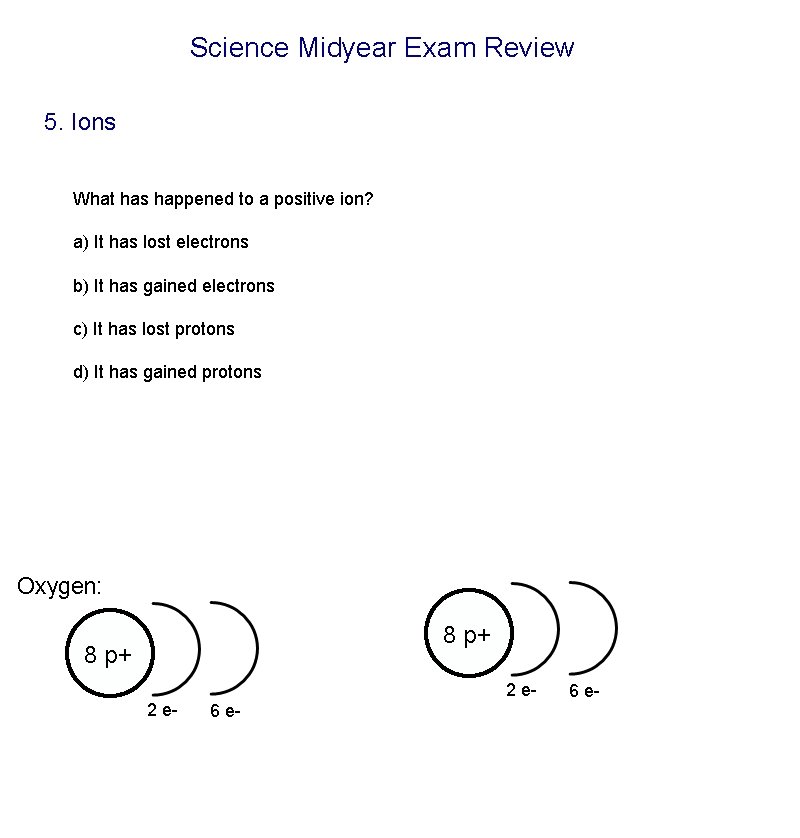
Science Midyear Exam Review 5. Ions What has happened to a positive ion? a) It has lost electrons b) It has gained electrons c) It has lost protons d) It has gained protons Oxygen: 8 p+ 2 e- 2 e 6 e-

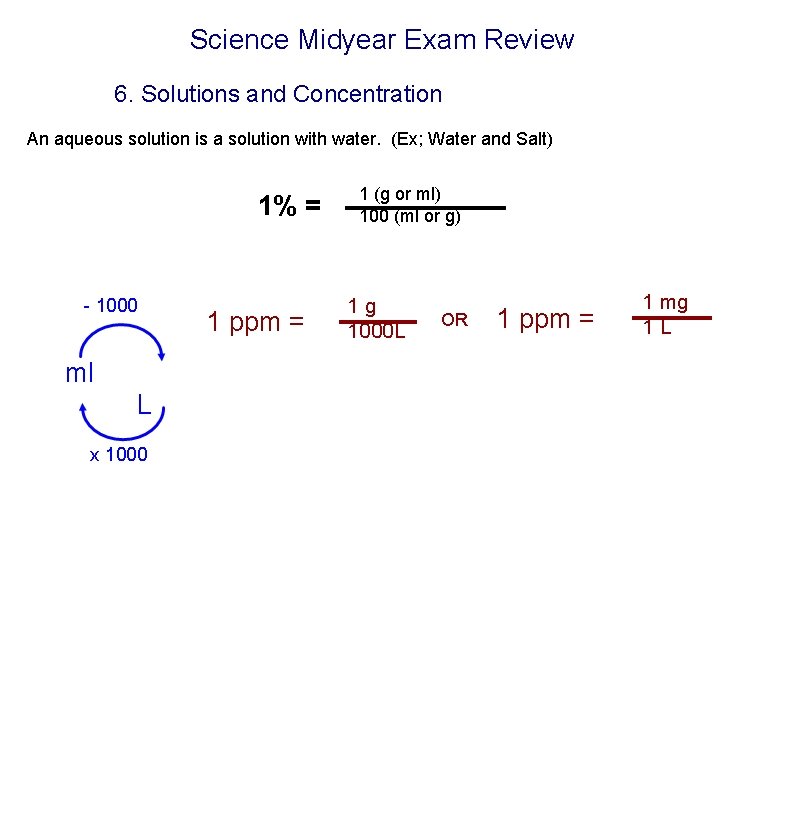
Science Midyear Exam Review 6. Solutions and Concentration An aqueous solution is a solution with water. (Ex; Water and Salt) 1% = - 1000 ml L x 1000 1 ppm = 1 (g or ml) 100 (ml or g) 1 g 1000 L OR 1 ppm = 1 mg 1 L

- 1000 6. Solutions and Concentration ml 1 ppm = 1 g OR 1 ppm = 1 mg 1 L 1% = 1000 L 1 (g or ml) 100 (ml or g) L x 1000 1. Calculate the concentration (g/L) if 30 g are dissolved in 250 ml of solution. 120 g/L 2. What is the PPM concentration if 0. 06 g are dissolved in 3000 ml of solution? 20 PPM 3. Analysis of a 25 L sample of well water shows that it contains 6 mg of dissolved arsenic. What is the concentration in ppm of dissolved arsenic? 0. 24 PPM

Science Midyear Exam Review 7. Electrolytes An electrolyte is a substance that allows an electric current to flow through the solution (when it's dissolved in water). Acids: Releases H+ ions. Starts with H Ex: HCl and H 2 SO 4 Bases: Releases OH- ions. Ends in OH Ex: Na. OH and Mg(OH)2 Salts: Produced by the chemical bonding of a metal and non-metal. Doesn't start with H, doesn't end in OH Ex: Na. Cl and Ag. NO

Science Midyear Exam Review 7. Electrolytes: PH PH Scale ranges from 0 to 14 If the PH level is less than 7, the solution is acidic If the PH level is 7, the solution is neutral. If the PH level is greater than 7, the solution is basic. or Basic The difference of one unit between two substances actually indicates that one of the substances is 10 times more acidic than the other. For example, a solution with a p. H of 3 is _10__ times more acidic than a solution with a p. H of 4. A solution with a p. H of 9 is _100_ times less basic than a solution with a p. H of 11.

Science Midyear Exam Review 8. Energy a. The Law of Conservation of Energy: Energy cannot be created nor destroyed; it can only be transferred or transformed. b. Energy efficiency: the percent of energy consumed by a machine or system that was transformed into useful energy. Energy efficiency = Amount of useful energy x 100 Amount of energy consumed Example: A fluorescent light bulb uses 100 joules of electrical energy. 25 Joules is light energy, how energy efficient is this light bulb? 25 %

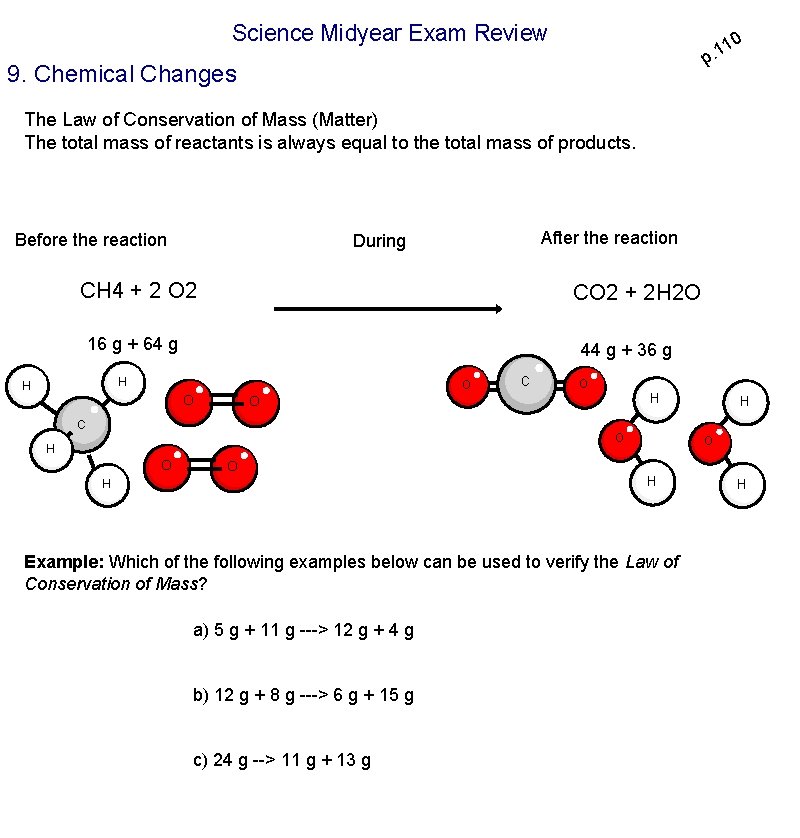
Science Midyear Exam Review 10 1. p 9. Chemical Changes The Law of Conservation of Mass (Matter) The total mass of reactants is always equal to the total mass of products. Before the reaction After the reaction During CH 4 + 2 O 2 CO 2 + 2 H 2 O 16 g + 64 g 44 g + 36 g H H O O C O H O H O O H H Example: Which of the following examples below can be used to verify the Law of Conservation of Mass? a) 5 g + 11 g ---> 12 g + 4 g b) 12 g + 8 g ---> 6 g + 15 g c) 24 g --> 11 g + 13 g H

Science Midyear Exam Review 9. Chemical Changes Example: Which of the following examples below can be used to verify the Law of Conservation of Mass? a) 5 g + 11 g ---> 12 g + 4 g b) 12 g + 8 g ---> 6 g + 15 g c) 24 g --> 11 g + 13 g 10 1. p

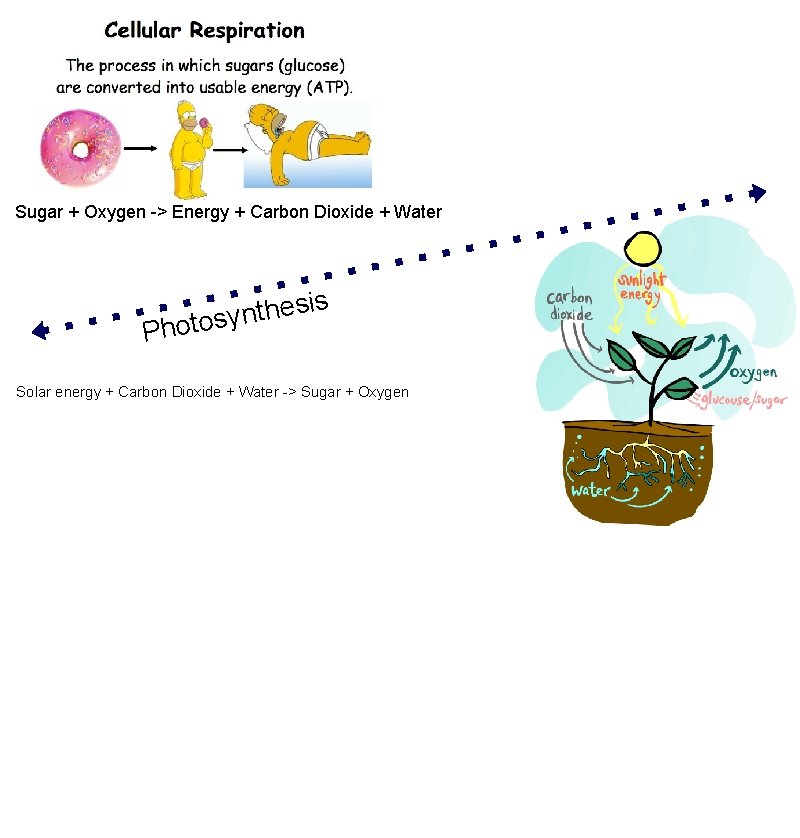
Science Midyear Exam Review 10. Types of Chemical Change Acid-Base Neutralization: a chemical change involving the reaction of an acid with a base, producing a salt and water Oxidation: a chemical change involving oxygen (or with properties similar to oxygen) Combustion : a form of oxidation that releases a large amount of energy Cellular-Respiration: a chemical change where glucose and oxygen are used to generate energy, the reaction also produces carbon dioxide and water Photosynthesis: a chemical change that produces glucose and oxygen from solar energy, carbon dioxide and water

Sugar + Oxygen -> Energy + Carbon Dioxide + Water Ph is s e h t n otosy Solar energy + Carbon Dioxide + Water -> Sugar + Oxygen

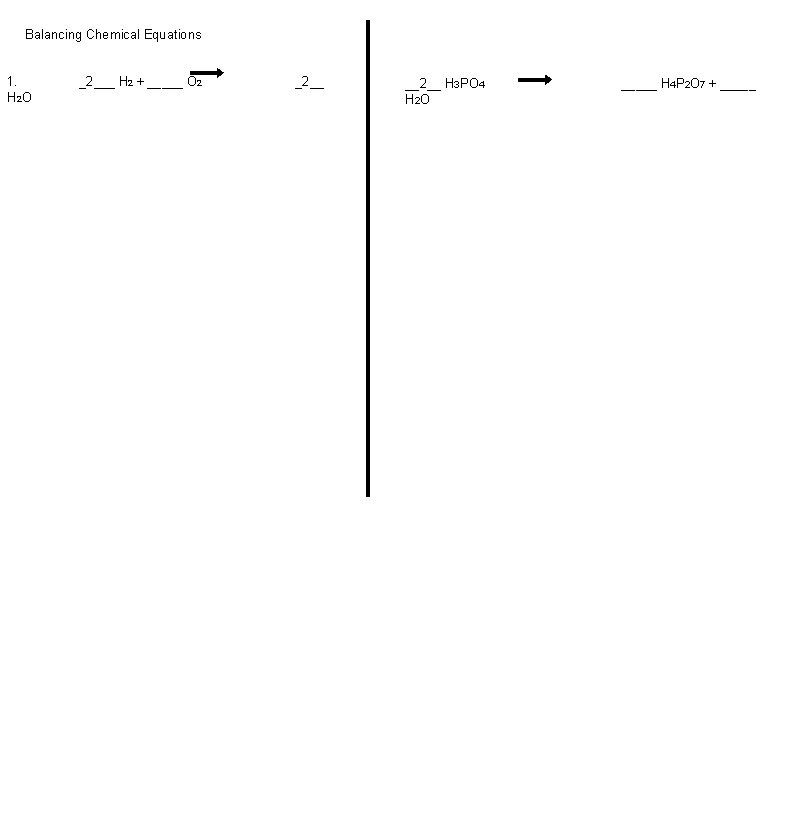
Balancing Chemical Equations 1. H 2 O _2___ H 2 + _____ O 2 _2__ __2__ H 3 PO 4 H 2 O _____ H 4 P 2 O 7 + _____

Science Midyear Exam Review 11. Electricity Negatively Charged Bodies: have more electrons then protons Positively Charged Bodies: have less electrons then protons Remember that the number of protons never changes! Substances can only lose or gain electrons. Conductors allow the free flow of electrical charges Insulators hinder the free flow of electrical charges

Science Midyear Exam Review 11. Electricity Opposite charges attract charges repel Like + - + If A is negatively charged, what charge does C have? A B B C If A is positively charged, what charge does D have? A B B C C D +

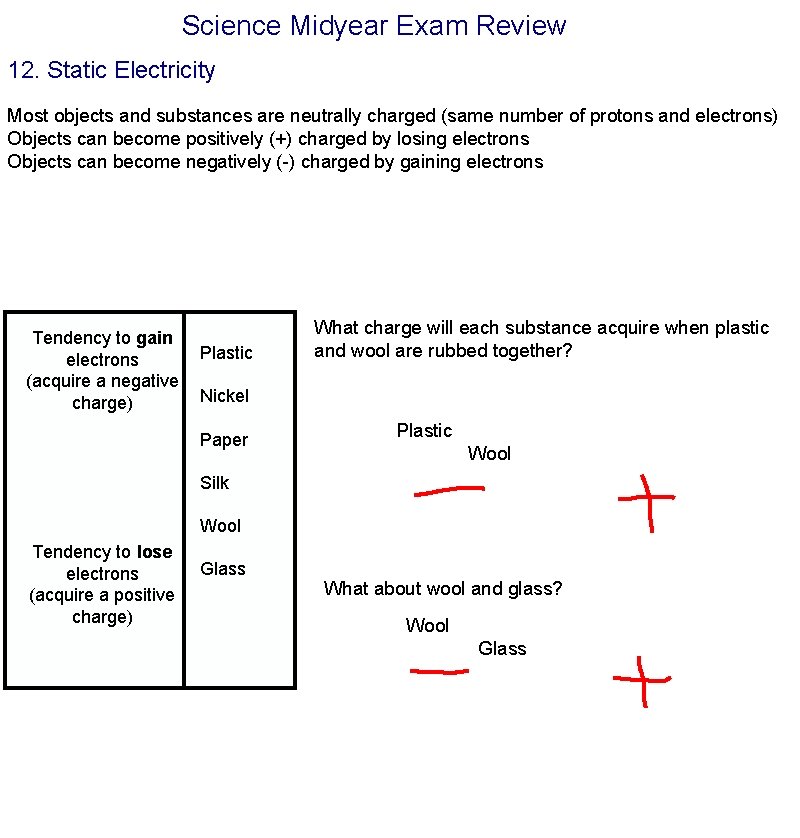
Science Midyear Exam Review 12. Static Electricity Most objects and substances are neutrally charged (same number of protons and electrons) Objects can become positively (+) charged by losing electrons Objects can become negatively (-) charged by gaining electrons Tendency to gain electrons (acquire a negative charge) Plastic What charge will each substance acquire when plastic and wool are rubbed together? Nickel Paper Plastic Wool Silk Wool Tendency to lose electrons (acquire a positive charge) Glass What about wool and glass? Wool Glass

Science Midyear Exam Review 13. Ohm's Law V = IR 1. Find the current through a 12 -ohm resistive circuit when 24 volts is applied. 2 A 2. Find the resistance of a circuit that draws 0. 06 amperes with 12 volts applied. 3. Find the applied voltage of a circuit that draws 0. 2 amperes through a 4800 -ohm resistance. 200 Ohms 960 V

How should you prepare for your midyear science exam? 1. Study! Read through your notes and worksheets, ask yourself questions about what you are reading, explain it to someone else because teaching is a great way to learn. 2. Visit facilelearning Look through the topics we have covered, answer the questions. Take your time! 3. Get a good night's sleep Teenagers should be sleeping 8. 5 to 9 hours a night. Go to bed early, keep yourself energized. 4. Eat well Have a big and healthy breakfast before your exams. Bring healthy snacks to school.

 Writ of certiorari ap gov example
Writ of certiorari ap gov example Pa state tree
Pa state tree Physical science jeopardy
Physical science jeopardy Earth science final
Earth science final My favourite subject is c
My favourite subject is c World history spring final exam review answers
World history spring final exam review answers You template
You template Spanish 2 final exam review
Spanish 2 final exam review Spanish 1 answers
Spanish 1 answers Pltw human body systems final exam review
Pltw human body systems final exam review Poe practice test kinematics answers
Poe practice test kinematics answers Innovait akt questions
Innovait akt questions Ied final exam
Ied final exam Hbs eoc practice test
Hbs eoc practice test Us history semester exam review answers
Us history semester exam review answers Entrepreneurship 1 final exam review
Entrepreneurship 1 final exam review Spanish 2 final exam review answer key
Spanish 2 final exam review answer key Apes ap exam review
Apes ap exam review World history final exam review
World history final exam review Us history semester 2 final exam
Us history semester 2 final exam English 11 a semester exam
English 11 a semester exam Review for exam pronouns
Review for exam pronouns Physics fall final exam review
Physics fall final exam review Zoology semester 1 exam review answers
Zoology semester 1 exam review answers Eduqas online exam review
Eduqas online exam review