Electrical Engineering Materials EE 3219 Dr Md Sherajul


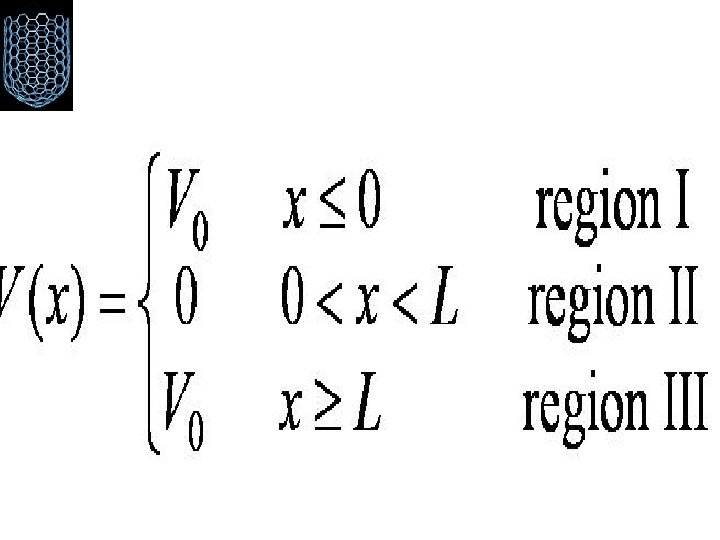





- Slides: 28

Electrical Engineering Materials EE 3219 Dr. Md. Sherajul Islam Department of Electrical and Electronic Engineering Khulna University of Engineering & Technology Khulna, Bangladesh

LECTURE - 5 Modern Theory of Solids

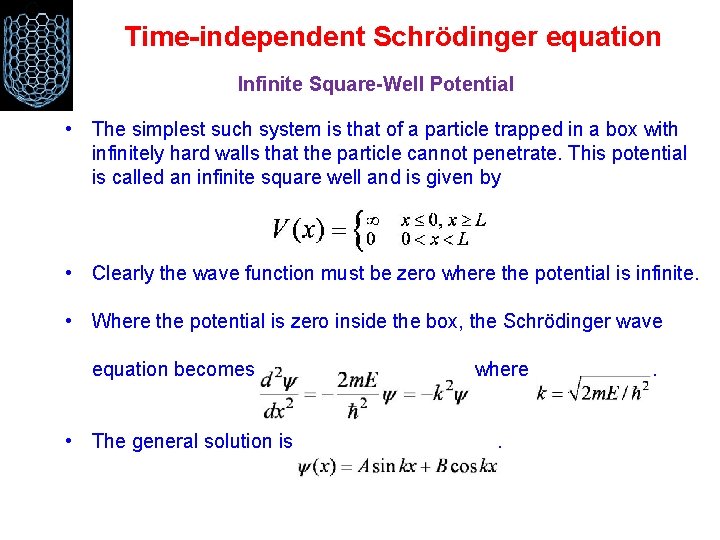
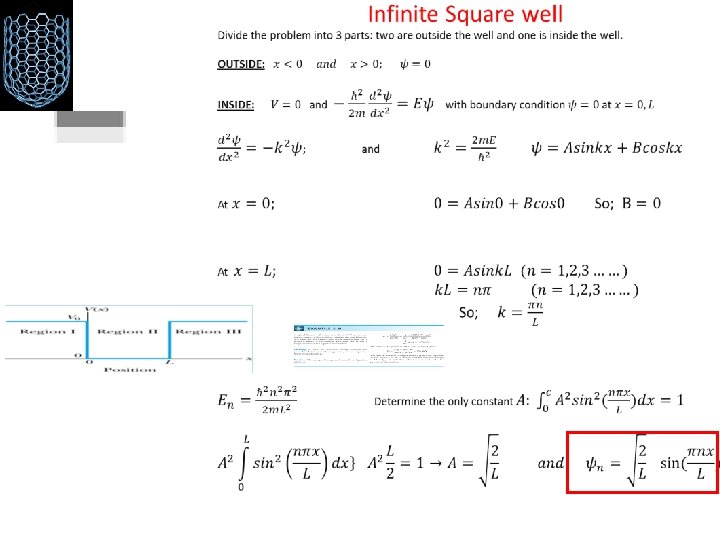
Time-independent Schrödinger equation Infinite Square-Well Potential • The simplest such system is that of a particle trapped in a box with infinitely hard walls that the particle cannot penetrate. This potential is called an infinite square well and is given by • Clearly the wave function must be zero where the potential is infinite. • Where the potential is zero inside the box, the Schrödinger wave equation becomes • The general solution is where. .

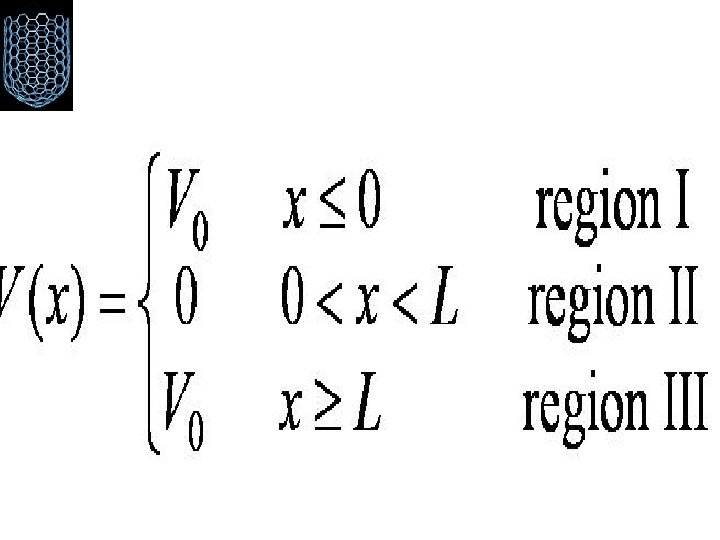

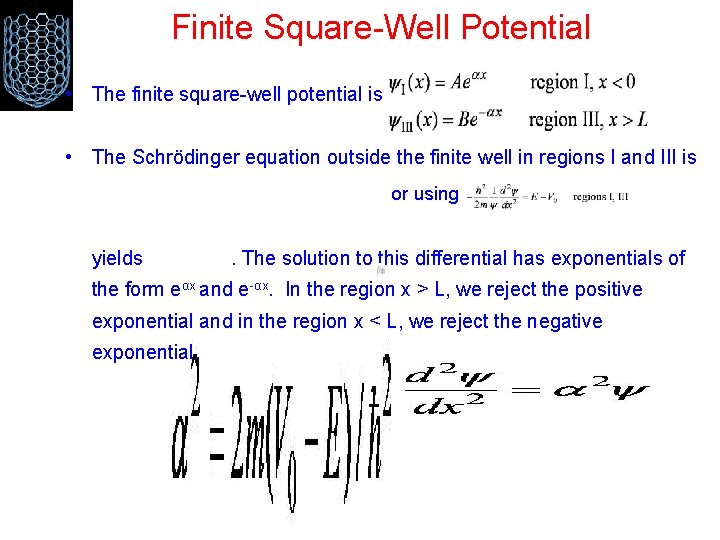
Finite Square-Well Potential • The finite square-well potential is • The Schrödinger equation outside the finite well in regions I and III is or using yields . The solution to this differential has exponentials of the form eαx and e-αx. In the region x > L, we reject the positive exponential and in the region x < L, we reject the negative exponential.

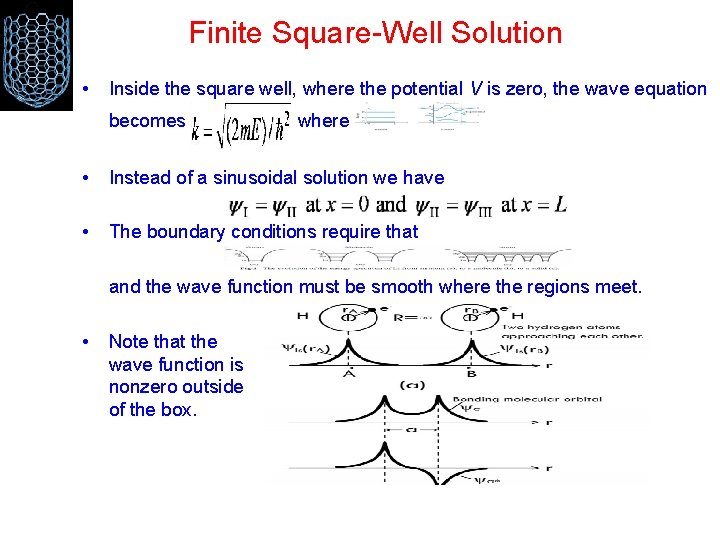
Finite Square-Well Solution • Inside the square well, where the potential V is zero, the wave equation becomes where • Instead of a sinusoidal solution we have • The boundary conditions require that and the wave function must be smooth where the regions meet. • Note that the wave function is nonzero outside of the box.

Band structure of Solids The energy spectrum gradually changes as atoms are assembled to form the solid

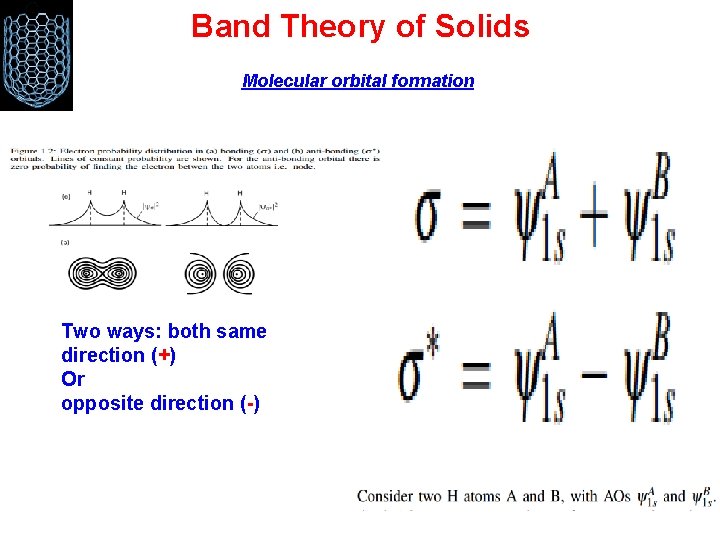
Band Theory of Solids Molecular orbital formation Two ways: both same direction (+) Or opposite direction (-)

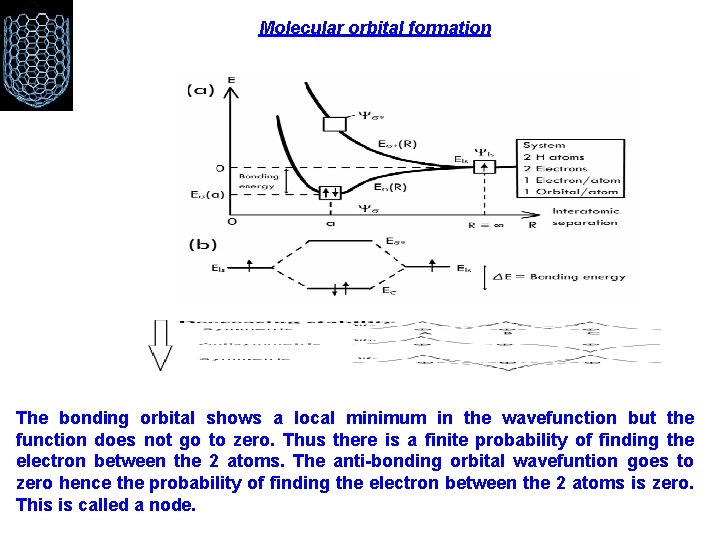
Molecular orbital formation The bonding orbital shows a local minimum in the wavefunction but the function does not go to zero. Thus there is a finite probability of finding the electron between the 2 atoms. The anti-bonding orbital wavefuntion goes to zero hence the probability of finding the electron between the 2 atoms is zero. This is called a node.

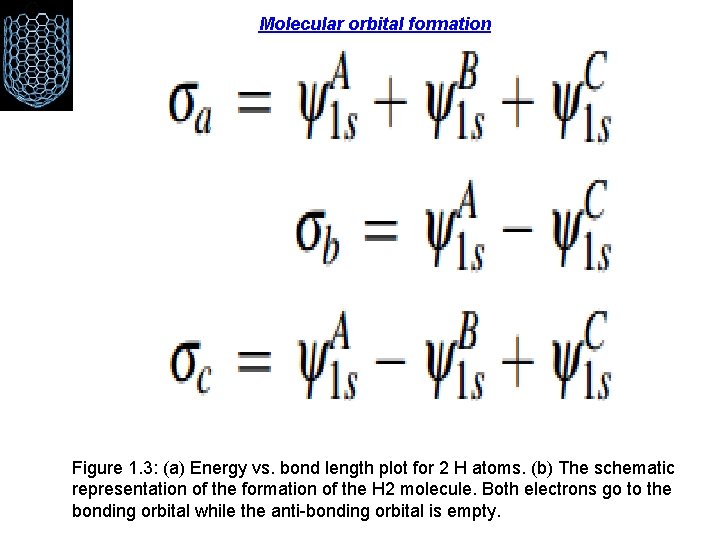
Molecular orbital formation Figure 1. 3: (a) Energy vs. bond length plot for 2 H atoms. (b) The schematic representation of the formation of the H 2 molecule. Both electrons go to the bonding orbital while the anti-bonding orbital is empty.

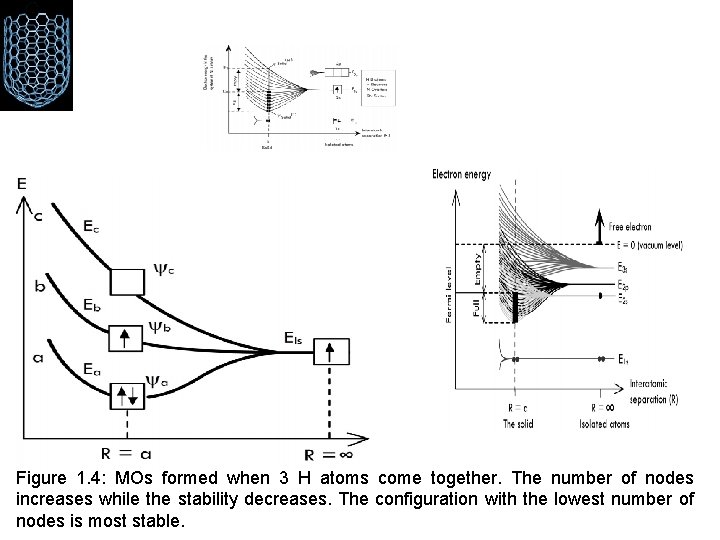
Figure 1. 4: MOs formed when 3 H atoms come together. The number of nodes increases while the stability decreases. The configuration with the lowest number of nodes is most stable.

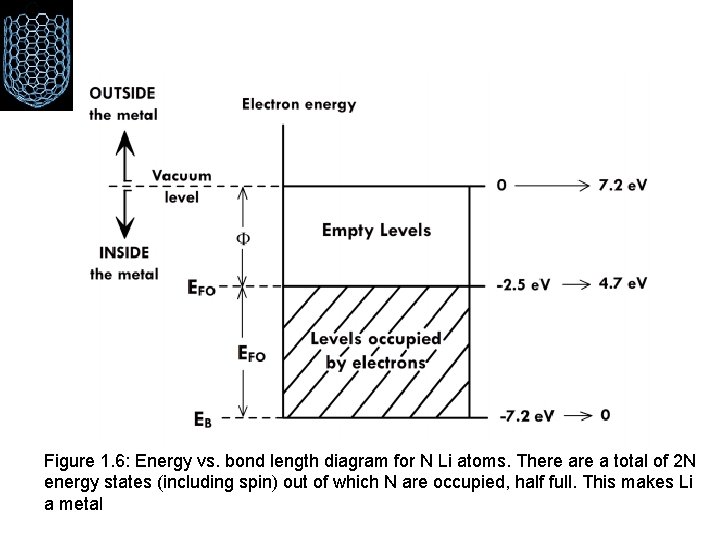
Figure 1. 6: Energy vs. bond length diagram for N Li atoms. There a total of 2 N energy states (including spin) out of which N are occupied, half full. This makes Li a metal

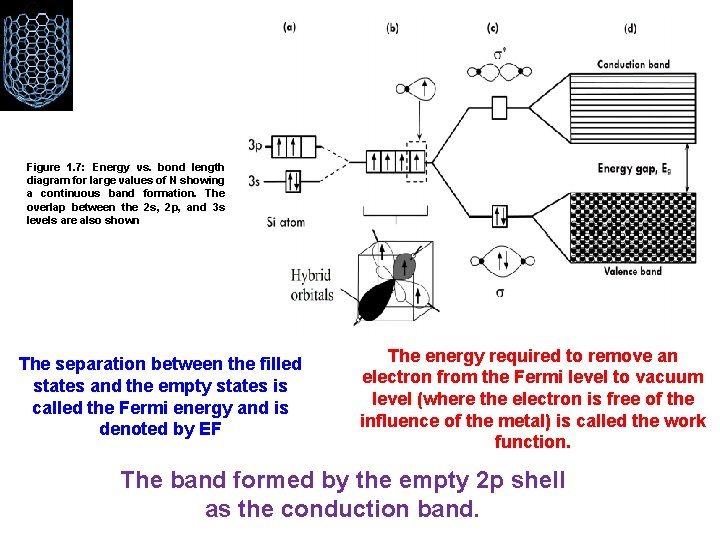
Figure 1. 7: Energy vs. bond length diagram for large values of N showing a continuous band formation. The overlap between the 2 s, 2 p, and 3 s levels are also shown The separation between the filled states and the empty states is called the Fermi energy and is denoted by EF The energy required to remove an electron from the Fermi level to vacuum level (where the electron is free of the influence of the metal) is called the work function. The band formed by the empty 2 p shell as the conduction band.

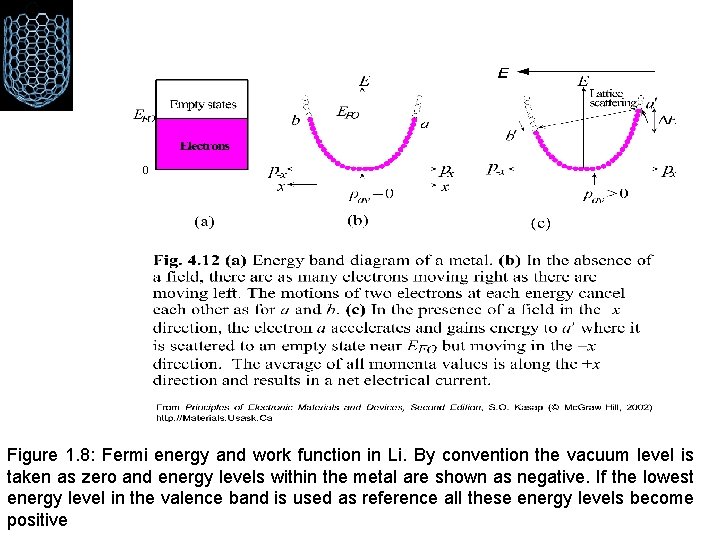
Figure 1. 8: Fermi energy and work function in Li. By convention the vacuum level is taken as zero and energy levels within the metal are shown as negative. If the lowest energy level in the valence band is used as reference all these energy levels become positive

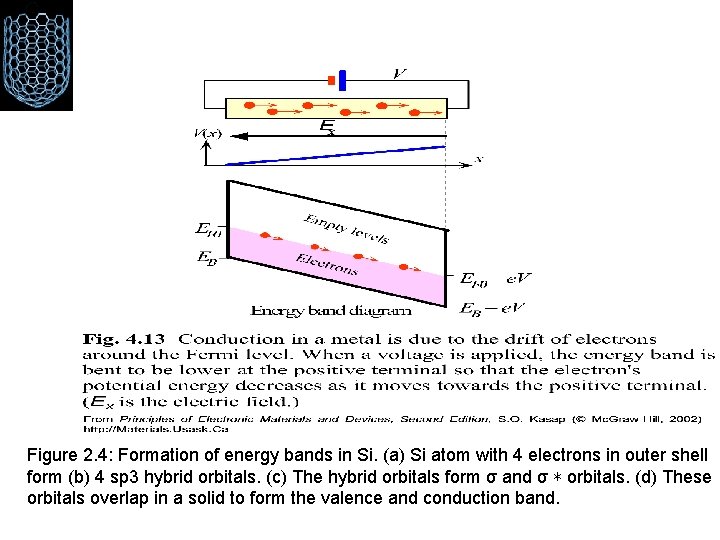
Figure 2. 4: Formation of energy bands in Si. (a) Si atom with 4 electrons in outer shell form (b) 4 sp 3 hybrid orbitals. (c) The hybrid orbitals form σ and σ ∗ orbitals. (d) These orbitals overlap in a solid to form the valence and conduction band.

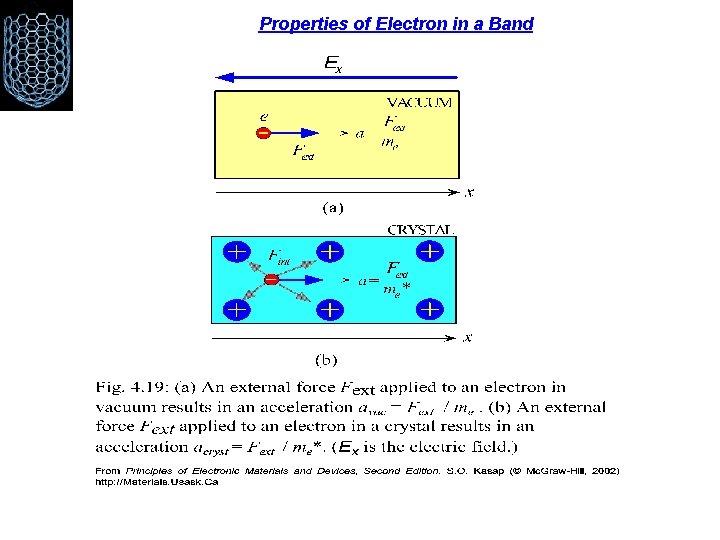
Properties of Electron in a Band

Properties of Electron in a Band

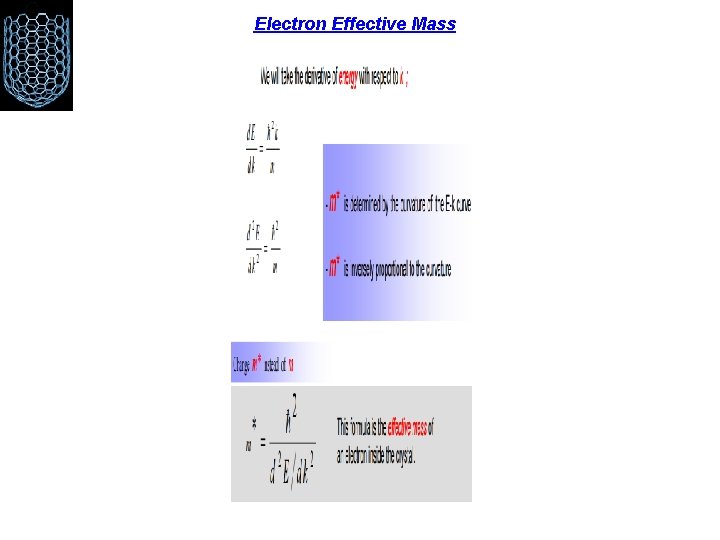
Electron Effective Mass

Electron Effective Mass

E-K Diagram E

Calculation of Effective mass from E-K Diagram What is the significance of E-K Diagram ? ?

Density of states in an Energy Band The density of states (DOS) of a system describes the number of available states per unit energy per unit volume

Density of states in an Energy Band

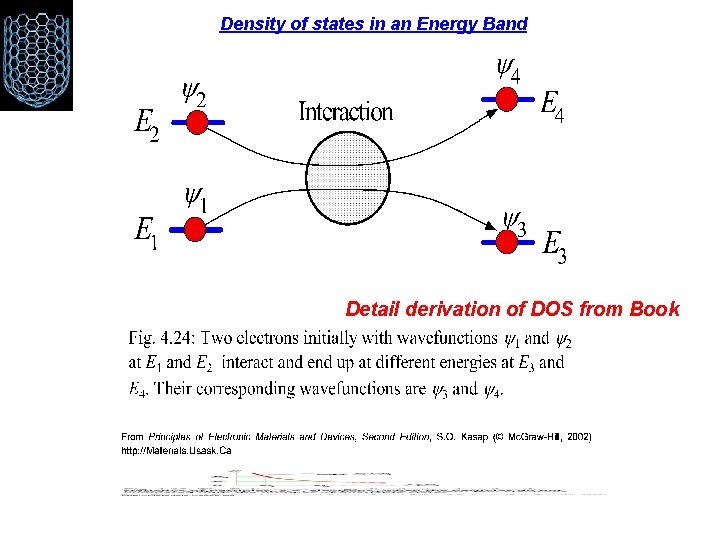
Density of states in an Energy Band Detail derivation of DOS from Book

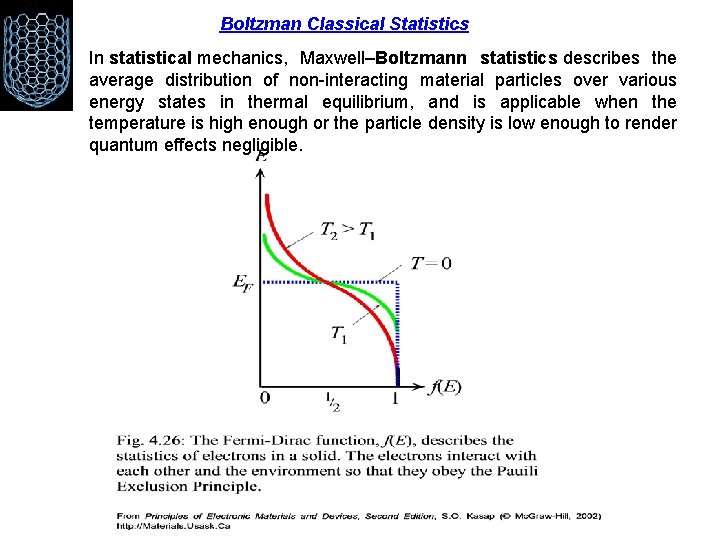
Boltzman Classical Statistics In statistical mechanics, Maxwell–Boltzmann statistics describes the average distribution of non-interacting material particles over various energy states in thermal equilibrium, and is applicable when the temperature is high enough or the particle density is low enough to render quantum effects negligible.

Boltzman Classical Statistics

Fermi-Dirac Statistics In quantum statistics, a branch of physics, Fermi–Diracstatistics describe a distribution of particles over energy states in systems consisting of many identical particles that obey the Pauli exclusion principle.