Element Quiz 1 Quietly study for your quiz









- Slides: 24

Element Quiz #1 Quietly study for your quiz – spelling counts! 2. Make sure you write “Element Quiz #1” at the top of the paper, portrait layout, and your name & bell. 3. Number 1 – 11. 1.

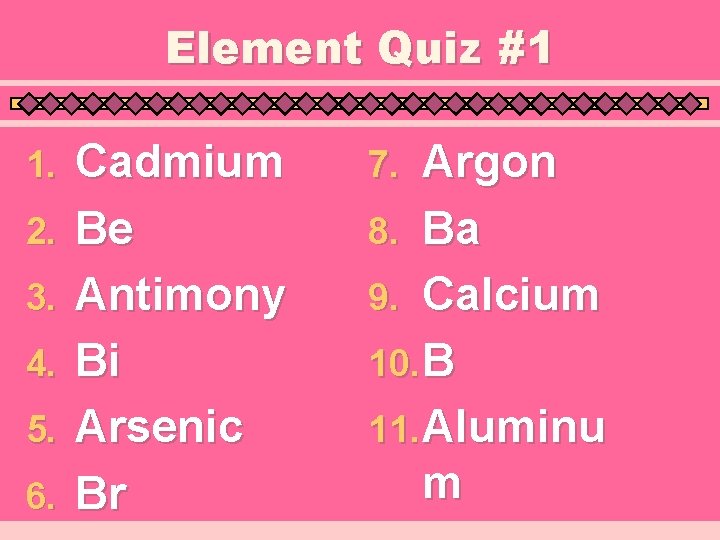
Element Quiz #1 1. 2. 3. 4. 5. 6. Cadmium Be Antimony Bi Arsenic Br Argon 8. Ba 9. Calcium 10. B 11. Aluminu m 7.

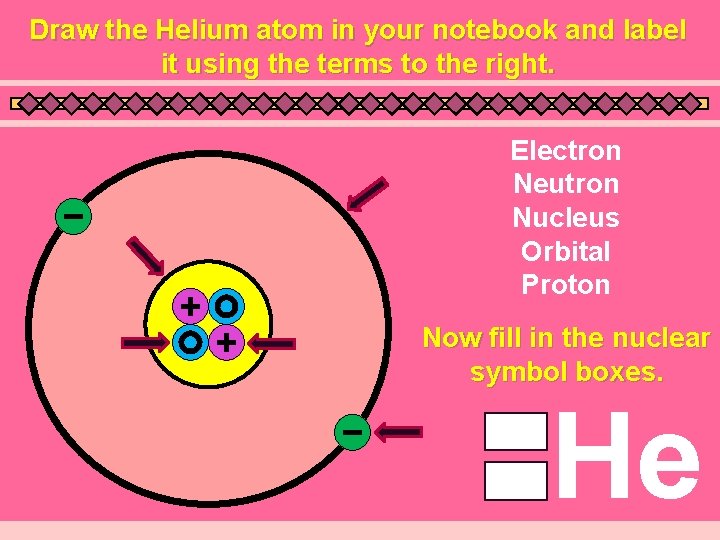
Draw the Helium atom in your notebook and label it using the terms to the right. Electron Neutron Nucleus Orbital Proton Now fill in the nuclear symbol boxes. He

Atomic Theories Follow along in your text Chapter 3 Section 1 Pages 74 - 78

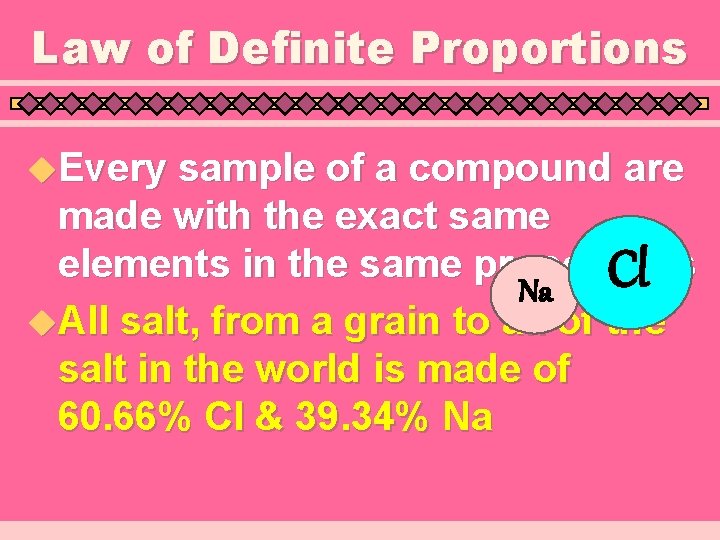
Law of Definite Proportions u. Every sample of a compound are made with the exact same elements in the same proportions Cl Na u. All salt, from a grain to all of the salt in the world is made of 60. 66% Cl & 39. 34% Na

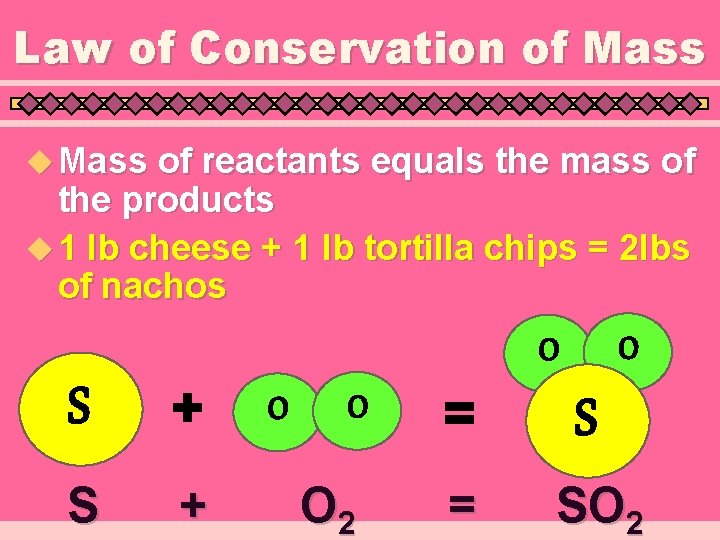
Law of Conservation of Mass u Mass of reactants equals the mass of the products u 1 lb cheese + 1 lb tortilla chips = 2 lbs of nachos S + O O O 2 = = S SO 2

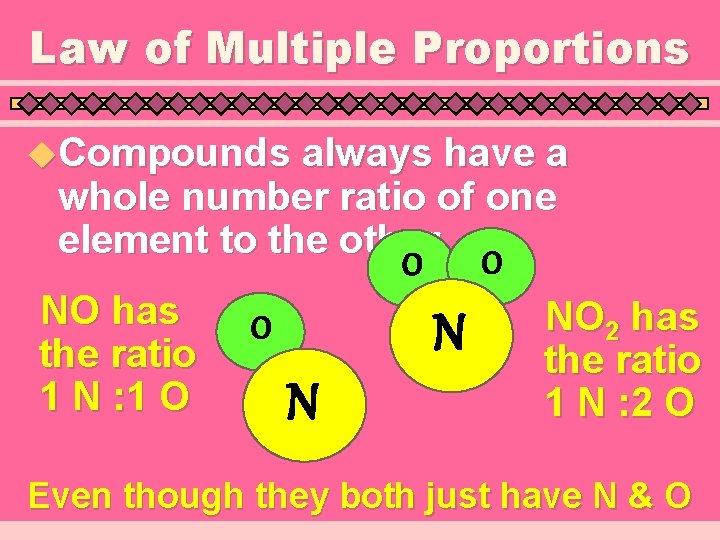
Law of Multiple Proportions u. Compounds always have a whole number ratio of one element to the other O O NO has the ratio 1 N : 1 O N NO 2 has the ratio 1 N : 2 O Even though they both just have N & O

Dalton’s Atomic Theory All matter is made of atoms, which can not be divided, created, or destroyed 2. Atoms of the same element are identical in all properties 3. Atoms of different elements differ in all properties 1.

Dalton’s Atomic Theory Atoms of different compounds combine in whole number ratios 5. Chemical Rxns combine, separate, and rearrange atoms but those atoms are never destroyed, created, or changed 4.

Warm-Up Pick the colored sticky note you want and put it on the board under the number you like best. When everyone has posted their note… 1. Find the percent of each number chosen. 2. Multiply the percent of each times each number. 3. Add these two answers together and then divide by 100 Does your final answer reflect an average of these two numbers? Justify.

Atomic Structure Follow along in your text Chapter 3 Section 2 Pages 84 - 89

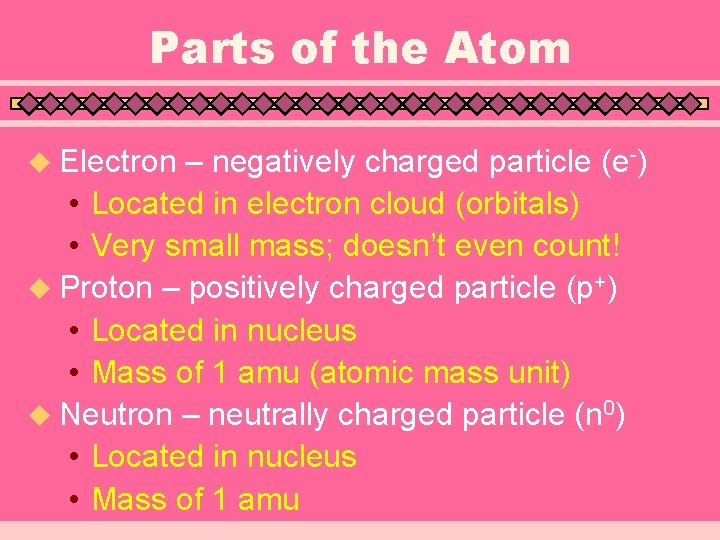
Parts of the Atom u Electron – negatively charged particle (e-) • Located in electron cloud (orbitals) • Very small mass; doesn’t even count! u Proton – positively charged particle (p+) • Located in nucleus • Mass of 1 amu (atomic mass unit) u Neutron – neutrally charged particle (n 0) • Located in nucleus • Mass of 1 amu

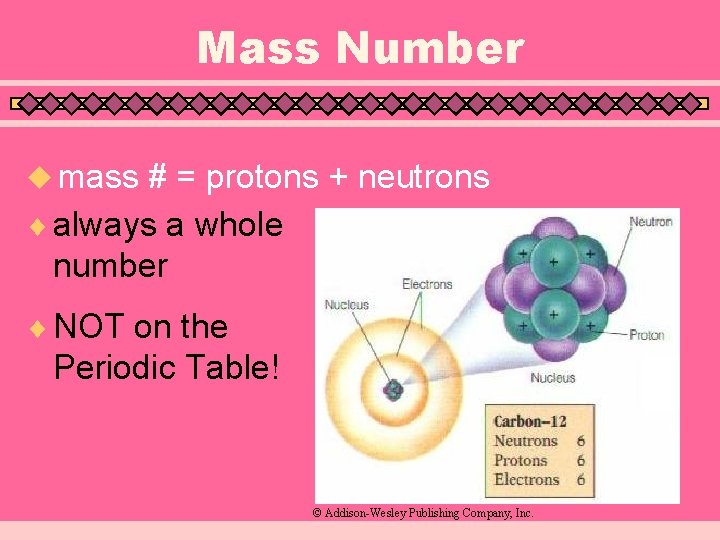
Mass Number u mass # = protons + neutrons ¨ always a whole number ¨ NOT on the Periodic Table! © Addison-Wesley Publishing Company, Inc.

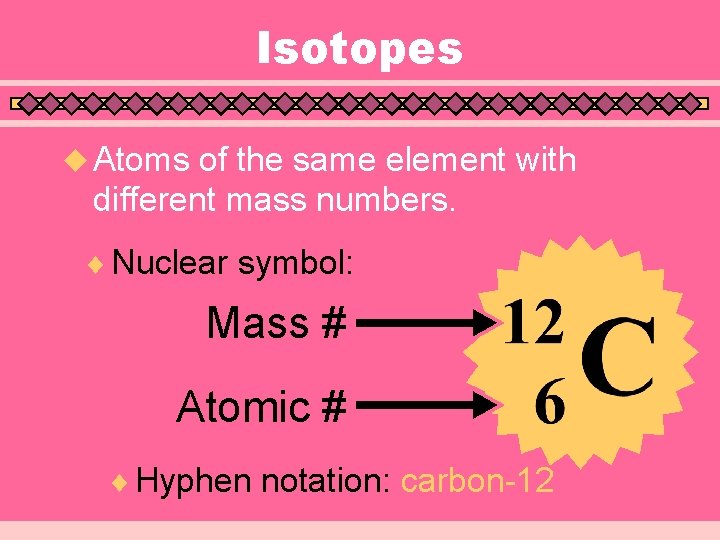
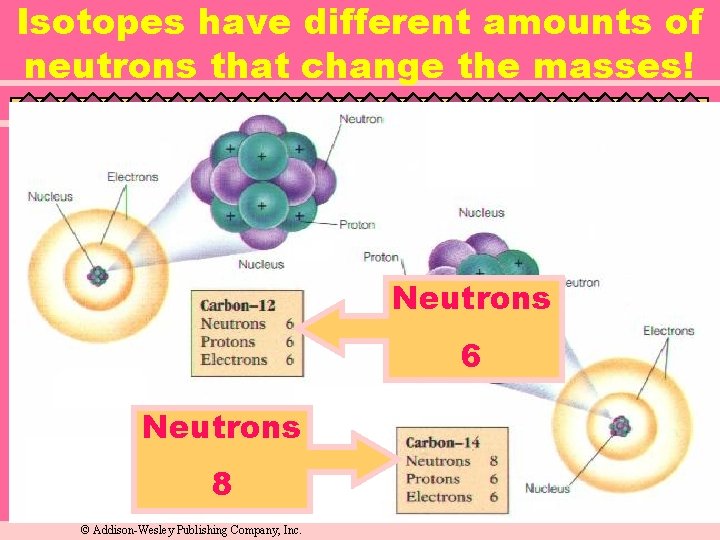
Isotopes u Atoms of the same element with different mass numbers. ¨ Nuclear symbol: Mass # Atomic # ¨ Hyphen notation: carbon-12

Isotopes have different amounts of neutrons that change the masses! Neutrons 6 Neutrons 8 © Addison-Wesley Publishing Company, Inc.

Isotopes u Chlorine-37 • atomic #: 17 • mass #: 37 • # of protons: 17 • # of electrons: 17 • # of neutrons: 20

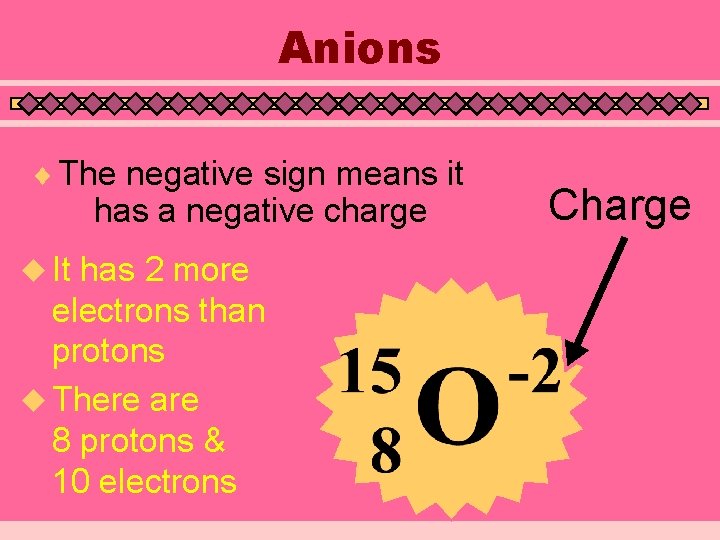
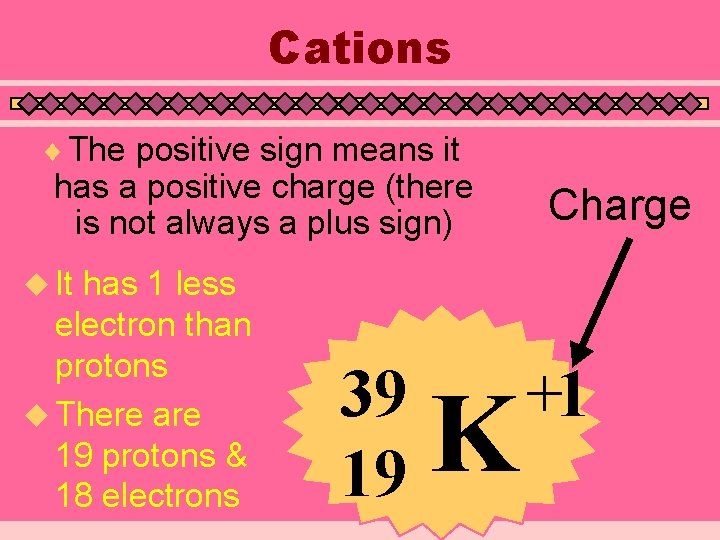
Ions (Charged Atoms) u Atoms of the same element with different amounts of electrons u Negative Ions (Anions) have more electrons than protons • Add the charge to the number of protons to get the number of electrons u Positive Ions (Cations) have less electrons than protons • Subtract the charge from the number of protons to get the number of electrons

Anions ¨ The negative sign means it has a negative charge u It has 2 more electrons than protons u There are 8 protons & 10 electrons Charge

Cations ¨ The positive sign means it has a positive charge (there is not always a plus sign) has 1 less electron than protons u There are 19 protons & 18 electrons Charge u It 39 19 K +1

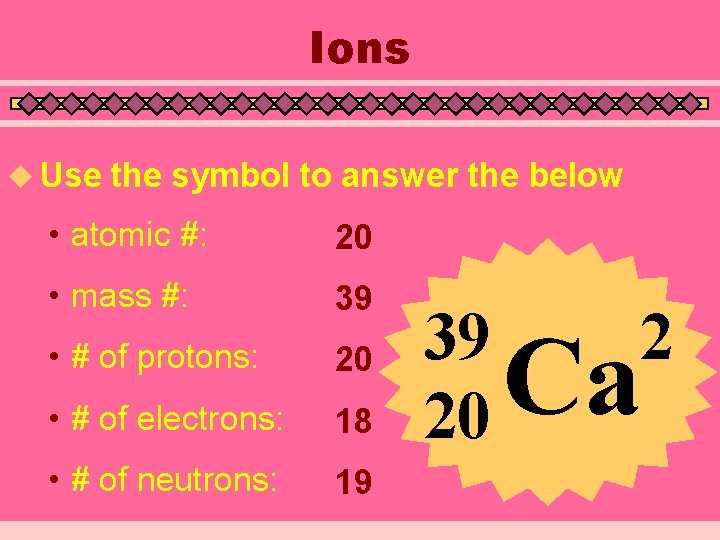
Ions u Use the symbol to answer the below • atomic #: 20 • mass #: 39 • # of protons: 20 • # of electrons: 18 • # of neutrons: 19 39 20 2 Ca

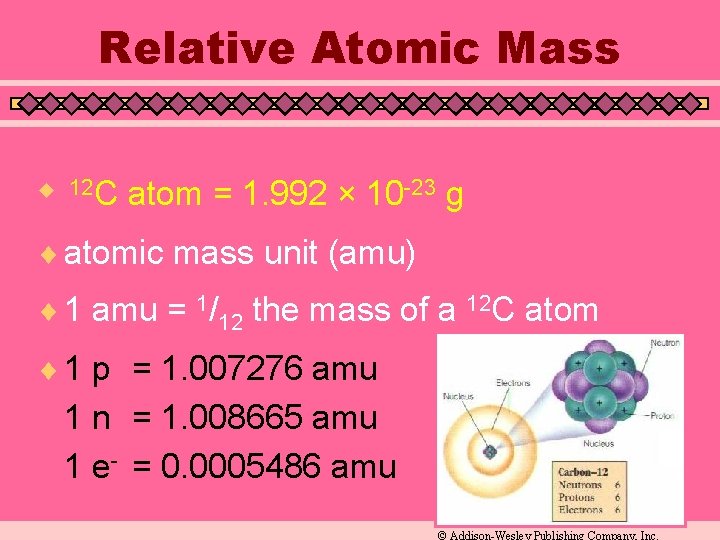
Relative Atomic Mass u 12 C atom = 1. 992 × 10 -23 g ¨ atomic mass unit (amu) ¨ 1 amu = 1/12 the mass of a 12 C atom ¨ 1 p = 1. 007276 amu 1 n = 1. 008665 amu 1 e- = 0. 0005486 amu © Addison-Wesley Publishing Company, Inc.

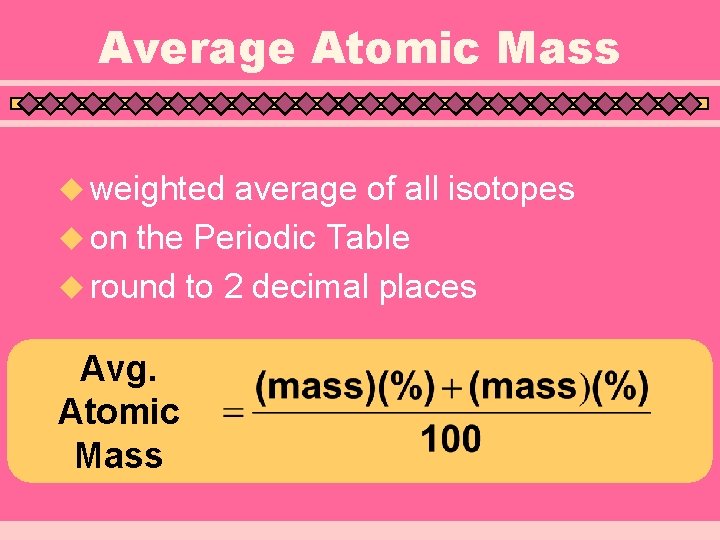
Average Atomic Mass u weighted average of all isotopes u on the Periodic Table u round to 2 decimal places Avg. Atomic Mass

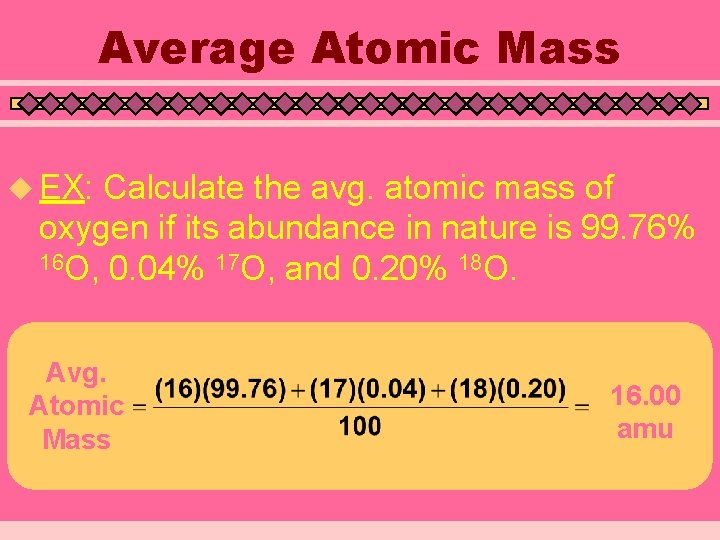
Average Atomic Mass u EX: Calculate the avg. atomic mass of oxygen if its abundance in nature is 99. 76% 16 O, 0. 04% 17 O, and 0. 20% 18 O. Avg. Atomic Mass 16. 00 amu

Average Atomic Mass u EX: Find chlorine’s average atomic mass if approximately 8 of every 10 atoms are chlorine-35 and 2 are chlorine-37. Avg. Atomic Mass 35. 40 amu