ENR 116 Mod 4 Slide No ENR 116














- Slides: 29

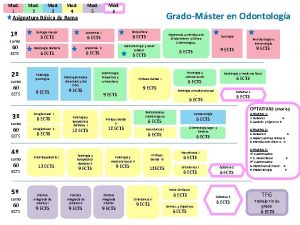
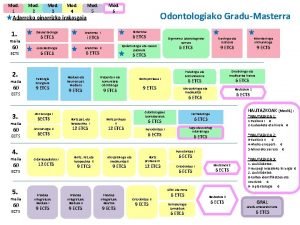
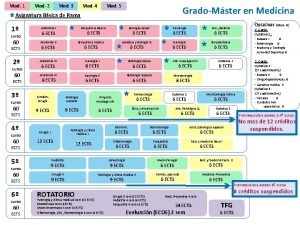
ENR 116 – Mod. 4 - Slide No. ENR 116 Engineering Materials Module 4 Non-metals and Corrosion Dr Drew Evans School of Advanced Manufacturing and Mechanical Engineering

ENR 116 – Mod. 4 - Slide No. 2 Copyright Notice Do not remove this notice. COMMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING This material has been produced and communicated to you by or on behalf of the University of South Australia pursuant to Part VB of the Copyright Act 1968 (the Act). The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice.

ENR 116 – Mod. 4 - Slide No. Ceramic processing and applications

ENR 116 – Mod. 4 - Slide No. 4 Intended Learning Outcomes At the end of this section, students will be able to: - • Identify the classes of ceramics. • Understand how and why ceramics are used. • Describe ceramic processing and how it differs from that of metals.

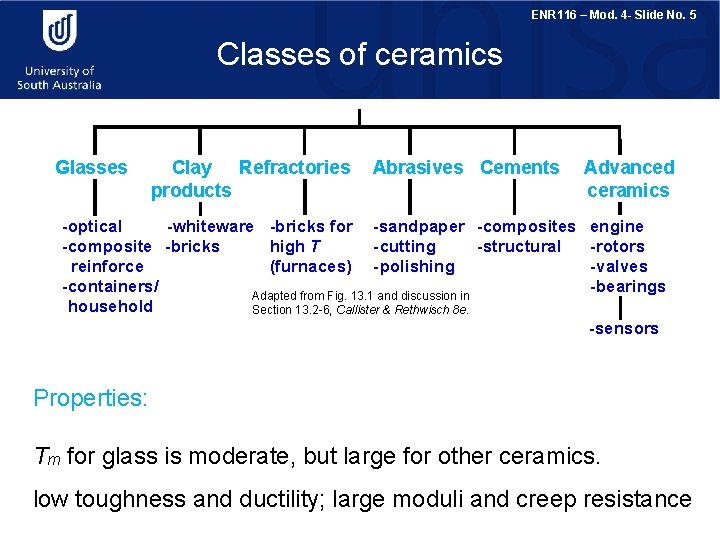
ENR 116 – Mod. 4 - Slide No. 5 Classes of ceramics Glasses Clay Refractories products Abrasives Cements Advanced ceramics -optical -whiteware -bricks for -sandpaper -composites engine high T -composite -bricks -cutting -structural -rotors (furnaces) -polishing reinforce -valves -containers/ -bearings Adapted from Fig. 13. 1 and discussion in household Section 13. 2 -6, Callister & Rethwisch 8 e. -sensors Properties: Tm for glass is moderate, but large for other ceramics. low toughness and ductility; large moduli and creep resistance

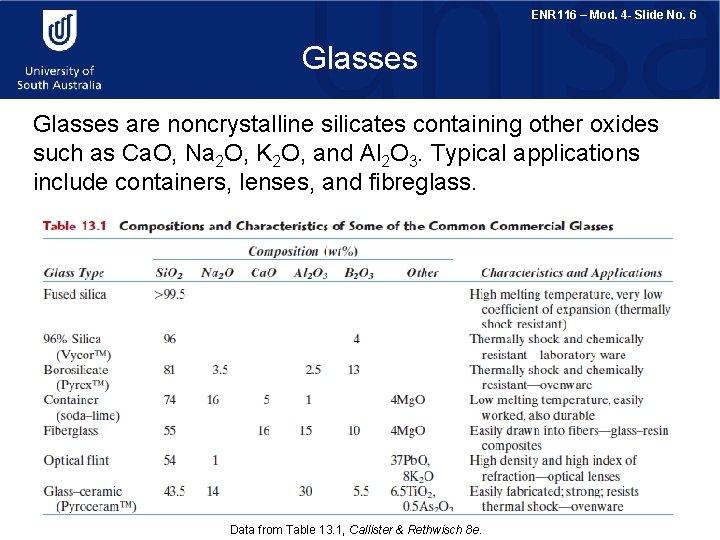
ENR 116 – Mod. 4 - Slide No. 6 Glasses are noncrystalline silicates containing other oxides such as Ca. O, Na 2 O, K 2 O, and Al 2 O 3. Typical applications include containers, lenses, and fibreglass. Data from Table 13. 1, Callister & Rethwisch 8 e.

ENR 116 – Mod. 4 - Slide No. 7 Glass-ceramics Noncrystalline to crystalline by the proper hightemperature heat treatment Fig. 13. 02, Callister & Rethwisch 8 e. Yields a fine-grained polycrystalline material which is often called a glass– ceramic. The cooling rate represented by curve 2 is much greater than that for curve 1. Cooling rate 1 will lead to formation of glass-ceramic. Continuous cooling transformation diagram for the crystallization of a lunar glass (35. 5 wt% Si. O 2, 14. 3 wt% Ti. O 2, 3. 7 wt% Al 2 O 3, 23. 5 wt% Fe. O, 11. 6 wt% Mg. O, 11. 1 wt% Ca. O, and 0. 2 wt% Na 2 O). Also superimposed on this plot are two cooling curves, labelled “ 1” and “ 2”.

ENR 116 – Mod. 4 - Slide No. 8 Glass-ceramics: properties and applications Properties: • Relatively high mechanical strengths • Low coefficients of thermal expansion • High temperature capabilities • Good dielectric properties • Good biological compatibility. Ceramic rangetop By fras 1977, released under CC BY-NC 2. 0 license Applications: Ovenware, tableware, oven windows, and rangetops • Strength and excellent resistance to thermal shock Electrical insulators, substrates for printed circuit boards, architectural cladding, heat exchangers and regenerators.

ENR 116 – Mod. 4 - Slide No. 9 Refractories: Properties Have the capacity to withstand high T’s without melting or decomposing. Adapted from Fig. 12. 27, Callister & Rethwisch 8 e. Remain unreactive and inert when exposed to severe environments. Also able to provide thermal insulation. Upgrading the alumina content will increase the maximum service temperature.

ENR 116 – Mod. 4 - Slide No. 10 Refractories: Applications Typical applications include furnace linings for metal refining, glass manufacturing, metallurgical heat treatment, and power generation. Metal pouring Power line insulators Data from Table 13. 2, Callister & Rethwisch 8 e.

ENR 116 – Mod. 4 - Slide No. 11 Abrasives: Properties Abrasive ceramics are used to wear, grind, or cut away other materials, which necessarily are softer. Properties: Materials: Hardness Diamond (both natural and synthetic) Wear resistance Silicon carbide (Si. C) Toughness Tungsten carbide (WC) Refractoriness Aluminum oxide (or corundum) Silica sand

ENR 116 – Mod. 4 - Slide No. 12 Abrasives: Applications Tools for: Oil drill bits grinding polishing cutting Coated single crystal diamonds drilling Cutting blades Polycrystalline diamonds in a resin matrix. Photos courtesy Martin Deakins, GE Superabrasives, Worthington, OH. Used with permission.

ENR 116 – Mod. 4 - Slide No. 13 Cements Characteristic feature of these materials is that when mixed with water they form a paste that subsequently sets and hardens. By joanna 8555 , released under CC BY-NC-ND 2. 0 license By Odalaigh, released under CC BY-NC-ND 2. 0 license Portland cement is consumed in the largest tonnages. The principal constituents are tricalcium silicate (3 Ca. O–Si. O 2) and dicalcium silicate (2 Ca. O–Si. O 2).

ENR 116 – Mod. 4 - Slide No. 14 Cements Produced by: 1. Grinding and intimately mixing clay and lime-bearing minerals in the proper proportions. 2. Heat mixture to about 1400 o. C in a rotary kiln (calcination). 3. Resulting “clinker” ground into a very fine powder to which a small amount of gypsum (Ca. SO 4– 2 H 2 O) is added to retard the setting process. The setting and hardening results, not from drying but from hydration reactions that occur among the various cement constituents and the water that is added. 2 Ca. O–Si. O 2 + x. H 2 O = 2 Ca. O–Si. O 2–x. H 2 O

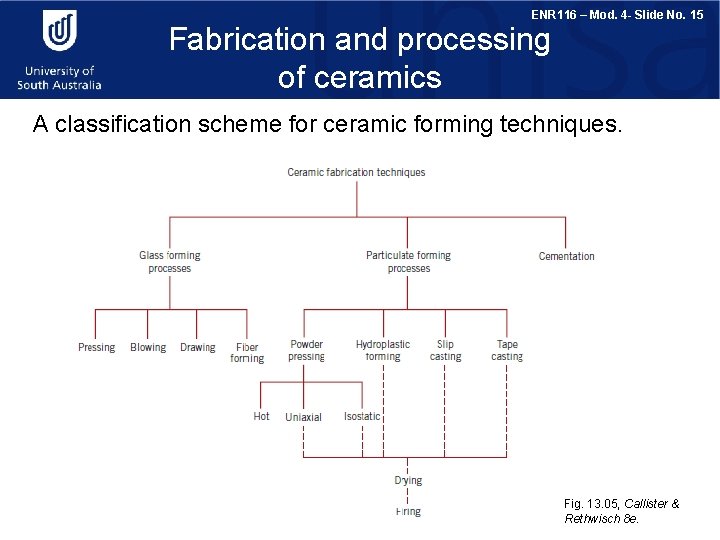
ENR 116 – Mod. 4 - Slide No. 15 Fabrication and processing of ceramics A classification scheme for ceramic forming techniques. Fig. 13. 05, Callister & Rethwisch 8 e.

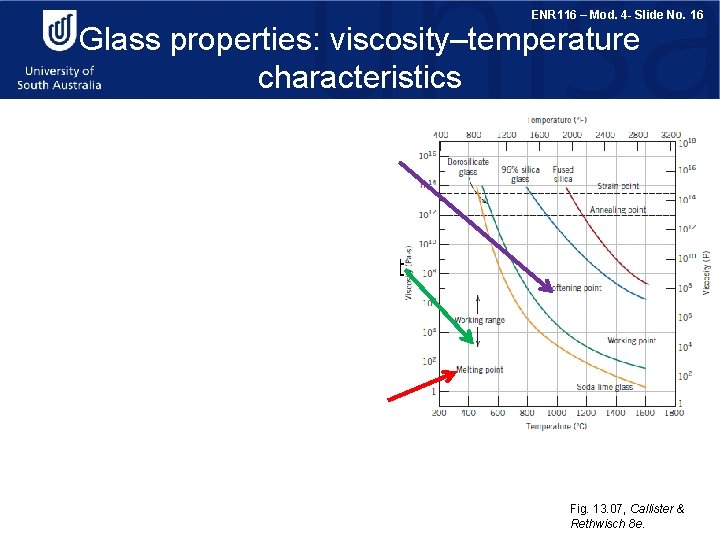
ENR 116 – Mod. 4 - Slide No. 16 Glass properties: viscosity–temperature characteristics Softening point: T at which the viscosity is 4 x 106 Pa·s, the maximum T at which a glass piece may be handled without causing significant dimensional alterations. Working point: Represents the T at which the viscosity is 103 Pa·s; the glass is easily deformed at this viscosity. Melting point: Corresponds to the T at which the viscosity is 10 Pa·s; the glass is fluid enough to be considered a liquid. Fig. 13. 07, Callister & Rethwisch 8 e.

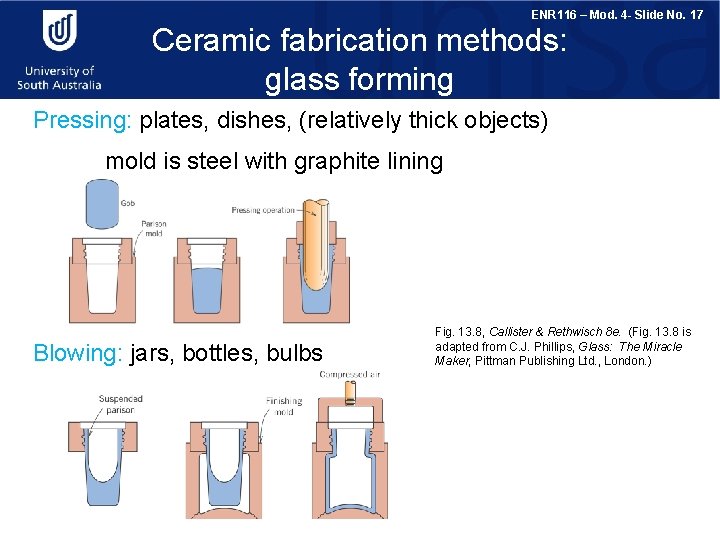
ENR 116 – Mod. 4 - Slide No. 17 Ceramic fabrication methods: glass forming Pressing: plates, dishes, (relatively thick objects) mold is steel with graphite lining Blowing: jars, bottles, bulbs Fig. 13. 8, Callister & Rethwisch 8 e. (Fig. 13. 8 is adapted from C. J. Phillips, Glass: The Miracle Maker, Pittman Publishing Ltd. , London. )

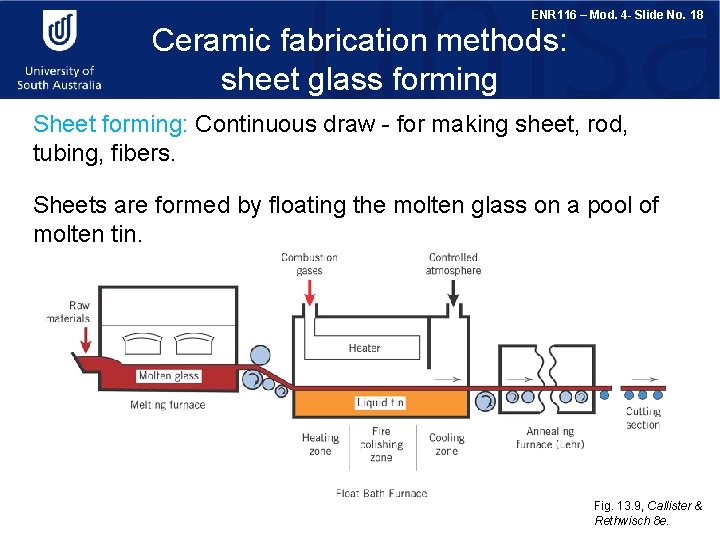
ENR 116 – Mod. 4 - Slide No. 18 Ceramic fabrication methods: sheet glass forming Sheet forming: Continuous draw - for making sheet, rod, tubing, fibers. Sheets are formed by floating the molten glass on a pool of molten tin. Fig. 13. 9, Callister & Rethwisch 8 e.

ENR 116 – Mod. 4 - Slide No. 19 Heat treating glass Annealing: Removes internal stress caused by uneven cooling. Tempering: Puts surface of glass part into compression, suppressing surface crack propagation Sequence: before cooling surface cooling further cooled hot cooler compression tension compression Result: surface crack growth is suppressed. Fig. 13. 10, Callister & Rethwisch 8 e.

ENR 116 – Mod. 4 - Slide No. 20 Fabrication and processing of clay products: Clay composition A mixture of components used i. e. typical porcelain: 1. Clay - aluminosilicates (50%). 2. Filler - e. g. quartz (finely ground) – inexpensive, relatively hard and chemically unreactive (25%). 3. Fluxing agent (Feldspar) - aluminosilicate materials that contain K+, Na+, and Ca 2+ ions (25%). Melts at relatively low temperature and during firing binds all the components together.

ENR 116 – Mod. 4 - Slide No. 21 Characteristics of clays Hydroplasticity: Becomes plastic when water is added. Shear Adding water to clay: Allows material to shear easily along weak van der Waals bonds. charge neutral enables extrusion enables slip casting Structure of kaolinite clay: Adapted from Fig. 12. 14, Callister & Rethwisch 8 e. (Fig. 12. 14 is adapted from W. E. Hauth, "Crystal Chemistry of Ceramics", American Ceramic Society Bulletin, Vol. 30 (4), 1951, p. 140. ) weak van der Waals bonding 4+ charge neutral Si 3+ Al OH 2 O Shear

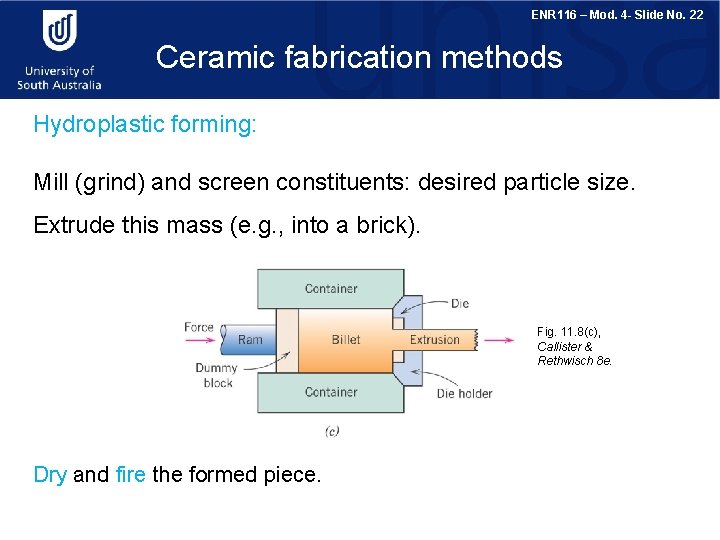
ENR 116 – Mod. 4 - Slide No. 22 Ceramic fabrication methods Hydroplastic forming: Mill (grind) and screen constituents: desired particle size. Extrude this mass (e. g. , into a brick). Fig. 11. 8(c), Callister & Rethwisch 8 e. Dry and fire the formed piece.

ENR 116 – Mod. 4 - Slide No. 23 Ceramic fabrication methods Slip casting: Mill (grind) and screen constituents: desired particle size. Mix with water and other constituents to form slip. Slip casting operation Solid Hollow Dry and fire the formed piece. Fig. 13. 12, Callister & Rethwisch 8 e. (Fig. 13. 12 is from W. D. Kingery, Introduction to Ceramics, John Wiley and Sons, Inc. , 1960. )

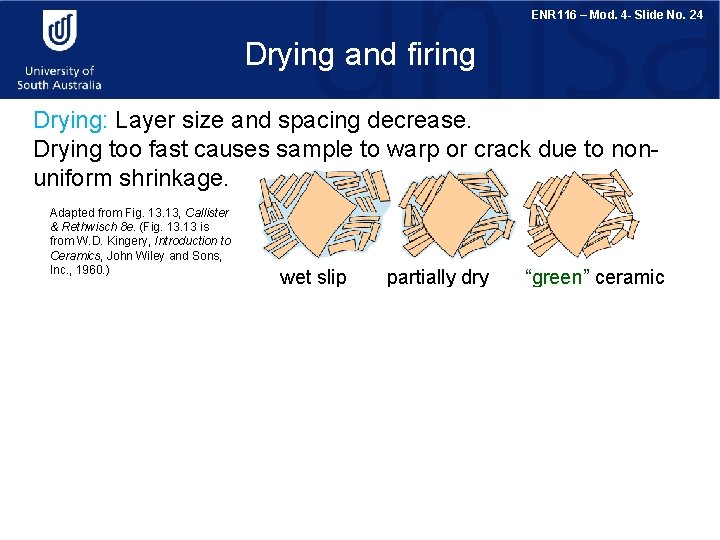
ENR 116 – Mod. 4 - Slide No. 24 Drying and firing Drying: Layer size and spacing decrease. Drying too fast causes sample to warp or crack due to nonuniform shrinkage. Adapted from Fig. 13, Callister & Rethwisch 8 e. (Fig. 13 is from W. D. Kingery, Introduction to Ceramics, John Wiley and Sons, Inc. , 1960. ) wet slip partially dry Firing: T raised to (9001400°C) vitrification (liquid glass forms from clay and flows between Si. O 2 particles). Flux melts at lower T. Adapted from Fig. 13. 14, Callister & Rethwisch 8 e. (Fig. 13. 14 is courtesy H. G. Brinkies, Swinburne University of Technology, Hawthorn Campus, Hawthorn, Victoria, Australia. ) “green” ceramic Si 02 particle (quartz) glass formed around the particle 70 mm micrograph of porcelain

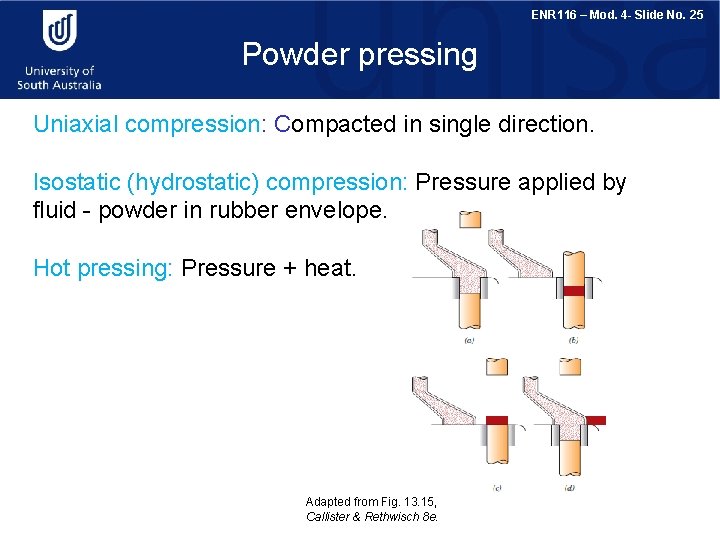
ENR 116 – Mod. 4 - Slide No. 25 Powder pressing Uniaxial compression: Compacted in single direction. Isostatic (hydrostatic) compression: Pressure applied by fluid - powder in rubber envelope. Hot pressing: Pressure + heat. Adapted from Fig. 13. 15, Callister & Rethwisch 8 e.

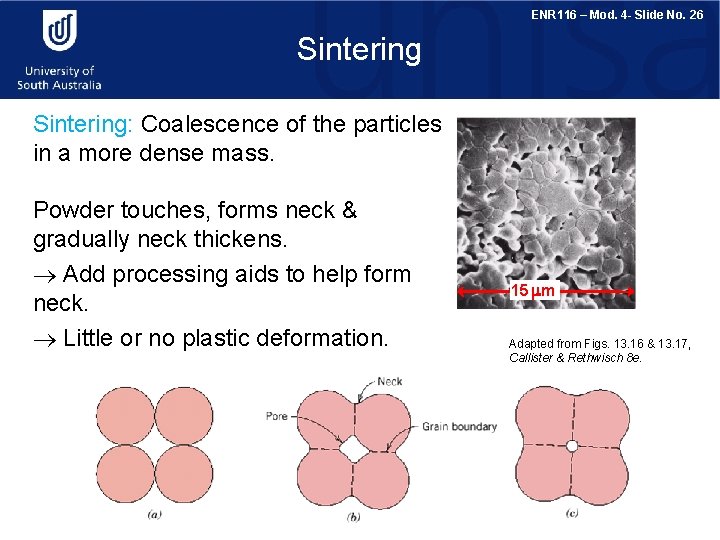
ENR 116 – Mod. 4 - Slide No. 26 Sintering: Coalescence of the particles in a more dense mass. Powder touches, forms neck & gradually neck thickens. Add processing aids to help form neck. Little or no plastic deformation. 15 m Adapted from Figs. 13. 16 & 13. 17, Callister & Rethwisch 8 e.

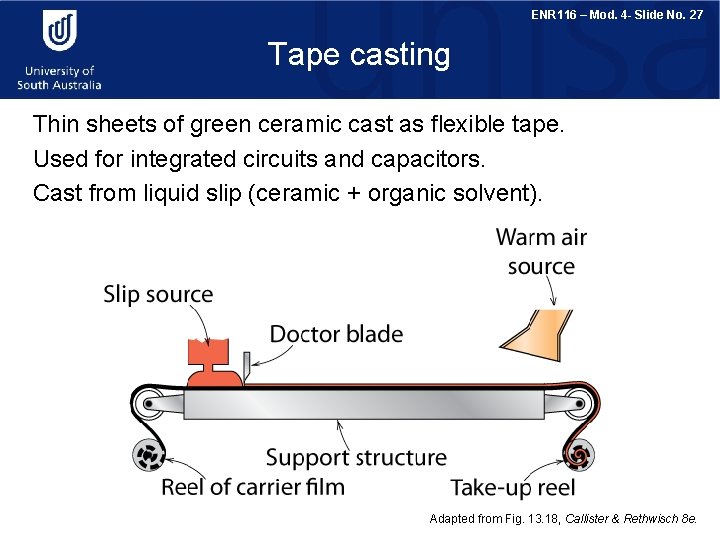
ENR 116 – Mod. 4 - Slide No. 27 Tape casting Thin sheets of green ceramic cast as flexible tape. Used for integrated circuits and capacitors. Cast from liquid slip (ceramic + organic solvent). Adapted from Fig. 13. 18, Callister & Rethwisch 8 e.

ENR 116 – Mod. 4 - Slide No. 28 Summary • Ceramics are classified by both structure and application. • Ceramics are processed as a glass (at high temperatures) and as powders under high pressures.

ENR 116 – Mod. 4 - Slide No. 29 Thank you
 No enr
No enr Fiche autocontrole qualit'enr
Fiche autocontrole qualit'enr Heel toe heel toe slide slide slide
Heel toe heel toe slide slide slide Slide divide slide factoring
Slide divide slide factoring 132 sonetas
132 sonetas Sonnet westminster bridge william wordsworth
Sonnet westminster bridge william wordsworth Quran 5 116
Quran 5 116 Cwe-89
Cwe-89 Salmo 116:15
Salmo 116:15 Psalm 23 niv
Psalm 23 niv Lucasmod
Lucasmod The diagram shows the circle with equation x^2+y^2=261
The diagram shows the circle with equation x^2+y^2=261 Keturiolikos eilučių eilėraštis
Keturiolikos eilučių eilėraštis Sonnet 116 stylistic devices
Sonnet 116 stylistic devices Psalms 116 3
Psalms 116 3 Cos 116
Cos 116 Sonnet 130 paraphrase
Sonnet 130 paraphrase Pg 116
Pg 116 Indas116 software
Indas116 software Salmos 116:1-9
Salmos 116:1-9 Villa hareskovby
Villa hareskovby 116-82
116-82 Sonnet 116 images
Sonnet 116 images Ra 9238
Ra 9238 Systolic mm hg
Systolic mm hg Afi 48-116
Afi 48-116 Vaaz dokumanlari
Vaaz dokumanlari Psalms 116:1-9
Psalms 116:1-9 116 qq
116 qq Sonnet 18 tpcastt
Sonnet 18 tpcastt