Eradicating MRSA and MSSA Prior to Inpatient Orthopedic





















- Slides: 31

Eradicating MRSA and MSSA Prior to Inpatient Orthopedic Surgery Maureen Spencer, RN, M. Ed. , CIC Infection Control Manager Diane Gulczynski, RN, MS, CNOR Senior Vice President, Patient Care Services Susan Cohen, MT, ASCP Manager, Microbiology Laboratory New England Baptist Hospital, Boston, Ma. 1

Who We Are New England Baptist Hospital Orthopedic Center of Excellence Acute inpatient discharges are divided among 3 service lines: Orthopedic = Medical = 74. 8% 17. 4% (Cardiology, Pulmonary, Gastroenterology, Nephrology) General Surgery = 7. 8% 2

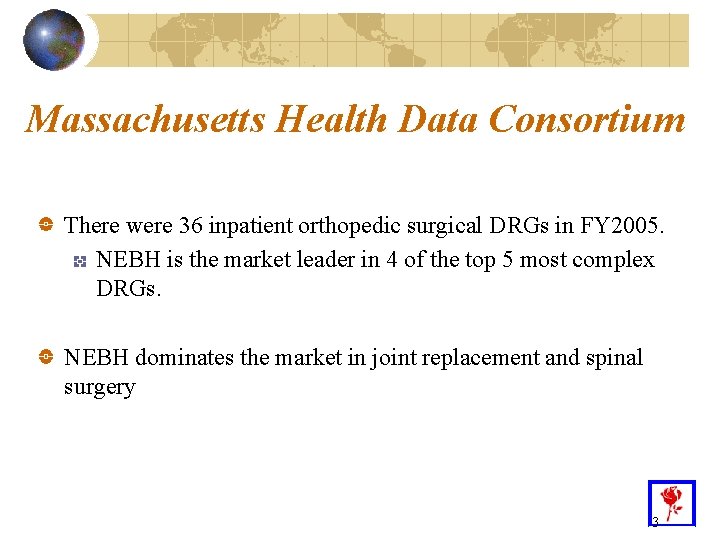
Massachusetts Health Data Consortium There were 36 inpatient orthopedic surgical DRGs in FY 2005. NEBH is the market leader in 4 of the top 5 most complex DRGs. NEBH dominates the market in joint replacement and spinal surgery 3

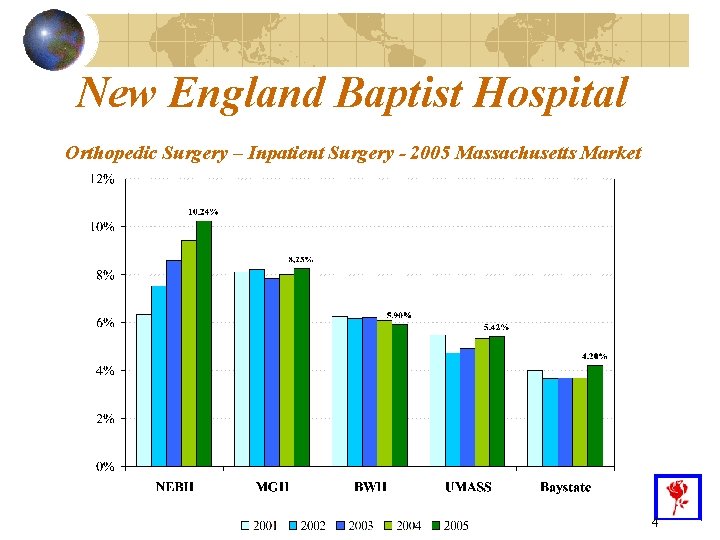
New England Baptist Hospital Orthopedic Surgery – Inpatient Surgery - 2005 Massachusetts Market 4

The inpatient orthopedic surgical market is growing and will continue – due to 1: Demographics – older population and more active lifestyles The emergence of new procedures (including minimally invasive surgery and artificial discs) Greater penetration of existing technologies Increase in the most complex DRGs 1. Herndon JH. The future of orthopaedics. AAOS Bulletin (online). June 2004; 52: 3. Available at http: //www. aaos. org/wordhtml/bulletin/jun 04/fline 3. htm. Accessed May 16, 2006. 5

The Implementation of an MRSA and MSSA Eradication Program at NEBH 6

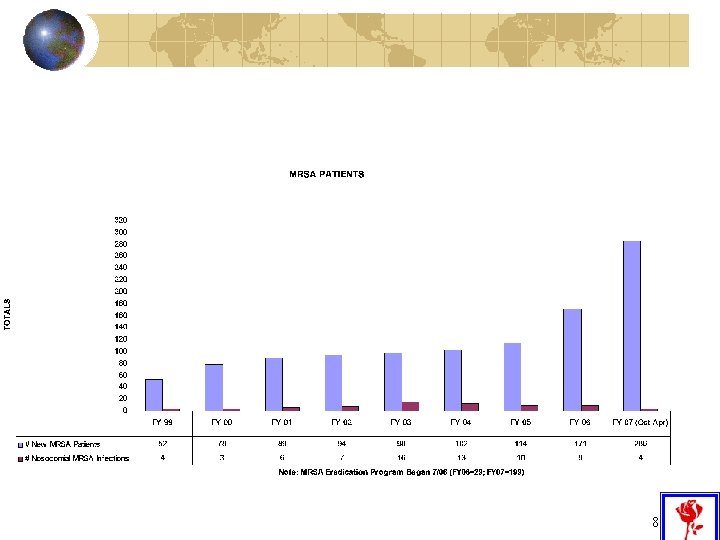
Reason #1: Increase in MRSA in Community Continued increase in community-acquired MRSA cases being admitted to NEBH 7

8

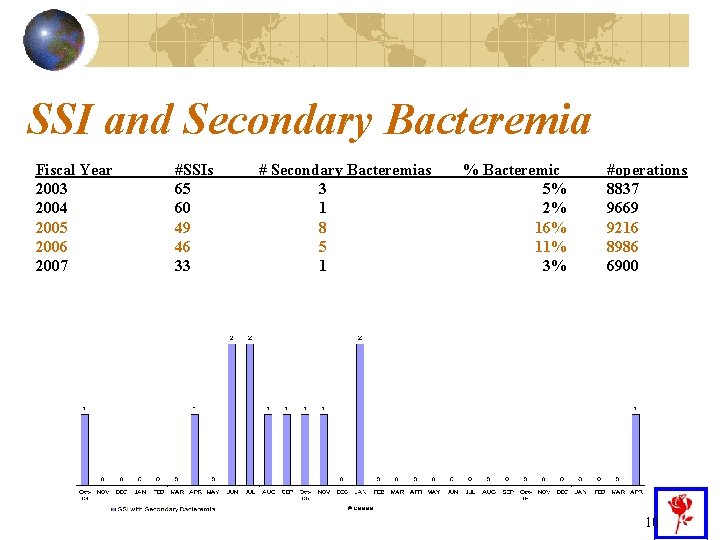
Reason #2 – Why We Implemented An Eradication Program FY 05 - 49 surgical site infections (SSI) in 9216 orthopedic surgeries (0. 5%) and in FY 06 – 46 SSI in 8986 (0. 5%) Very low rates since the NNIS national overall rate for orthopedic surgery is 1. 5% However, 8 patients in end of FY 05 and 5 in beginning of FY 06 developed a surgical site infection with secondary bacteremia post discharge. • Bacteremia is associated with an increase in morbidity and mortality 9

SSI and Secondary Bacteremia Fiscal Year 2003 2004 2005 2006 2007 #SSIs 65 60 49 46 33 # Secondary Bacteremias 3 1 8 5 1 % Bacteremic 5% 2% 16% 11% 3% #operations 8837 9669 9216 8986 6900 10

? Point Source Outbreak In October 2005 27 Staph aureus isolates (17 MSSA and 10 MRSA) were sent to the Mayo Clinic for pulsed field gel electrophoresis These included 15 nosocomial strains and 12 community-acquired strains Purpose: To determine if we were experiencing a point source outbreak related to SSI with bacteremia Results: 6 of 27 strains had similar number and size of bands 3 were community-acquired strains and 3 nosocomial The 3 nosocomial cases were unrelated in terms of time, person and place 11

Program Implementation The Infection Control Committee recommended implementation of an MSSA/MRSA eradication program to reduce nasal colonization in patients scheduled for inpatient surgery and treat MRSA positive screens with vancomycin for surgical prophylaxis Administrative support was elicited from the Senior Vice President of Patient Care Services to fund a program included nasal screens with rapid polymerase chain reaction (PCR) technology, which enabled 2 -hour results for MRSA and one day for MSSA. 12

Senior VP Patient Care Services Researched MRSA problem and developed a “White Paper” January 2006 - prepared a letter to the Infection Control Committee regarding eradicating MRSA in all surgeries February 2006 – conducted an anonymous active surveillance culture study in the operating room February 2006 – prepared three testing proposals with budgetary cost for Board of Trustees traditional 3 day process for results rapid test – purchasing equipment rapid test – leasing equipment 13

14

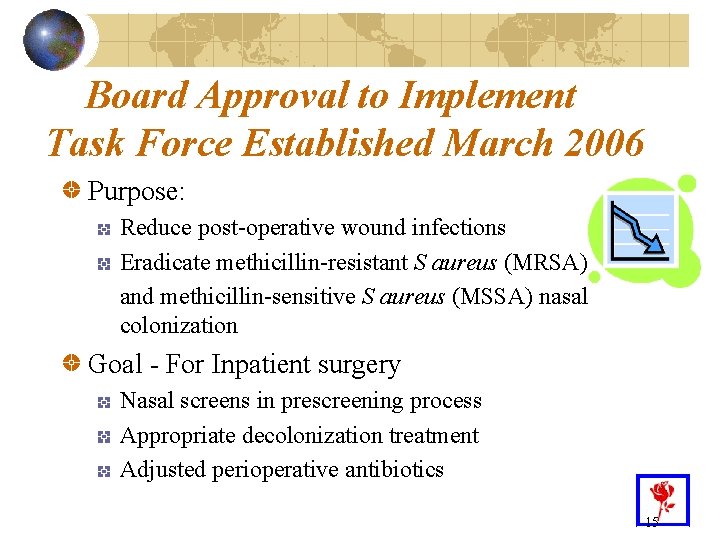
Board Approval to Implement Task Force Established March 2006 Purpose: Reduce post-operative wound infections Eradicate methicillin-resistant S aureus (MRSA) and methicillin-sensitive S aureus (MSSA) nasal colonization Goal - For Inpatient surgery Nasal screens in prescreening process Appropriate decolonization treatment Adjusted perioperative antibiotics 15

Implementation Steps March 2006 – October 2006 – weekly meetings with surgical services, infection control, micro, administration, and medical staff members July 2006 – letter to surgeons July 17, 2006 – initiated pilot on Spine Service August 2006 - presentation to the Patient Care Assessment Committee August 2006 – letter to all medical staff August 2006 – letter to OR Scheduling September 2006 – initiated program for all inpatient surgeries 16

Policy and Procedure Developed procedural steps for departments and units affected by the implementation • • • Patient Access Operating Room Scheduling Prescreening Unit Pre-surgical unit (Bond Center) Operating Room Post Anesthesia Care Unit Nursing Units Microbiology Lab Ancillary Departments: Housekeeping, Central Transport 17

Implementation Steps May 2006 - Microbiology Lab Purchased rapid polymerase chain reaction equipment Hired a full-time technologist June 2006 - The prescreening unit (PASU) Hired a full-time MRSA Coordinating Medical Technician 18

19

20

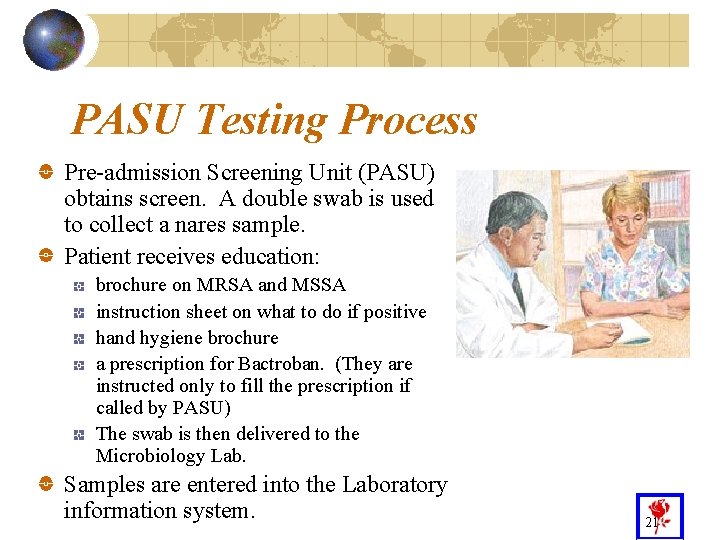
PASU Testing Process Pre-admission Screening Unit (PASU) obtains screen. A double swab is used to collect a nares sample. Patient receives education: brochure on MRSA and MSSA instruction sheet on what to do if positive hand hygiene brochure a prescription for Bactroban. (They are instructed only to fill the prescription if called by PASU) The swab is then delivered to the Microbiology Lab. Samples are entered into the Laboratory information system. 21

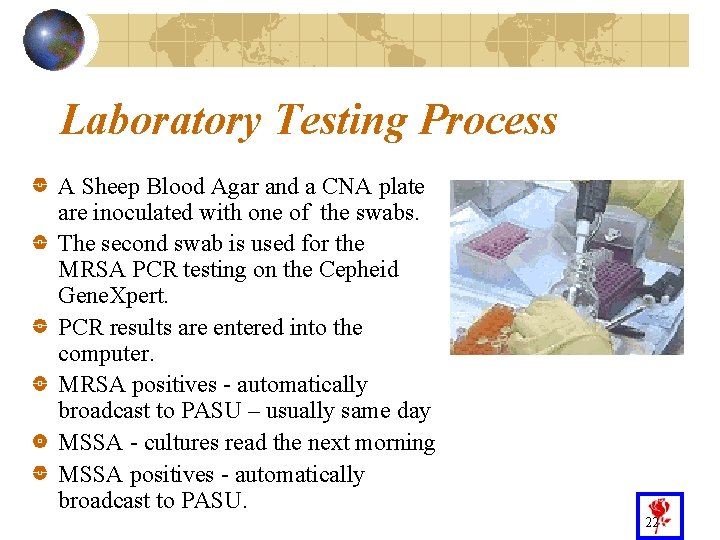
Laboratory Testing Process A Sheep Blood Agar and a CNA plate are inoculated with one of the swabs. The second swab is used for the MRSA PCR testing on the Cepheid Gene. Xpert. PCR results are entered into the computer. MRSA positives - automatically broadcast to PASU – usually same day MSSA - cultures read the next morning MSSA positives - automatically broadcast to PASU. 22

23

Laboratory Challenges Instructing staff on the proper swabs to use and how to obtain a nares specimen How to differentiate patients colonized from patients infected in the lab. Getting a Molecular Lab up and running in a short time frame. How to notify PASU and Infection Control of positive results. 24

Equipment We began using the Cepheid’s Smart. Cycler in May 2006 and conducted validity testing and training of staff. In July 2006 we started the pilot program In September 2006 we went live for all inpatient surgeries In June of 2007 we began using Cepheid’s Gene. Xpert 25

Validation Smart Cycler: The first 100 samples run were screened by conventional culture for MRSA. Gene. Xpert: 75 samples were run on both the Smart Cycler and the Gene. Xpert. This required PASU to collect swabs from patients using the Smart Cycler swabs and the Gene. Xpert swabs. 26

Teamwork Microbiology, PASU, Infection Control, Surgical Services, Nursing, Pharmacy and Information Systems are all involved with the MRSA eradication process. PASU – obtaining screens and delivering to Microbiology Lab in a timely fashion Microbiology – results to PASU as soon as they are available. Information Systems - setting up systems for automatic broadcasting Nursing - make sure the correct swabs are used. 27

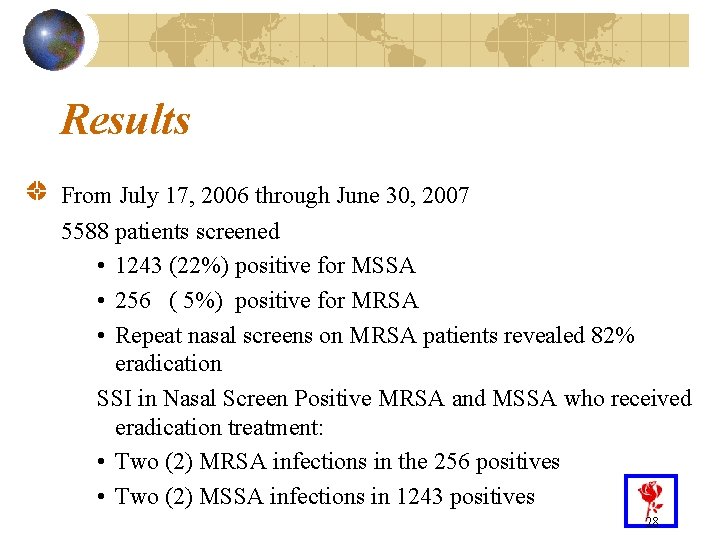
Results From July 17, 2006 through June 30, 2007 5588 patients screened • 1243 (22%) positive for MSSA • 256 ( 5%) positive for MRSA • Repeat nasal screens on MRSA patients revealed 82% eradication SSI in Nasal Screen Positive MRSA and MSSA who received eradication treatment: • Two (2) MRSA infections in the 256 positives • Two (2) MSSA infections in 1243 positives 28

Conclusion A multidisciplinary approach strong administrative and financial support consistent communication and teamwork Outcome: Prescreening for MSSA and MRSA with decolonization treatment reduces post surgical site infections 29

What Is Next For NEBH? Screening of ~5000 Same Day Surgery Patients What are we thinking? ? Testing and Treatment by MD’s office prior to surgery? Testing on the day of surgery in order to provide appropriate surgical prophylaxis? Who is responsible for patient follow-up post same day surgery discharge? The nares is still positive! 30

Thank You M. R. S. A. Make Resistance Stay Away 31
 Mssa vs mrsa
Mssa vs mrsa Footbob
Footbob Miracles wichita kansas
Miracles wichita kansas Inpatient in nh
Inpatient in nh Thcic inpatient data
Thcic inpatient data Poa indicators are assigned in the inpatient setting for
Poa indicators are assigned in the inpatient setting for Inpatient pharmacy workflow
Inpatient pharmacy workflow Mrsa
Mrsa Baset zarrug
Baset zarrug Ca mrsa
Ca mrsa Mrsa
Mrsa Mrsa wound
Mrsa wound Mrsa de centro
Mrsa de centro Mrsa
Mrsa Mrsa infekce
Mrsa infekce Mrsa meddig fertőz
Mrsa meddig fertőz Scabies
Scabies Mrsa prenos
Mrsa prenos Hygieniaohjeet
Hygieniaohjeet What is mrsa in medical terms
What is mrsa in medical terms Spannhake orthodontics
Spannhake orthodontics Abbot saunders test
Abbot saunders test Bechterew's orthopedic test
Bechterew's orthopedic test Uva powerpoint
Uva powerpoint 2 minute orthopedic exam
2 minute orthopedic exam Search engine optimization for orthopedic practices
Search engine optimization for orthopedic practices Orthopedic history taking
Orthopedic history taking O'donoghue's maneuver
O'donoghue's maneuver Dr. craig shank orthopedic surgery
Dr. craig shank orthopedic surgery Orthopedic icats
Orthopedic icats Causes of orthopedic impairment
Causes of orthopedic impairment Orthopedic case presentation
Orthopedic case presentation