ENR 116 Mod 1 Slide No ENR 116




- Slides: 22

ENR 116 – Mod. 1 - Slide No. ENR 116 Engineering Materials Module 1 Introduction to Materials Dr Andrew Michelmore Unit Coordinator School of Advanced Manufacturing and Mechanical Engineering

ENR 116 – Mod. 1 - Slide No. 2 Copyright Notice Do not remove this notice. COMMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING This material has been produced and communicated to you by or on behalf of the University of South Australia pursuant to Part VB of the Copyright Act 1968 (the Act). The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice.

ENR 116 – Mod. 1 - Slide No. Crystallography

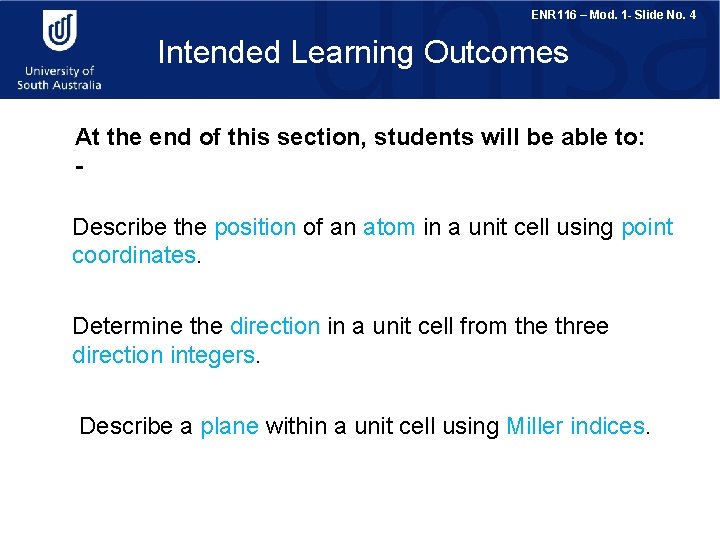
ENR 116 – Mod. 1 - Slide No. 4 Intended Learning Outcomes At the end of this section, students will be able to: Describe the position of an atom in a unit cell using point coordinates. Determine the direction in a unit cell from the three direction integers. Describe a plane within a unit cell using Miller indices.

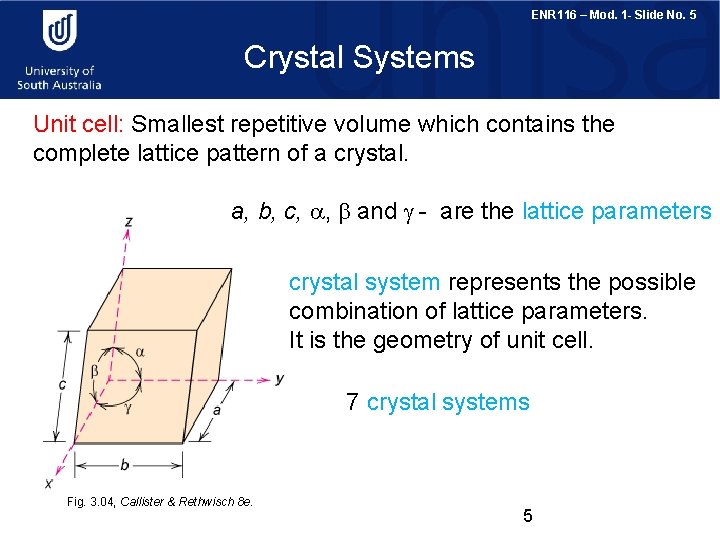
ENR 116 – Mod. 1 - Slide No. 5 Crystal Systems Unit cell: Smallest repetitive volume which contains the complete lattice pattern of a crystal. a, b, c, a, b and g - are the lattice parameters crystal system represents the possible combination of lattice parameters. It is the geometry of unit cell. 7 crystal systems Fig. 3. 04, Callister & Rethwisch 8 e. 5

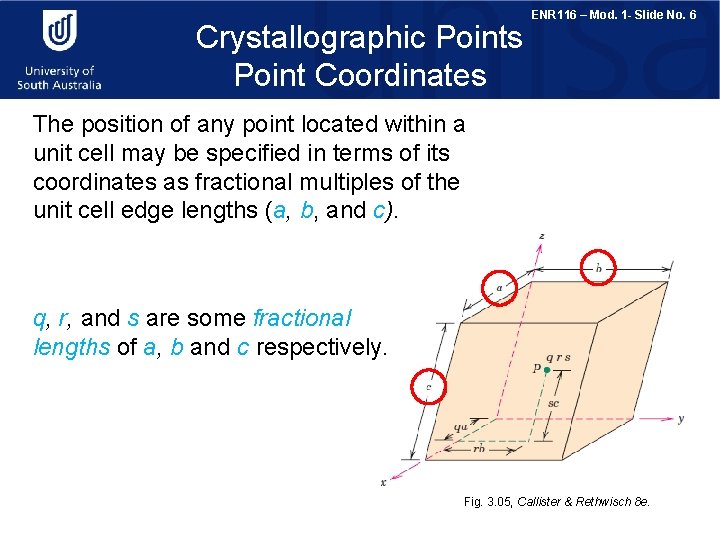
Crystallographic Points Point Coordinates ENR 116 – Mod. 1 - Slide No. 6 The position of any point located within a unit cell may be specified in terms of its coordinates as fractional multiples of the unit cell edge lengths (a, b, and c). q, r, and s are some fractional lengths of a, b and c respectively. Fig. 3. 05, Callister & Rethwisch 8 e.

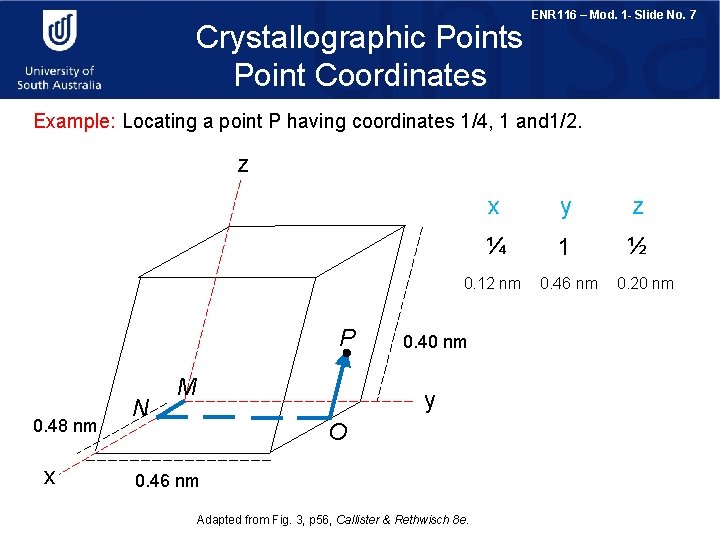
Crystallographic Points Point Coordinates ENR 116 – Mod. 1 - Slide No. 7 Example: Locating a point P having coordinates 1/4, 1 and 1/2. z x y z ¼ 1 ½ 0. 12 nm P 0. 48 nm x N M 0. 40 nm y O 0. 46 nm Adapted from Fig. 3, p 56, Callister & Rethwisch 8 e. 0. 46 nm 0. 20 nm

ENR 116 – Mod. 1 - Slide No. 8 Point coordinates for atoms in BCC unit cell Fig. 3 and table, p 57, Callister & Rethwisch 8 e.

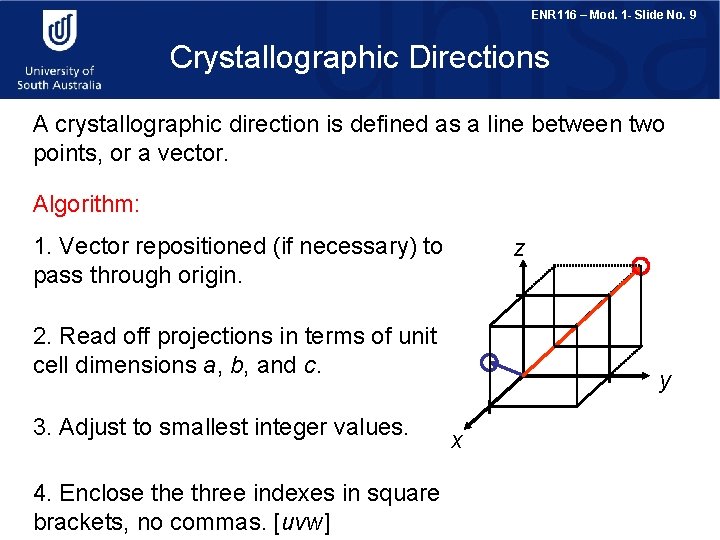
ENR 116 – Mod. 1 - Slide No. 9 Crystallographic Directions A crystallographic direction is defined as a line between two points, or a vector. Algorithm: 1. Vector repositioned (if necessary) to pass through origin. z 2. Read off projections in terms of unit cell dimensions a, b, and c. 3. Adjust to smallest integer values. 4. Enclose three indexes in square brackets, no commas. [uvw] y x

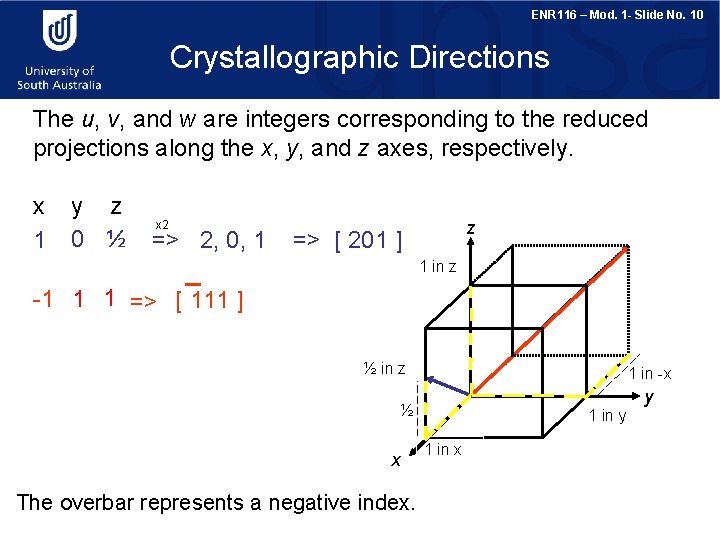
ENR 116 – Mod. 1 - Slide No. 10 Crystallographic Directions The u, v, and w are integers corresponding to the reduced projections along the x, y, and z axes, respectively. x 1 y z 0 ½ x 2 => 2, 0, 1 z => [ 201 ] 1 in z -1 1 1 => [ 111 ] ½ in z 1 in -x ½ x The overbar represents a negative index. 1 in y 1 in x y

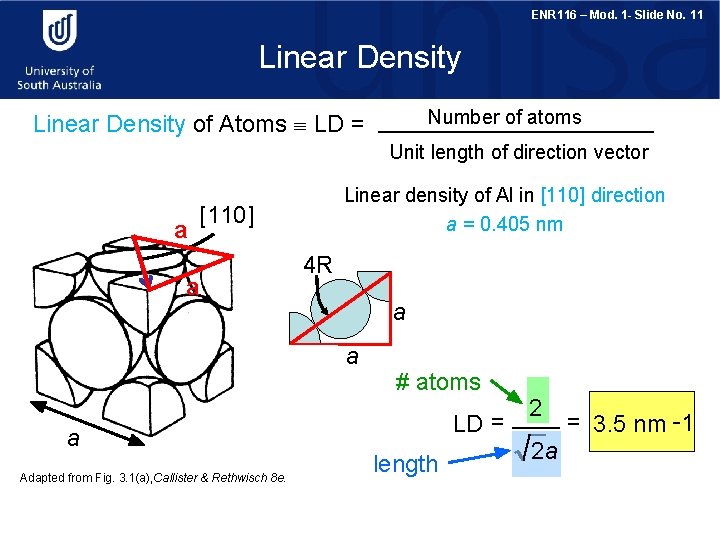
ENR 116 – Mod. 1 - Slide No. 11 Linear Density Number of atoms Linear Density of Atoms LD = Unit length of direction vector a Linear density of Al in [110] direction a = 0. 405 nm [110] a 4 R a a a Adapted from Fig. 3. 1(a), Callister & Rethwisch 8 e. # atoms LD = length 2 2 a = 3. 5 nm -1

ENR 116 – Mod. 1 - Slide No. 12 Crystallographic Planes Algorithm: 1. If the plane passes through the selected origin, either another parallel plane must be constructed within the unit cell by an appropriate translation, or a new origin must be established at the corner of another unit cell. 2. Read off intercepts of plane with axes in terms of a, b, c. 3. Take reciprocals of the intercepts. A plane that parallels an axis may be considered to have an infinite intercept, and, therefore, a zero index. 4. Reduce to smallest integer values by multiplication or division by a common factor. 5. Enclose in parentheses, no commas i. e. , (hkl)

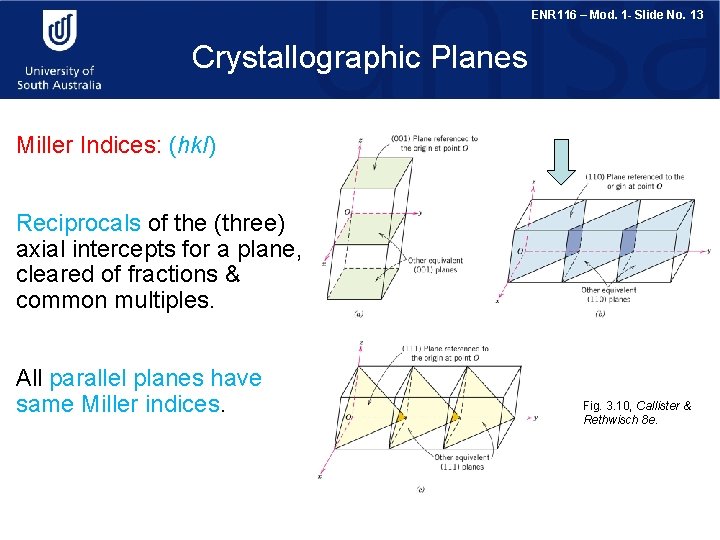
ENR 116 – Mod. 1 - Slide No. 13 Crystallographic Planes Miller Indices: (hkl) Reciprocals of the (three) axial intercepts for a plane, cleared of fractions & common multiples. All parallel planes have same Miller indices. Fig. 3. 10, Callister & Rethwisch 8 e.

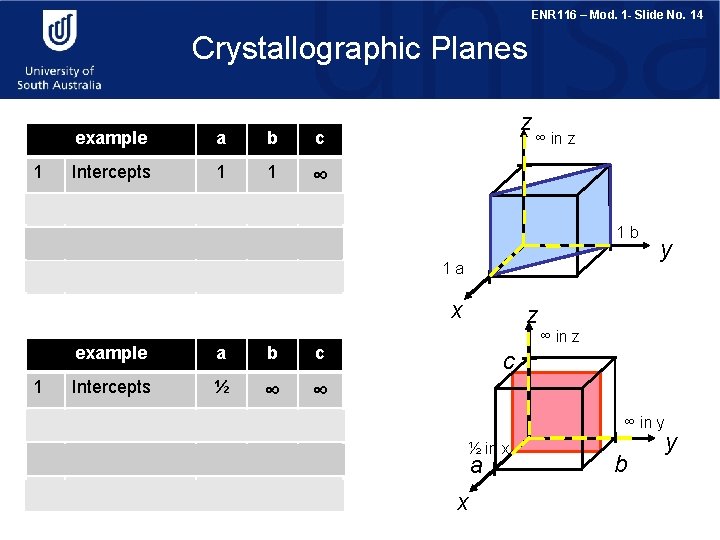
ENR 116 – Mod. 1 - Slide No. 14 Crystallographic Planes example a b c 1 Intercepts 1 1 ∞ 2 Reciprocals 1/1 1/∞ 3 Reduction 1 1 0 4 Miller Indices z 1 b 1 a (110) x example a b c 1 Intercepts ½ ∞ ∞ 2 Reciprocals 1/½ 1/∞ 3 Reduction 2 0 0 4 Miller Indices (200) ∞ in z z y ∞ in z c ∞ in y ½ in x a x b y

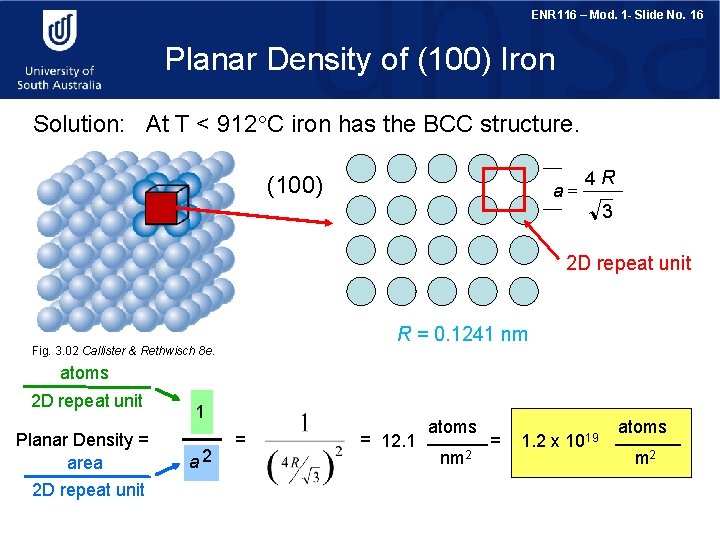
ENR 116 – Mod. 1 - Slide No. 15 Crystallographic Planes Iron foil can be used as a catalyst. The atomic packing of the exposed planes is important. We want to examine the atomic packing of crystallographic planes - the planar density (PD) number of atoms centred on a plane PD = area of plane Draw (100) crystallographic planes for Fe. Calculate the planar density for this plane

ENR 116 – Mod. 1 - Slide No. 16 Planar Density of (100) Iron Solution: At T < 912 C iron has the BCC structure. (100) a= 4 R 3 2 D repeat unit R = 0. 1241 nm Fig. 3. 02 Callister & Rethwisch 8 e. atoms 2 D repeat unit Planar Density = area 2 D repeat unit 1 a 2 = = 12. 1 atoms nm 2 = 1. 2 x 1019 atoms m 2

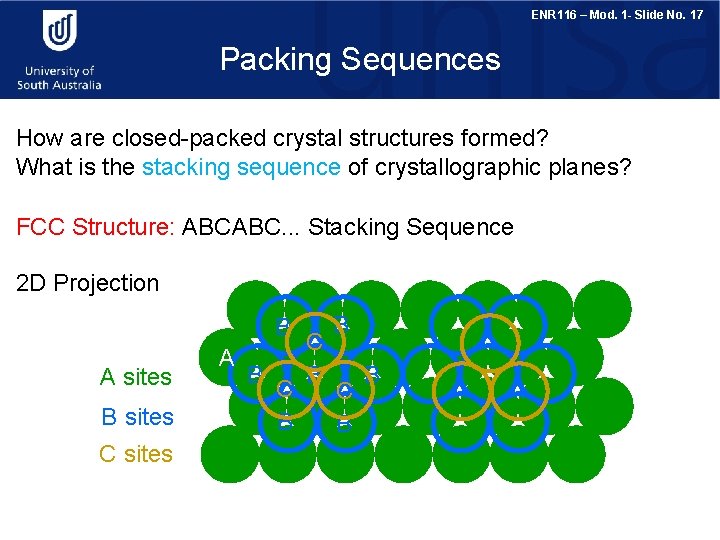
ENR 116 – Mod. 1 - Slide No. 17 Packing Sequences How are closed-packed crystal structures formed? What is the stacking sequence of crystallographic planes? FCC Structure: ABCABC. . . Stacking Sequence 2 D Projection B A sites B sites C sites A B C B B

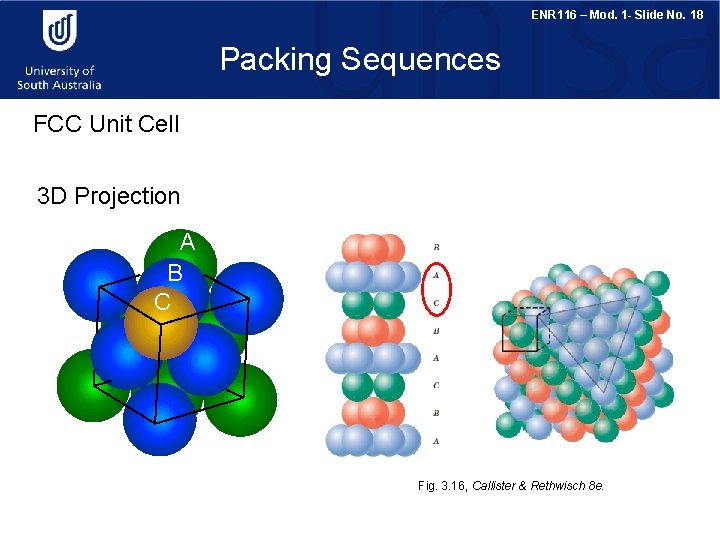
ENR 116 – Mod. 1 - Slide No. 18 Packing Sequences FCC Unit Cell 3 D Projection A B C Fig. 3. 16, Callister & Rethwisch 8 e.

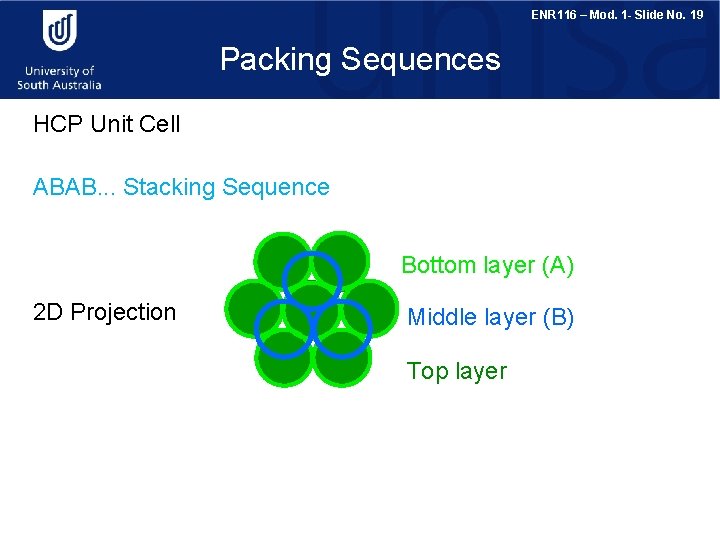
ENR 116 – Mod. 1 - Slide No. 19 Packing Sequences HCP Unit Cell ABAB. . . Stacking Sequence Bottom layer (A) 2 D Projection Middle layer (B) Top layer

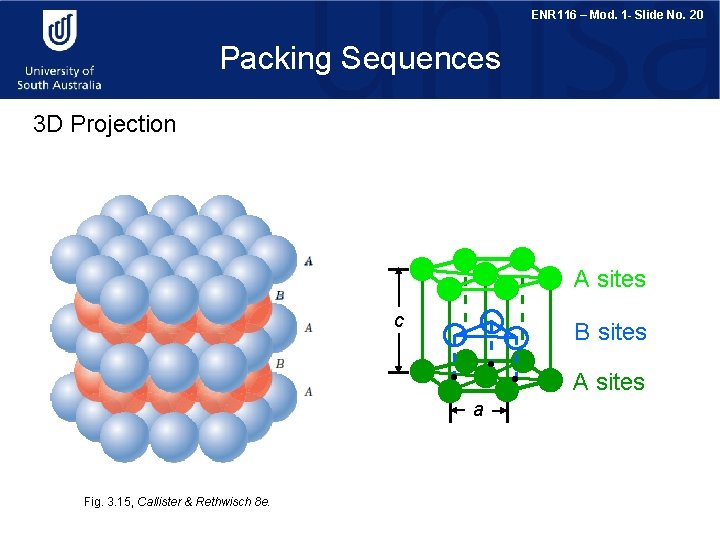
ENR 116 – Mod. 1 - Slide No. 20 Packing Sequences 3 D Projection A sites c B sites A sites a Fig. 3. 15, Callister & Rethwisch 8 e.

ENR 116 – Mod. 1 - Slide No. 21 Summary • A point within a unit cell is specified using coordinates. • Directions within a unit cell are described as a vector – a line between two points. • Crystallographic planes are specified by Miller indices.

ENR 116 – Mod. 1 - Slide No. 22 Thank you
 Fiche autocontrole qualit'enr
Fiche autocontrole qualit'enr No enr
No enr What is heel and toe polka
What is heel and toe polka Factoring checklist
Factoring checklist 116 qq
116 qq Sonnet 130 tpcastt
Sonnet 130 tpcastt Sonnet 116 theme
Sonnet 116 theme What is a sonnet? *
What is a sonnet? * Why does coelho open with the modified myth of narcissus?
Why does coelho open with the modified myth of narcissus? Wordsworth poem westminster bridge
Wordsworth poem westminster bridge Quran 5 116
Quran 5 116 116 sonetas
116 sonetas Cwe-116
Cwe-116 Salmo 116:15
Salmo 116:15 Lucasmod
Lucasmod Psalm 19 niv
Psalm 19 niv Sami asked 50 people which drinks they liked
Sami asked 50 people which drinks they liked 13 sonetas
13 sonetas Octet stanza
Octet stanza Psalms 116 3
Psalms 116 3 Cos 116
Cos 116 William shakespeare sonnet 30
William shakespeare sonnet 30 Vedic caste system
Vedic caste system