Element Quiz 2 1 Which element has an

























- Slides: 27

Element Quiz - 2 1. Which element has an atomic number of 14? How many neutrons this element has? 2. Write down the mass number, number of protons and number of neutrons for Calcium? 3. Which element has 16 protns, 16 electrons and 16 neutrons? 4. Write down the symbol for the element Sodium. Mark its atomic number and mass number with its symbol? 5. Which element has the symbol 'Mg '?

Write the Orbital Diagram for the following • Mg • Fe

Determine which of the following are diamagnetic or paramagnetic • 1. Ne • 2. Mg • 3. Mn • 4. Zn • 5. S • 6. Ar

Write the Noble Gases (Short) Electron Configuration • Sr • Ag

Periodic Trends • A periodic trend is a pattern that predicts general properties of elements

Atomic Size • Atomic size is measured using atomic radii • Atomic radius is half the distance between two identical nuclei • Example: Two bromine atoms are 228 picometers apart. The atomic radius is 114 picometers

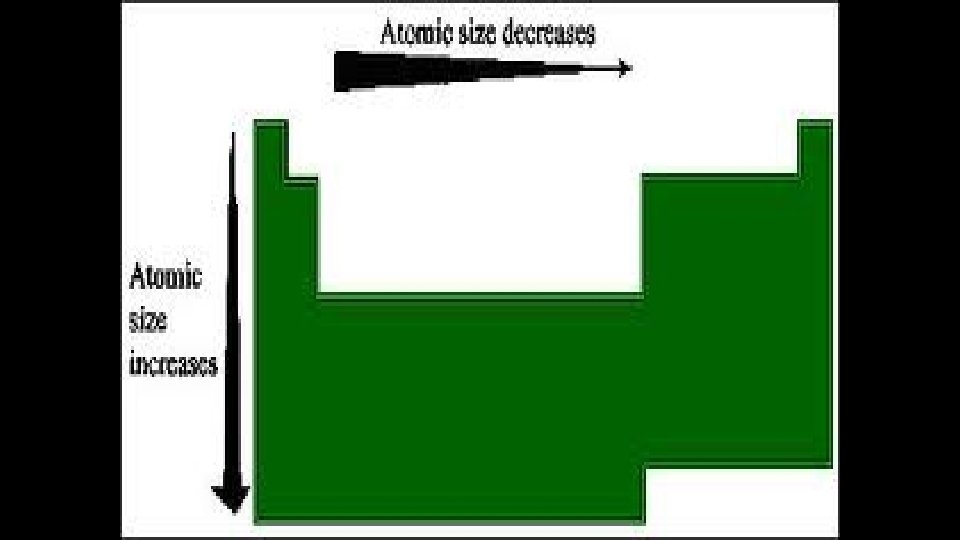
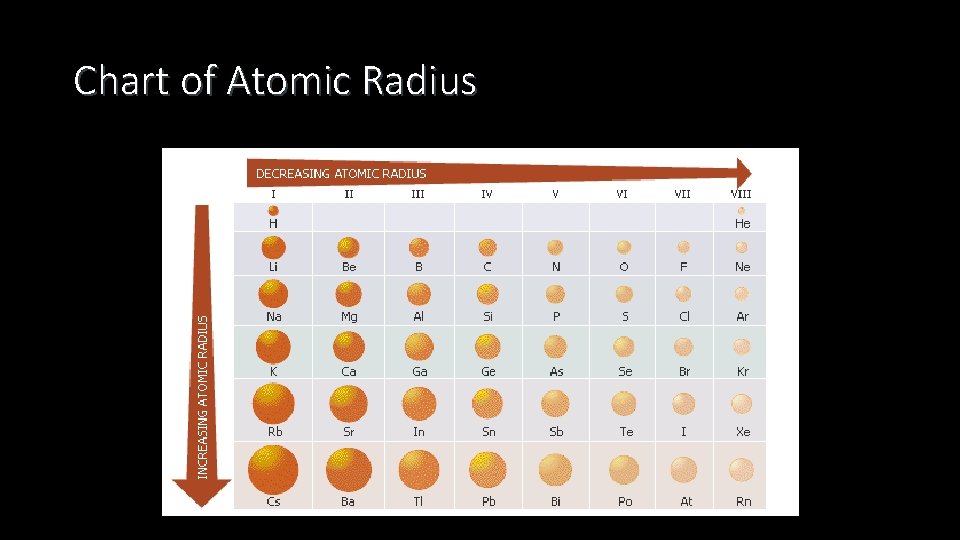
Atomic Radius Explanation • Atomic radius increases as you move down the periodic table • It becomes larger as you move down new periods, because you are adding additional energy levels

Atomic Radius • Atomic radius decreases as you move across the periodic table • The number of protons increase which creates a greater attraction to all electrons • This attraction pulls the electrons closer to nucleus thereby decreasing size


Chart of Atomic Radius


Practice • Arrange the following in terms of increasing atomic size: • a. Ca, V, Co, Br • b. Li, Na, K, Rb • c. O, Po, S, Te • d. Hg, Rn, Cs, Hf

Practice • Arrange according to increasing atomic size: • a. K, Na, P, Cs • b. Cl, At, Br, F • c. Fr, Na, Rb, P

Ionization Energy • Ionization Energy is the energy needed to remove an electron • 1 st Ionization Energy: energy required to remove the outermost electron

Ionization Energy • Ionization Energy depends on: • a. Distance an electron is from nucleus • The further the electron is away, the easier it is to remove

Ionization Energy Continued • b. Nuclear charge: refers to the attraction between the nucleus and electrons • A. K. A the Shielding Effect • The further away the electrons are, the less the attraction is between the nucleus and electrons

Trends with Ionization Energy • The first Ionization energy decreases as you move down a group • The first Ionization energy increases as you move across the periodic table

Ionization Energy Problems • Arrange the following in terms of increasing ionization energy: • A. Li, K, Cs, Na • B. Ca, Zn, Ge, Br • C. In, B, Al, Ga • D. Mg, P, Na, Cl

Practice • Place the following in increasing Ionization Energy • Rb, Mo, Br, Fr • Cs, O, C, Be, F • Au, Hf, Ba, Po, At

Electronegativity • Electronegativity is the tendency of elements to attract electrons • Nonmetals attract electrons more than metals

Electronegativity • Electronegativity is really the strength of an element to “steal and keep” electrons

Electronegativity • Electronegativity is measured on a scale from 0 to 4 • The most electronegative element is given a value of 4. 0

Electronegativity Continued • Fluorine is the most electronegative and given a value of 4. 0 • O = 3. 5 Cl = 3. 0 • S = 2. 5 H = 2. 1 • Li = 1. 0 Na = 0. 9

Effects of Electronegativity • The more electronegative an element is, the more nonmetallic it is

Electronegativity • The strength of other elements to steal electrons is compared to the most electronegative element

Trends in Electronegativity • Electronegativity increases as you move across the table • Electronegativity decreases as you move down the table • Electronegativity can be used to predict which type of bond that atoms will form

Problems • Arrange the following in terms of increasing electronegativity: • a. K, As, Cr, Zn • b. Sr, Y, Ag, I • c. F, O, N, Li • d. Ga, Ca, Fe, K • e. F, At, Cl, Br