Endothermic vs Exothermic Reactions Mrs Chan Energy Energy




- Slides: 12

Endothermic vs Exothermic Reactions Mrs. Chan

Energy • Energy is the capacity to do some kind of work, such as moving an object, forming a new compound, or generating light. • Energy is always involved when there is a change in matter. 2

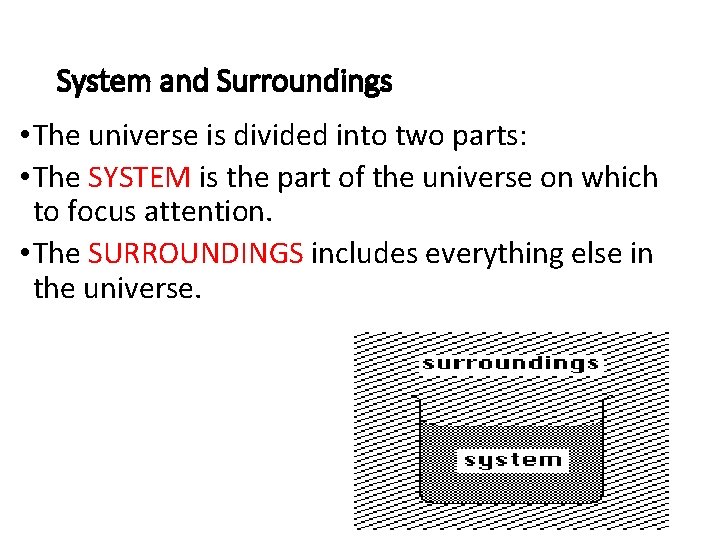
System and Surroundings • The universe is divided into two parts: • The SYSTEM is the part of the universe on which to focus attention. • The SURROUNDINGS includes everything else in the universe. 3

Every Change in Matter Involves a Change in Energy • All physical and chemical changes involve a change in energy. • Some involve releasing energy, some involve absorbing energy. 4

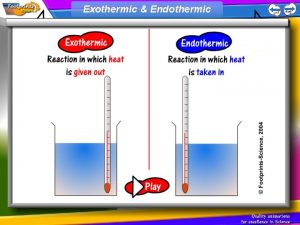
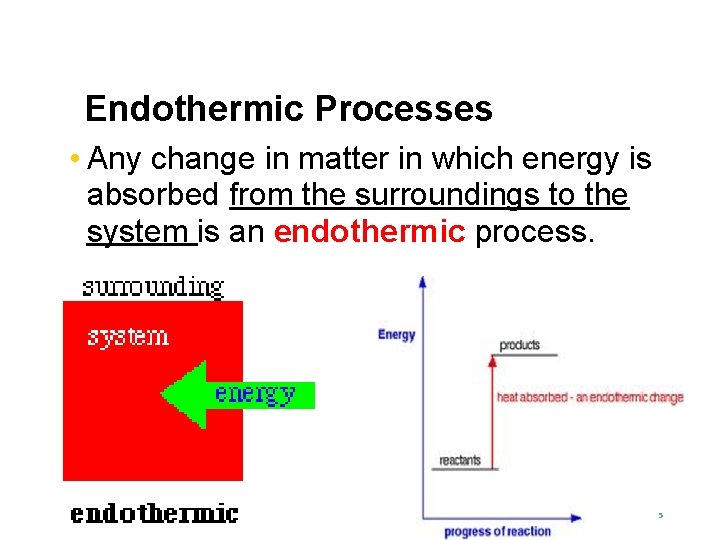
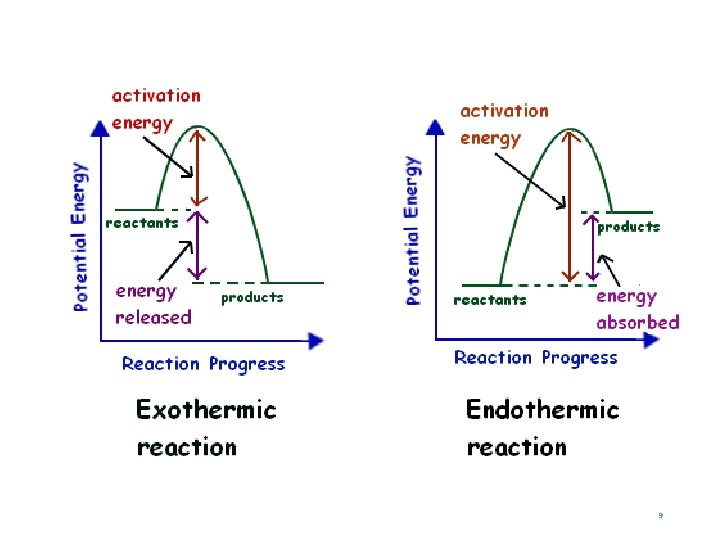
Endothermic Processes • Any change in matter in which energy is absorbed from the surroundings to the system is an endothermic process. 5

Endothermic Process Examples Physical Changes The melting of ice Chemical Changes The reaction between barium hydroxide and ammonium nitrate The boiling of water 6

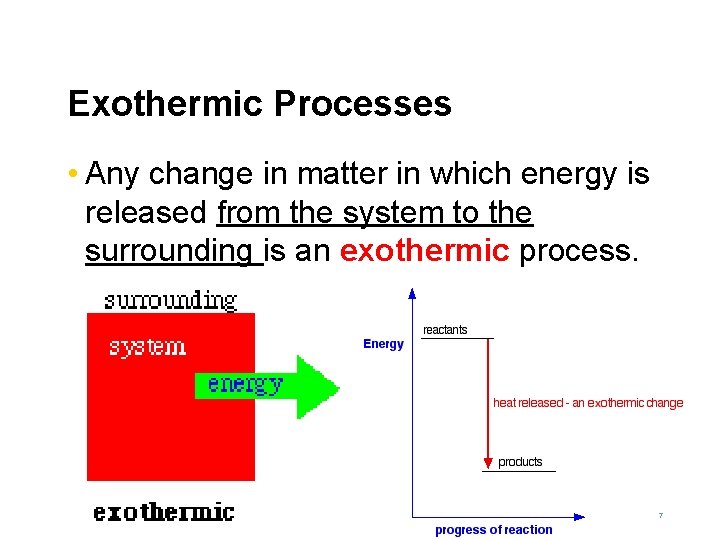
Exothermic Processes • Any change in matter in which energy is released from the system to the surrounding is an exothermic process. 7

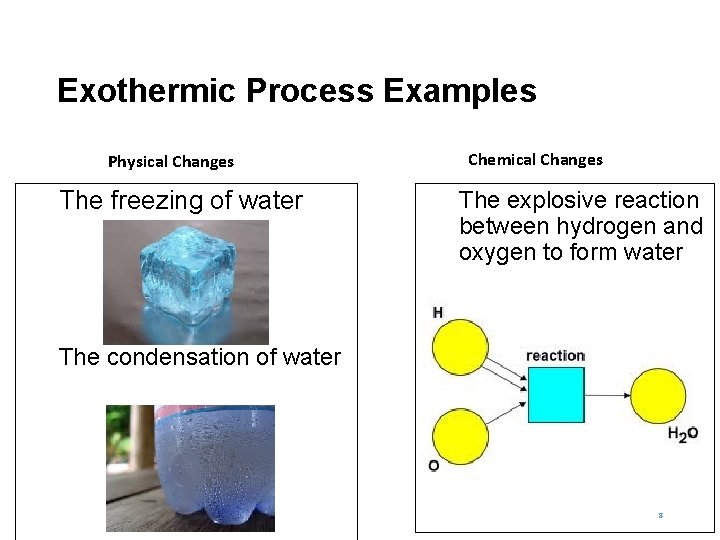
Exothermic Process Examples Physical Changes The freezing of water Chemical Changes The explosive reaction between hydrogen and oxygen to form water The condensation of water 8

9

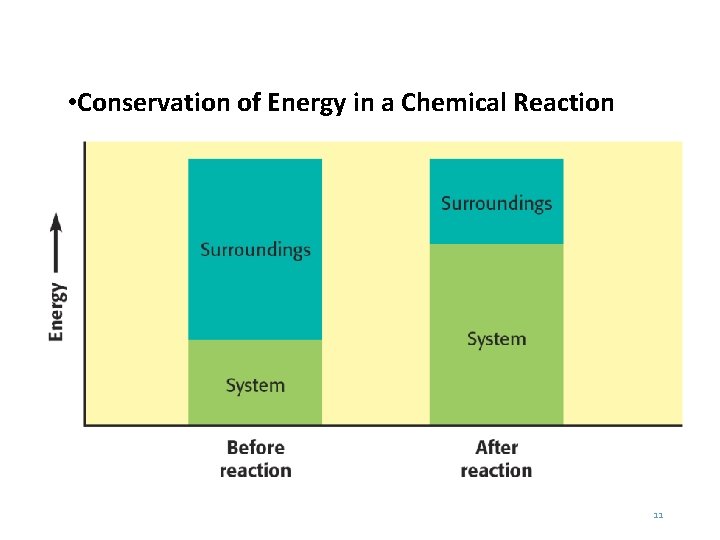
The Law of Conservation of Energy • Energy can be absorbed by the surroundings or released to the surroundings, but it cannot be created or destroyed. • The law of conservation of energy states that during any physical or chemical change, the total quantity of energy remains constant. 10

• Conservation of Energy in a Chemical Reaction 11

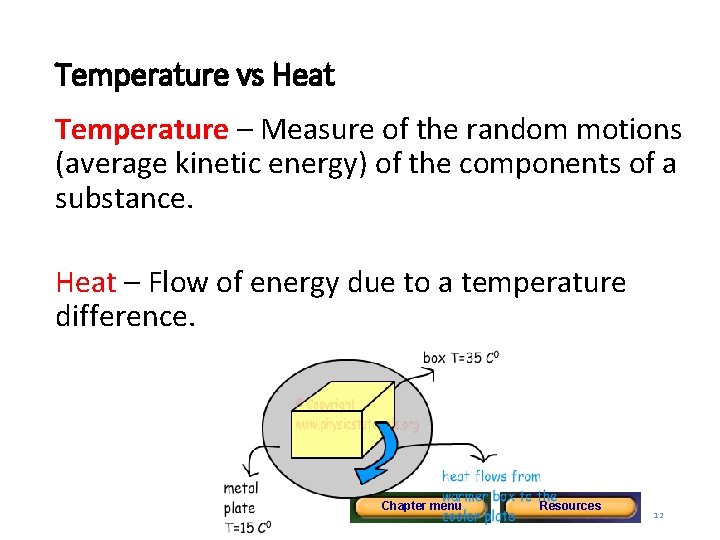
Temperature vs Heat Temperature – Measure of the random motions (average kinetic energy) of the components of a substance. Heat – Flow of energy due to a temperature difference. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. 12
 Endothermic and exothermic reaction experiment grade 11
Endothermic and exothermic reaction experiment grade 11 What is exothermic reaction
What is exothermic reaction Is vaporization endothermic or exothermic
Is vaporization endothermic or exothermic Endothermic vs exothermic
Endothermic vs exothermic Methane oxygen endothermic or exothermic
Methane oxygen endothermic or exothermic Exothermic v endothermic
Exothermic v endothermic Fusion chemistry phase change
Fusion chemistry phase change Endothermic reaction examples
Endothermic reaction examples Examples of ectotherms
Examples of ectotherms Endothermic and exothermic worksheet
Endothermic and exothermic worksheet Exothermic and endothermic homework
Exothermic and endothermic homework Cellular respiration endothermic or exothermic
Cellular respiration endothermic or exothermic Ins
Ins