Quiz 1 Each quiz sheet has a different











- Slides: 45

Quiz 1 • Each quiz sheet has a different 5 -digit symmetric number which must be filled in (as shown on the transparency, but NOT the same one!!!!!) • Please hand in both the exam and the answer sheets with your name on both • Question/answer sheets will be handed back on Wednesday after class • Please remain seated until we begin collecting (20 -25 minutes after start) • Class after quiz


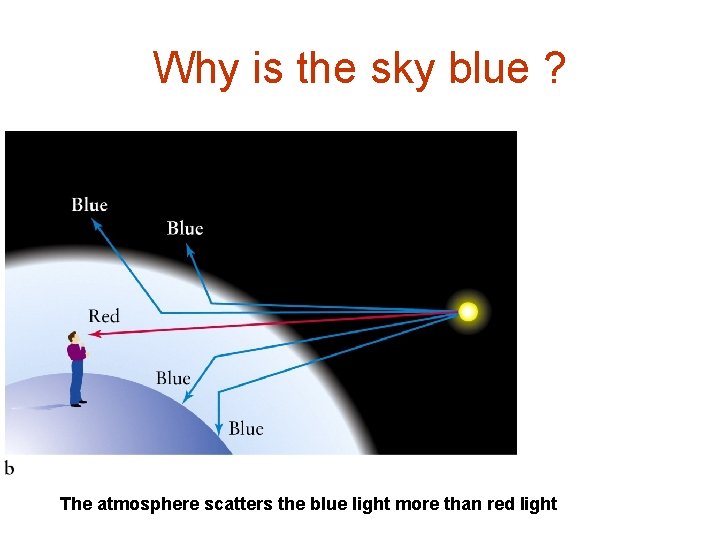
Why is the sky blue ? The atmosphere scatters the blue light more than red light

Light and Matter • Light is electromagnetic energy, due to interaction of electrical charges • Matter is made of atoms – equal number of positive and negative particles • An atom is the smallest particle of an element; natural element H to U • Atom Nucleus (protons + neutrons), with ‘orbiting’ electrons • No. of protons in nucleus = Atomic Number • Science of light Spectroscopy

Radiation and Spectroscopy • • Light is electromagnetic energy Propagates as both particles and waves Photons – particles of light Wavelength = Velocity / Frequency

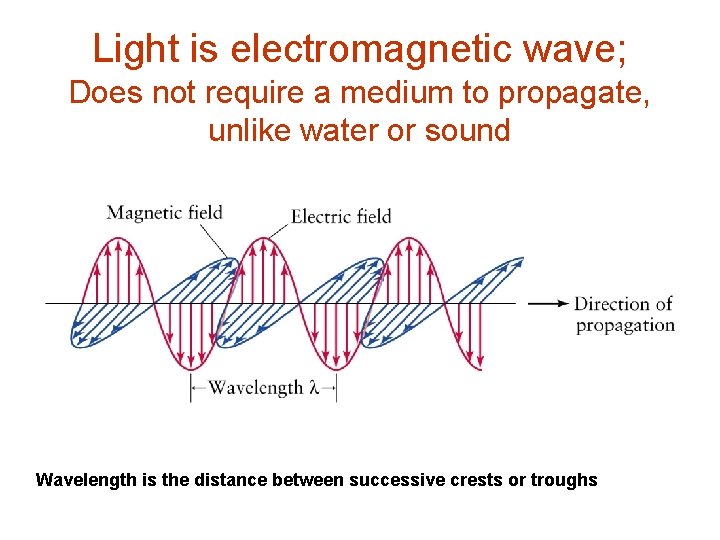
Light is electromagnetic wave; Does not require a medium to propagate, unlike water or sound Wavelength is the distance between successive crests or troughs

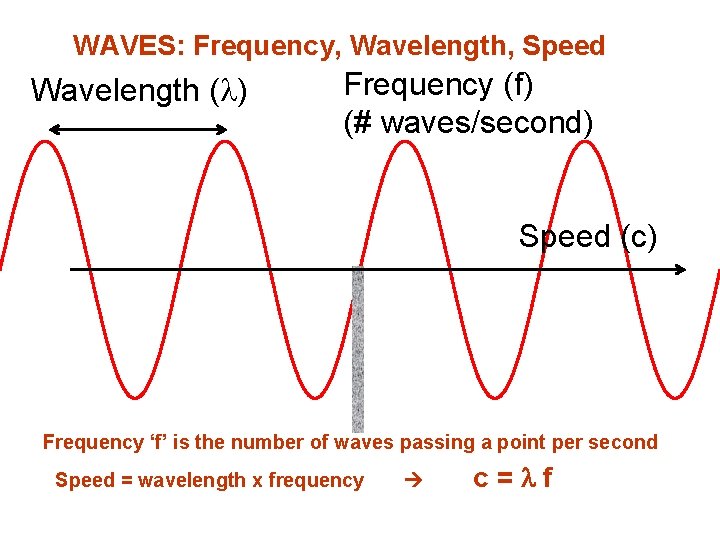
WAVES: Frequency, Wavelength, Speed Wavelength ( ) Frequency (f) (# waves/second) Speed (c) Frequency ‘f’ is the number of waves passing a point per second Speed = wavelength x frequency c=lf

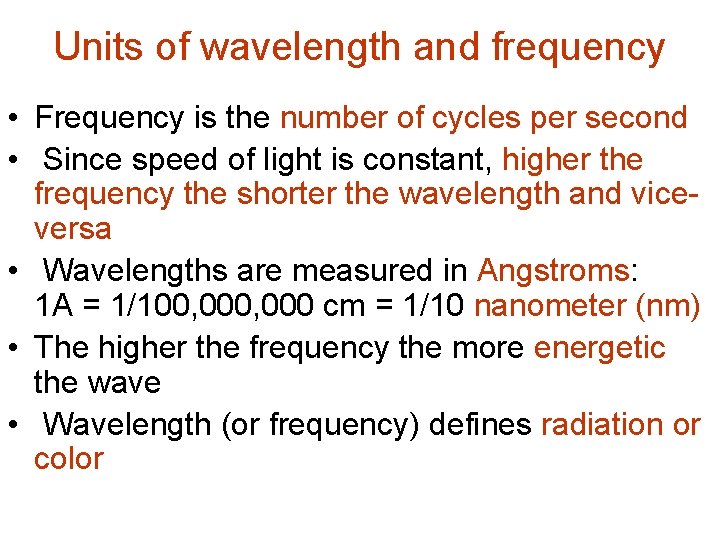
Units of wavelength and frequency • Frequency is the number of cycles per second • Since speed of light is constant, higher the frequency the shorter the wavelength and viceversa • Wavelengths are measured in Angstroms: 1 A = 1/100, 000 cm = 1/10 nanometer (nm) • The higher the frequency the more energetic the wave • Wavelength (or frequency) defines radiation or color

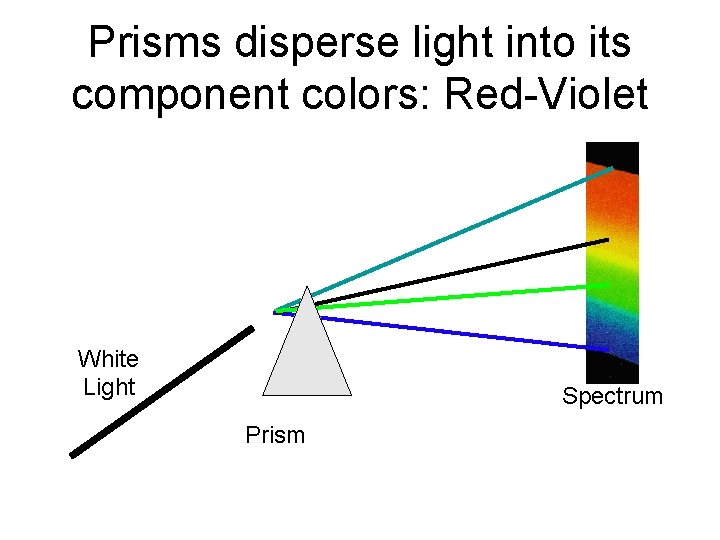
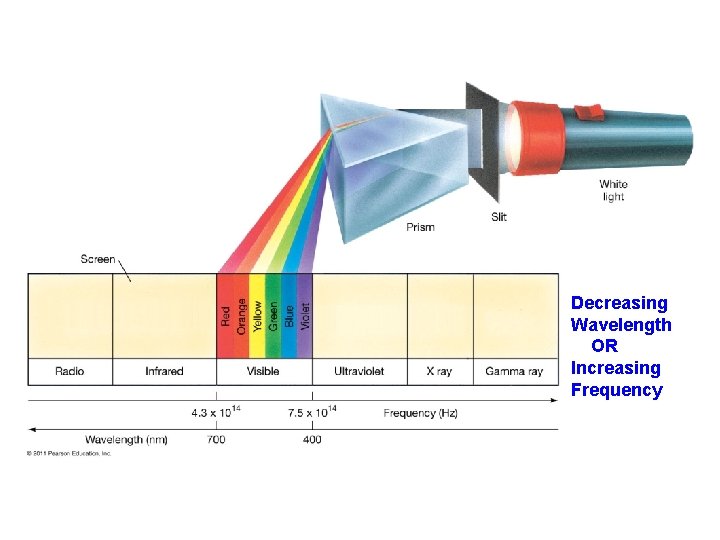
Prisms disperse light into its component colors: Red-Violet White Light Spectrum Prism

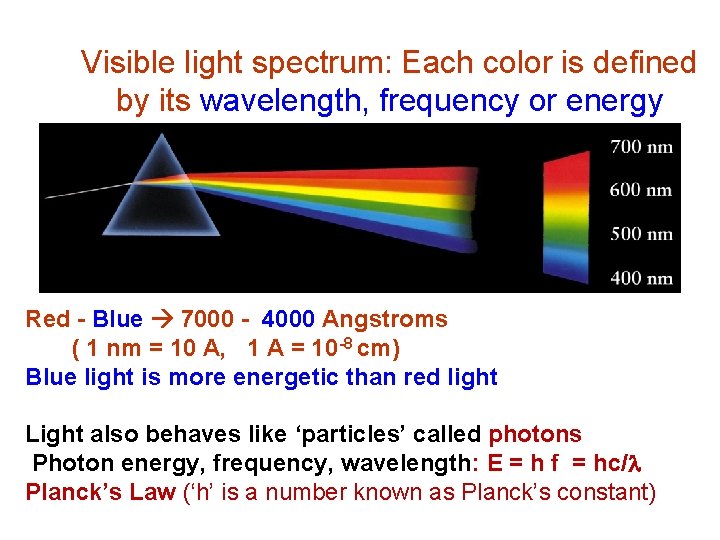
Visible light spectrum: Each color is defined by its wavelength, frequency or energy Red - Blue 7000 - 4000 Angstroms ( 1 nm = 10 A, 1 A = 10 -8 cm) Blue light is more energetic than red light Light also behaves like ‘particles’ called photons Photon energy, frequency, wavelength: E = h f = hc/l Planck’s Law (‘h’ is a number known as Planck’s constant)

Visible Light • Forms a narrow band within the electromagnetic spectrum ranging from gamma rays to radio waves • Human eye is most sensitive to which color? • Yellow. Why?

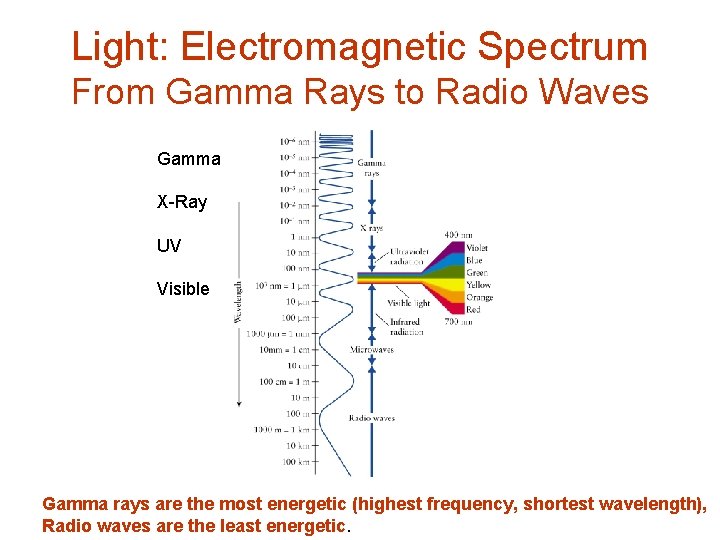
Light: Electromagnetic Spectrum From Gamma Rays to Radio Waves Gamma X-Ray UV Visible Gamma rays are the most energetic (highest frequency, shortest wavelength), Radio waves are the least energetic.

Decreasing Wavelength OR Increasing Frequency

Matter and Particles of Light: Quantum Theory • Light (energy) and matter in motion behave both as • • • waves and particles Wave-Particle Duality - Quantum Theory Energy particles: quanta or photons: E = hf = hc/l Photons of a specific wavelength may be absorbed or emitted by atoms in matter Matter is made of different natural elements: lightest Hydrogen (1 proton), heaviest Uranium (92 protons) Smallest particle of an element is atom, made up of a nucleus (protons and neutrons), and orbiting electrons Electrons and protons attract as opposite electrical charges, NOT gravitationally like planets and Sun


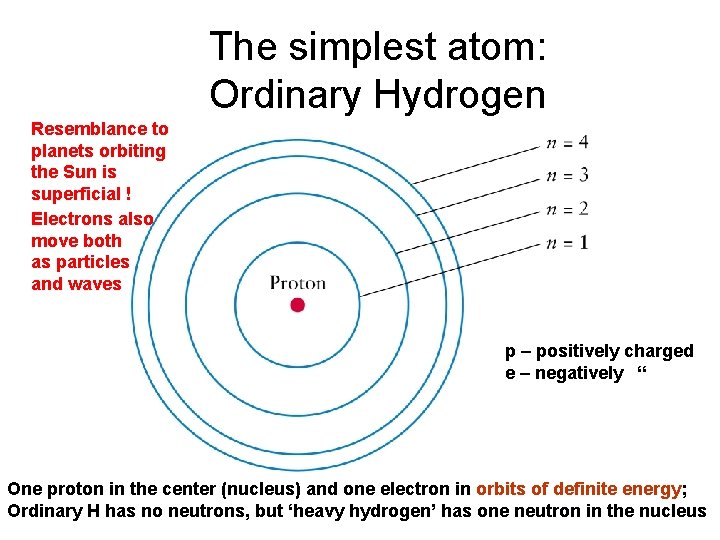
The simplest atom: Ordinary Hydrogen Resemblance to planets orbiting the Sun is superficial ! Electrons also move both as particles and waves p – positively charged e – negatively “ One proton in the center (nucleus) and one electron in orbits of definite energy; Ordinary H has no neutrons, but ‘heavy hydrogen’ has one neutron in the nucleus

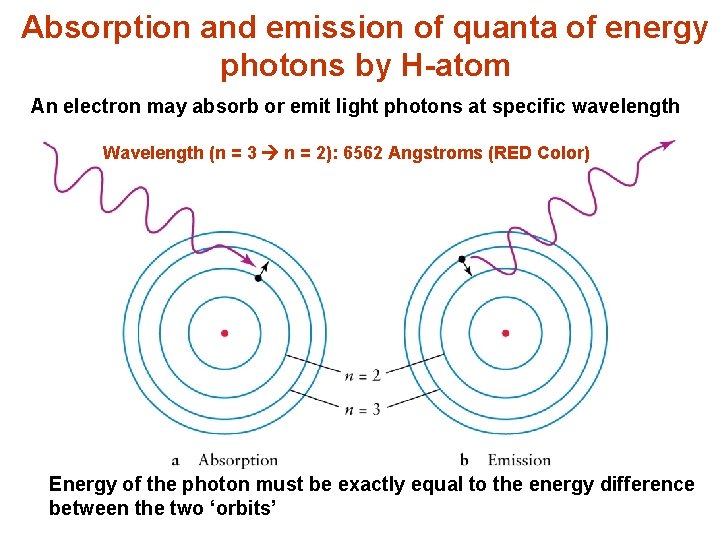
Absorption and emission of quanta of energy photons by H-atom An electron may absorb or emit light photons at specific wavelength Wavelength (n = 3 n = 2): 6562 Angstroms (RED Color) Energy of the photon must be exactly equal to the energy difference between the two ‘orbits’

file: ///E: /Univ 7 e/content/ch 05/0503002. html

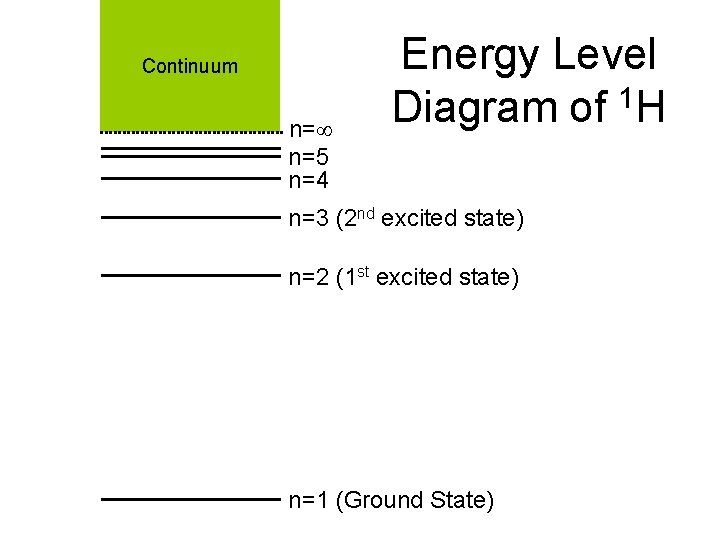
Continuum n= n=5 n=4 Energy Level Diagram of 1 H n=3 (2 nd excited state) n=2 (1 st excited state) n=1 (Ground State)

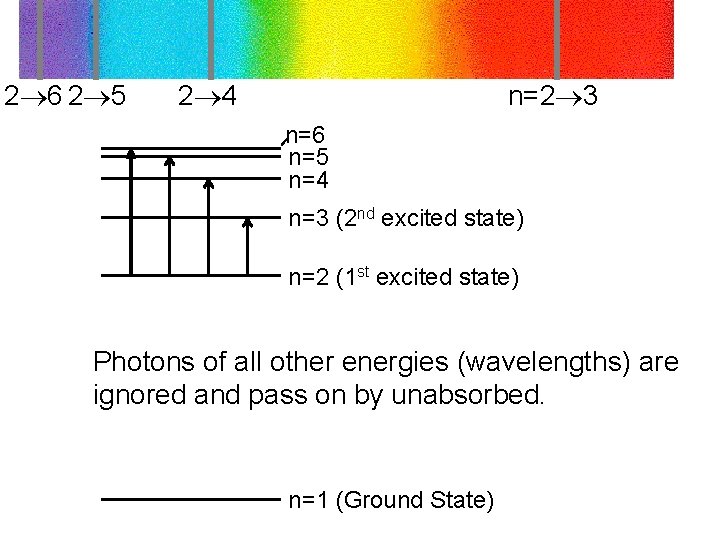
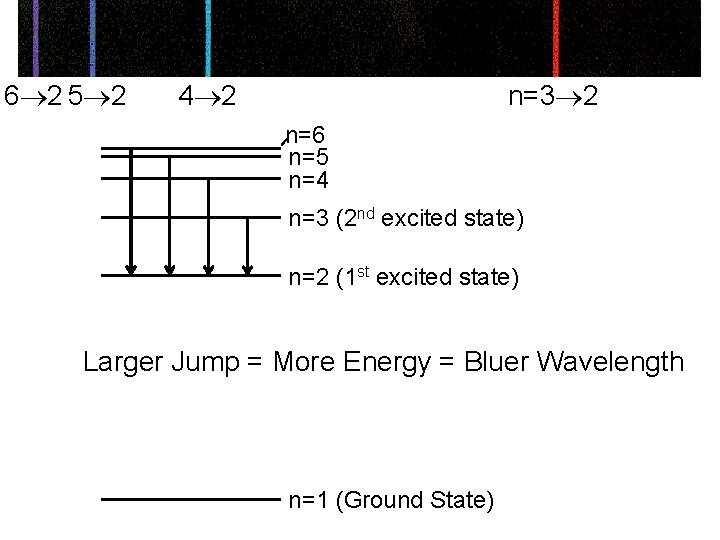
2 6 2 5 2 4 n=2 3 n=6 n=5 n=4 n=3 (2 nd excited state) n=2 (1 st excited state) Photons of all other energies (wavelengths) are ignored and pass on by unabsorbed. n=1 (Ground State)

6 2 5 2 4 2 n=3 2 n=6 n=5 n=4 n=3 (2 nd excited state) n=2 (1 st excited state) Larger Jump = More Energy = Bluer Wavelength n=1 (Ground State)

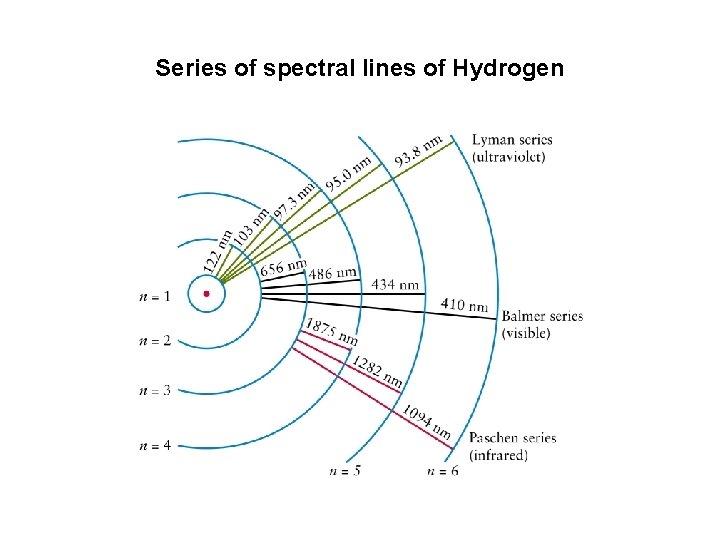
Series of spectral lines of Hydrogen

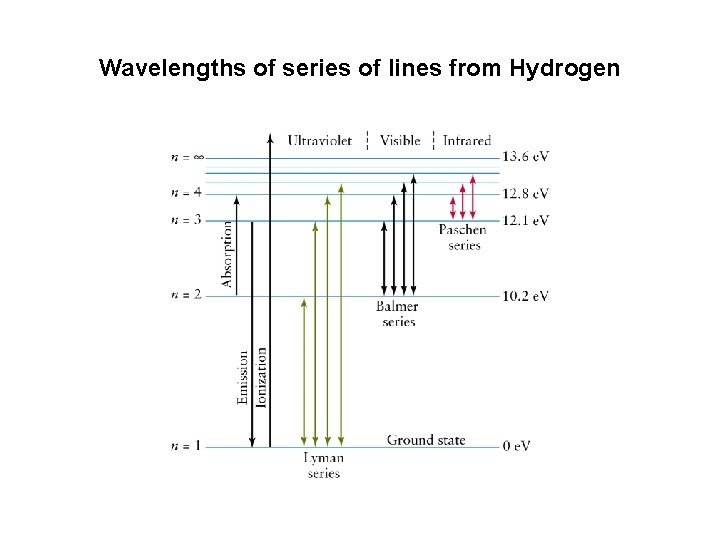
Wavelengths of series of lines from Hydrogen

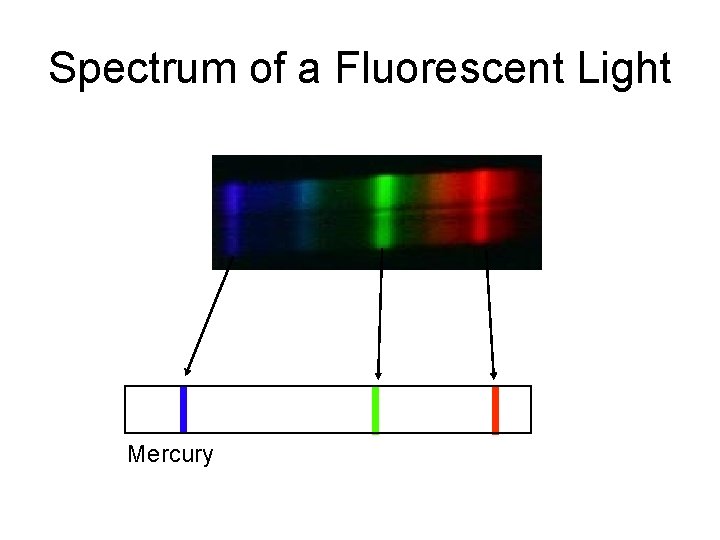
Spectrum of a Fluorescent Light Mercury


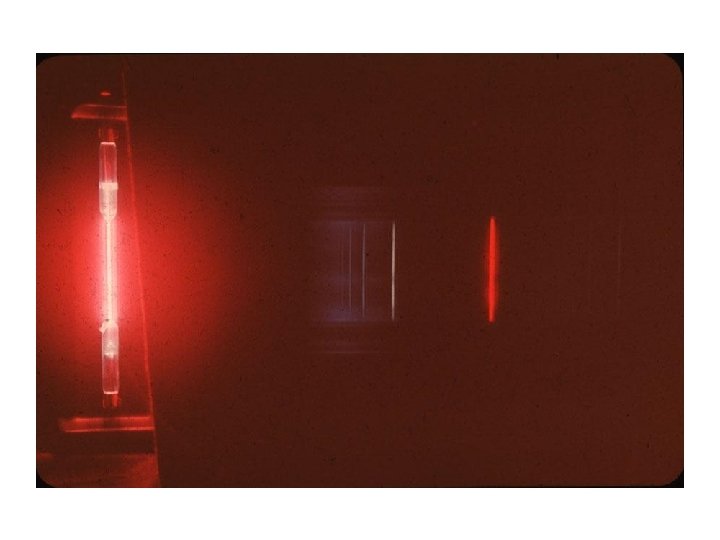
Spectroscopy Demo: Spectra of Elements • https: //www. youtube. com/watch? v=oae 5 fa -f 0 S 0&feature=youtu. be

Spectroscopy Demo – Spectra of Elements • https: //www. youtube. com/watch? v=oae 5 fa -f 0 S 0&feature=youtu. be

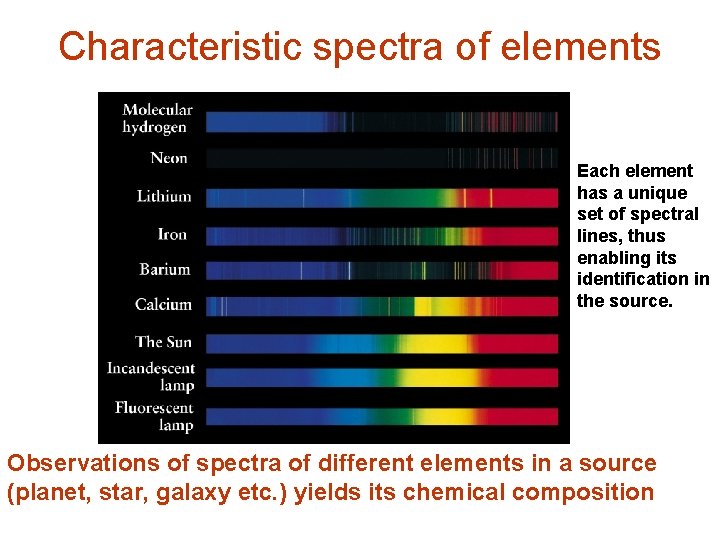
Characteristic spectra of elements Each element has a unique set of spectral lines, thus enabling its identification in the source. Observations of spectra of different elements in a source (planet, star, galaxy etc. ) yields its chemical composition

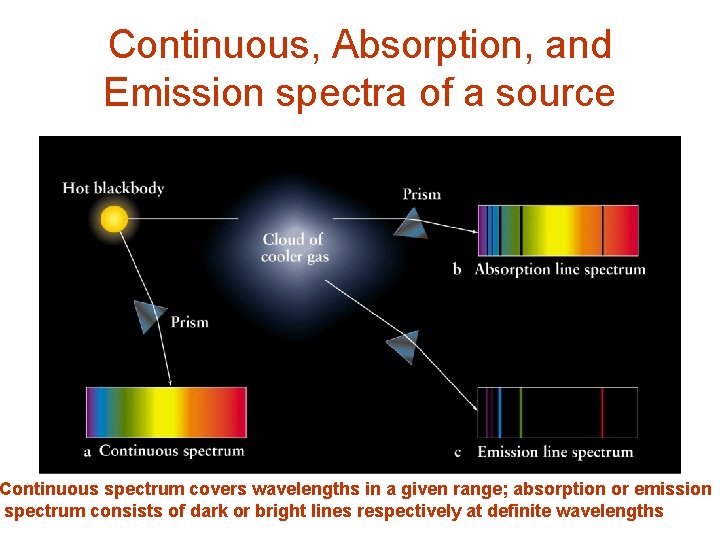
Continuous, Absorption, and Emission spectra of a source Continuous spectrum covers wavelengths in a given range; absorption or emission spectrum consists of dark or bright lines respectively at definite wavelengths

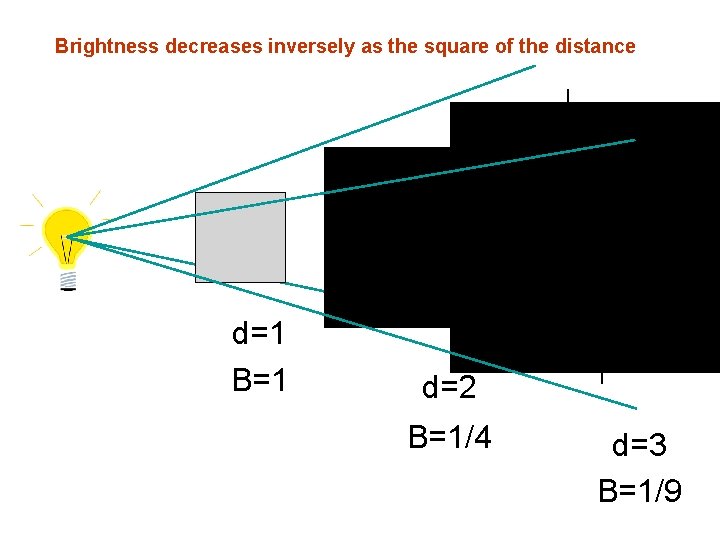
Brightness and Temperature • Brightness is related to the total energy emitted, or the luminosity of an object • The energy emitted is related to the temperature of the object • B = s T 4 (s is a constant) Stefan-Boltzmann Law

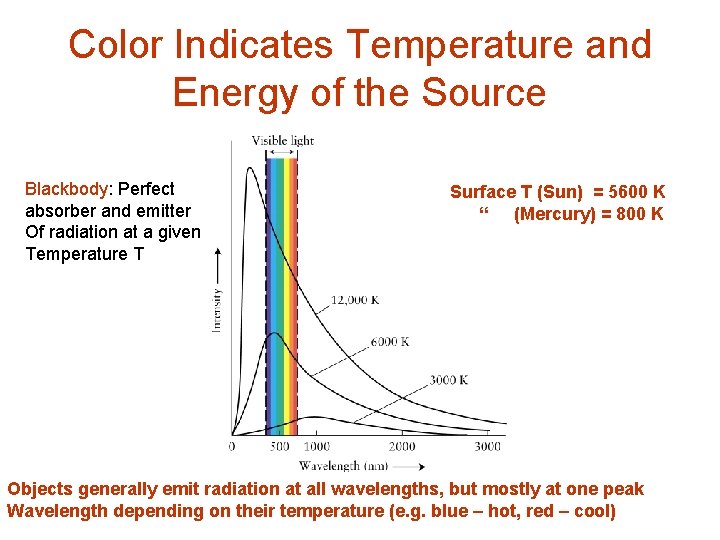
Color Indicates Temperature and Energy of the Source Blackbody: Perfect absorber and emitter Of radiation at a given Temperature T Surface T (Sun) = 5600 K “ (Mercury) = 800 K Objects generally emit radiation at all wavelengths, but mostly at one peak Wavelength depending on their temperature (e. g. blue – hot, red – cool)

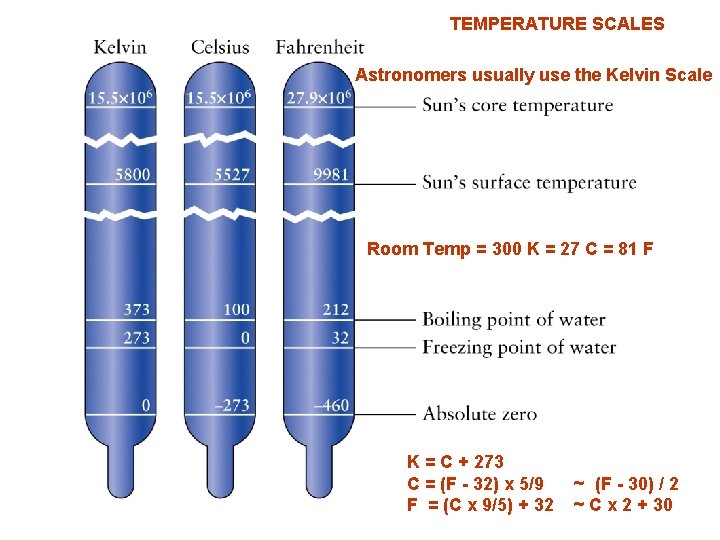
TEMPERATURE SCALES Astronomers usually use the Kelvin Scale Room Temp = 300 K = 27 C = 81 F K = C + 273 C = (F - 32) x 5/9 F = (C x 9/5) + 32 ~ (F - 30) / 2 ~ C x 2 + 30

Brightness decreases inversely as the square of the distance d=1 B=1 d=2 B=1/4 d=3 B=1/9

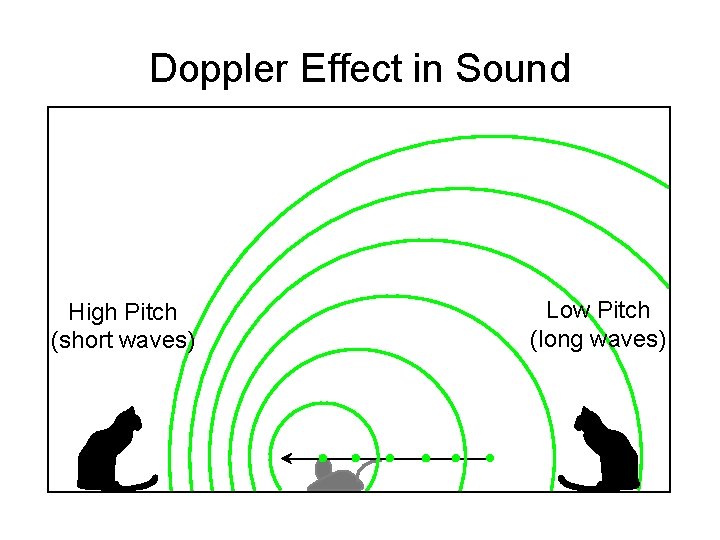
The Doppler Effect • Why does the “pitch” of a police siren differ when, say, a police car is approaching you, or when you are running away from the police (not recommended) ? • The frequency (the number of sound waves per second) is higher when approaching, and smaller when receding from the source

Doppler Effect in Sound High Pitch (short waves) Low Pitch (long waves)

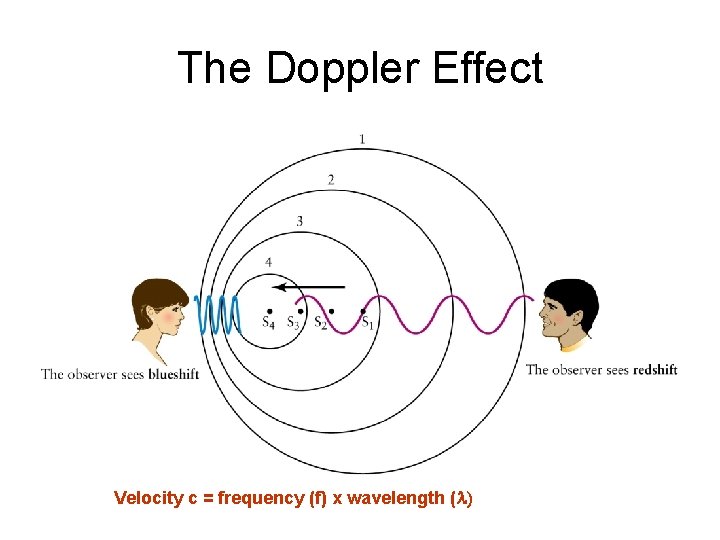
The Doppler Effect Velocity c = frequency (f) x wavelength (l)

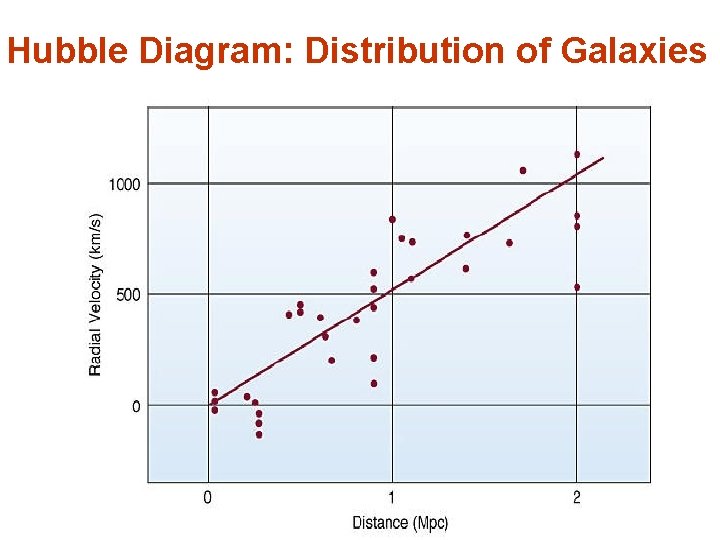
Doppler Shift of Wavelengths • • • What about the wavelength? What about light? Shorter wavelength Blue-shift, Longer wavelength Red-shift We can determine the velocity of astronomical objects, moving away or towards the Earth, by measuring the wavelength of light from the object • Observed red-shift of galaxies all over the sky shows that galaxies are moving away from one another the Universe is expanding (Hubble’s Law)

Hubble Diagram: Distribution of Galaxies

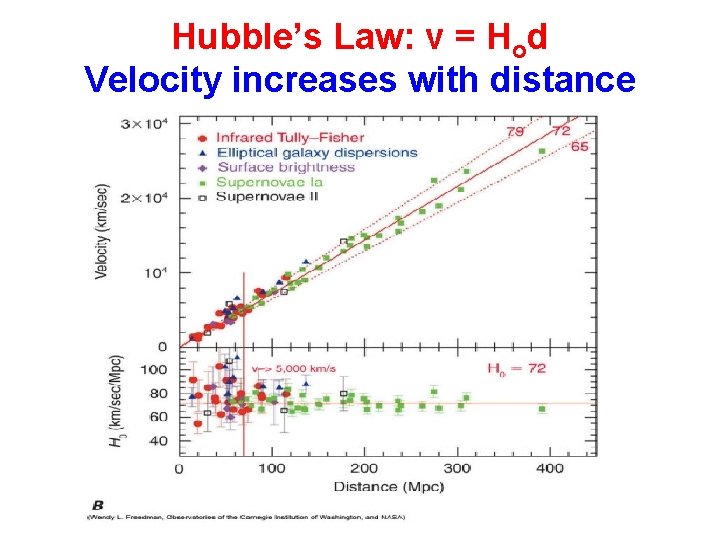
Hubble’s Law: v = Hod Velocity increases with distance

Expanding Universe • • Hubble’s law Universe is expanding Universe had a beginning ! How long ago? Distance/Velocity = time ! Ho = 71 Km/sec/Mpc (units of v/d = 1/t) Age of the universe: 1/Ho (units of time) Big Bang !! About 13. 7 billion years ago How does one determine distances? Redshift

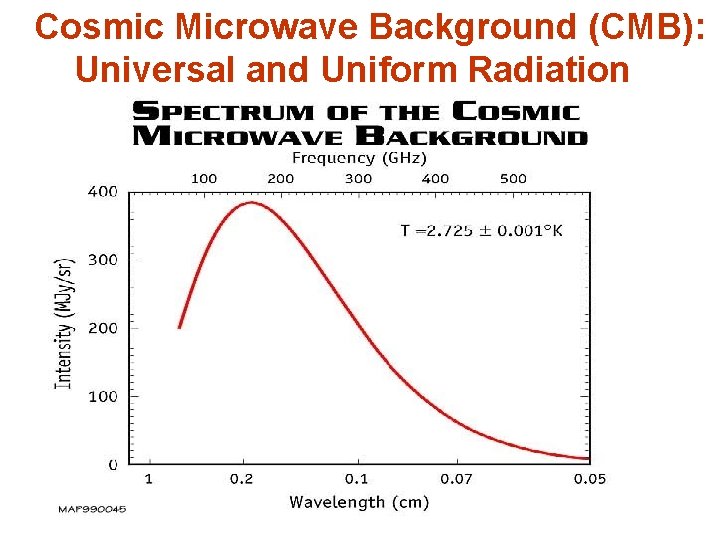
Cosmic Microwave Background (CMB): Universal and Uniform Radiation

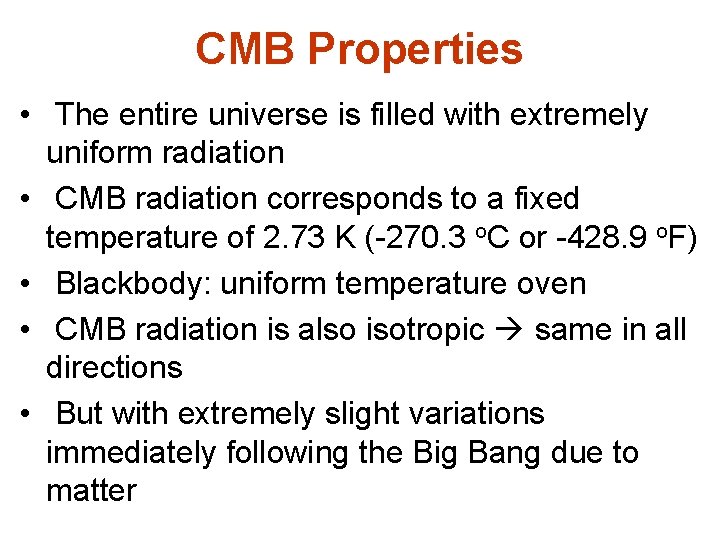
CMB Properties • The entire universe is filled with extremely uniform radiation • CMB radiation corresponds to a fixed temperature of 2. 73 K (-270. 3 o. C or -428. 9 o. F) • Blackbody: uniform temperature oven • CMB radiation is also isotropic same in all directions • But with extremely slight variations immediately following the Big Bang due to matter

Distribution of Matter in Galaxy • Stars rotate about the center of galaxy • Velocity determined by gravity: mass Mc and distance Rc from the center • KE = PE • ½ mstar v 2 = G Mc mstar / Rc • Velocity v should decrease with radius Rc • Surprise !

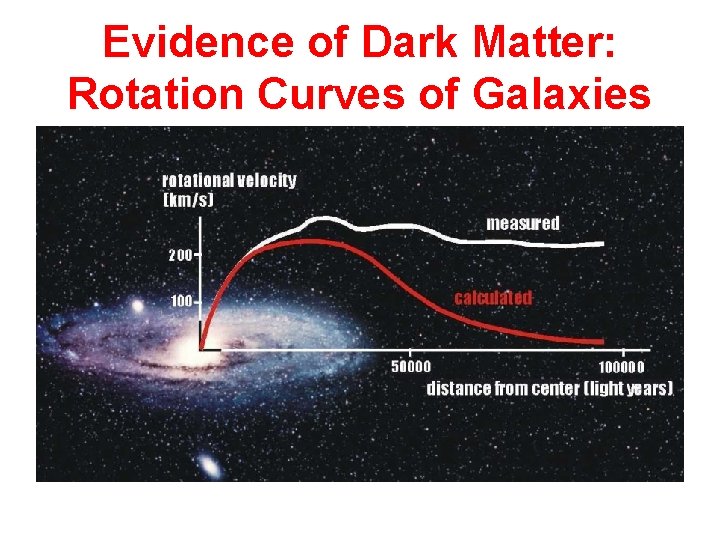
Evidence of Dark Matter: Rotation Curves of Galaxies

Dark Matter Halo • Rotation curves are flat out to distances beyond observable galaxies • Ergo: Galaxies have “dark matter” haloes • What is dark matter?