Electrical Engineering Materials EE 3219 Dr Md Sherajul








- Slides: 34

Electrical Engineering Materials EE 3219 Dr. Md. Sherajul Islam Associate Professor Department of Electrical and Electronics Engineering Khulna University of Engineering & Technology Khulna, Bangladesh

LECTURE - 4 Elementary quantum physics

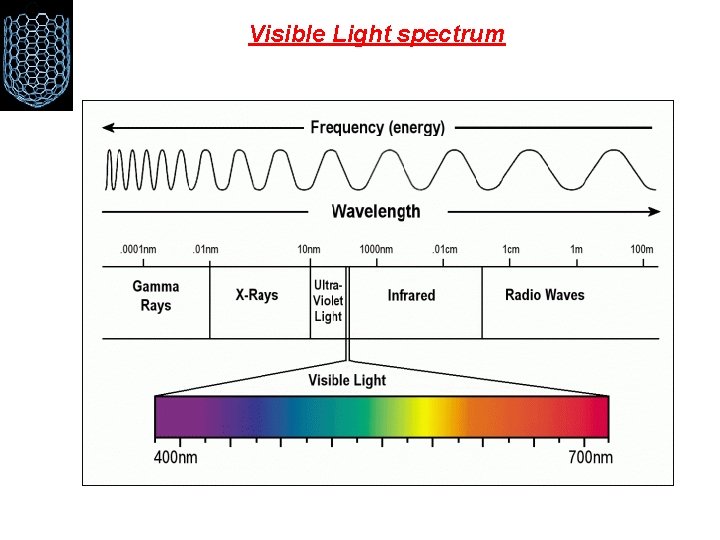
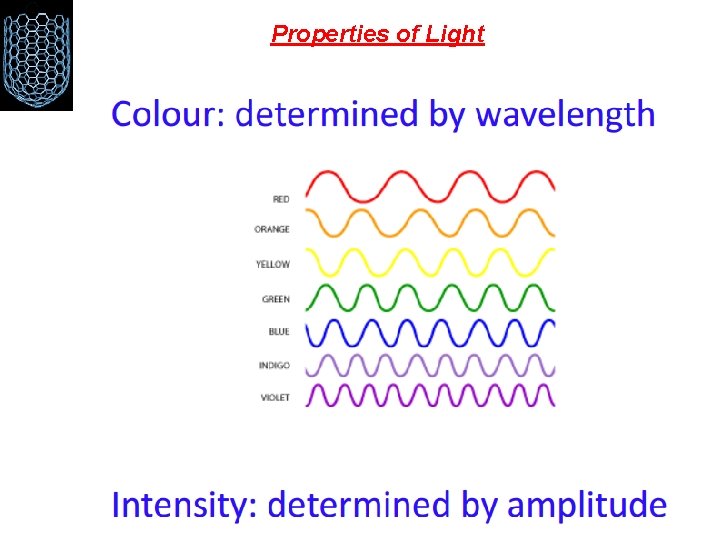
Visible Light spectrum

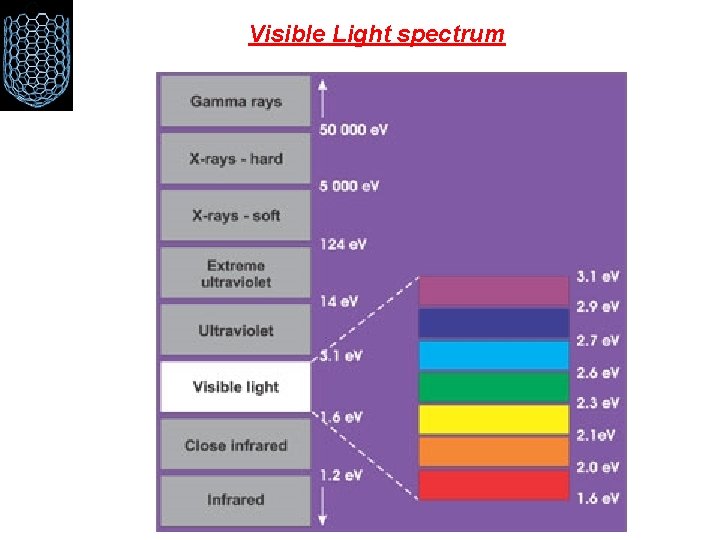
Visible Light spectrum

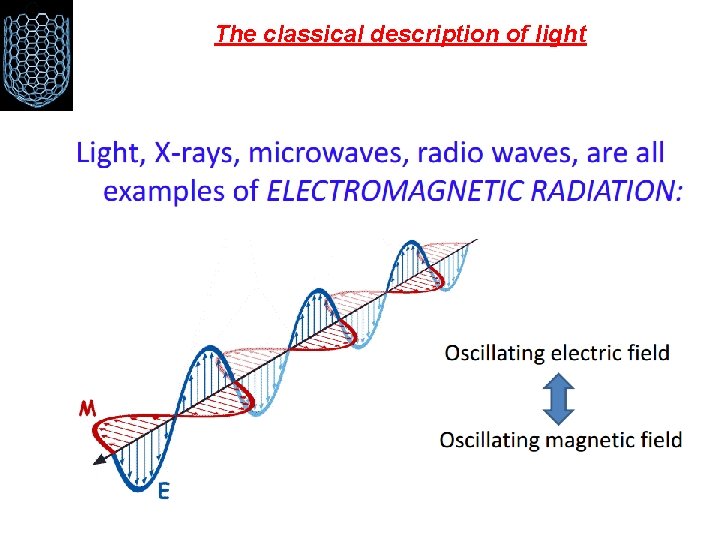
The classical description of light

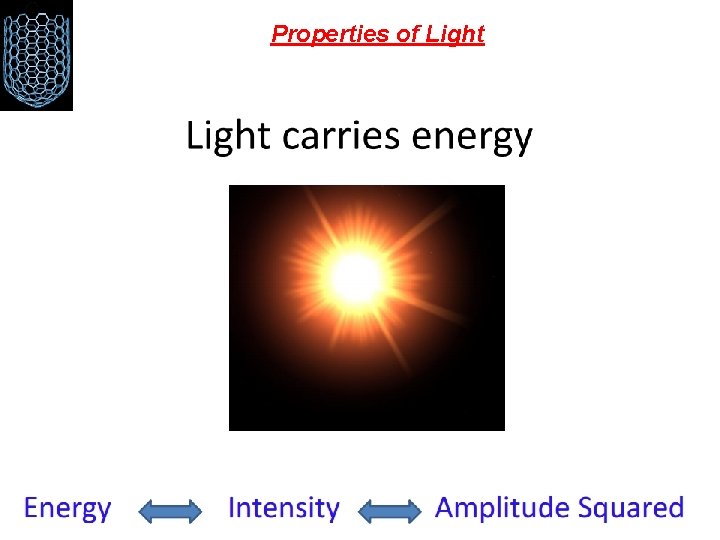
Properties of Light

Properties of Light

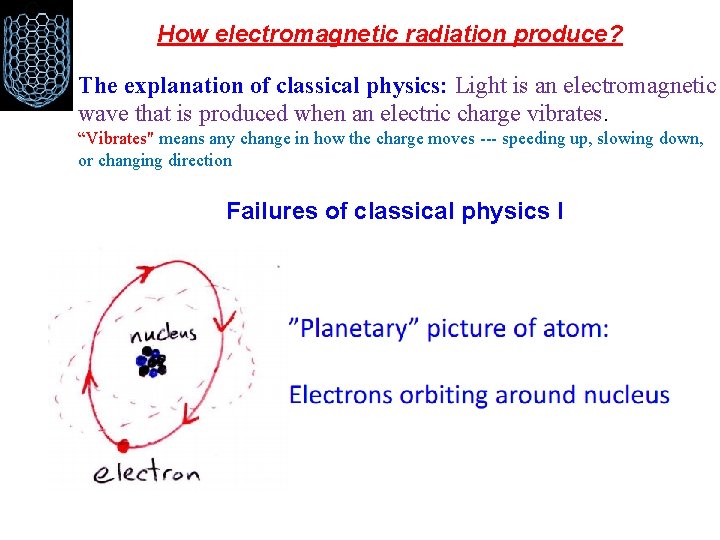
How electromagnetic radiation produce? The explanation of classical physics: Light is an electromagnetic wave that is produced when an electric charge vibrates. “Vibrates" means any change in how the charge moves --- speeding up, slowing down, or changing direction Failures of classical physics I

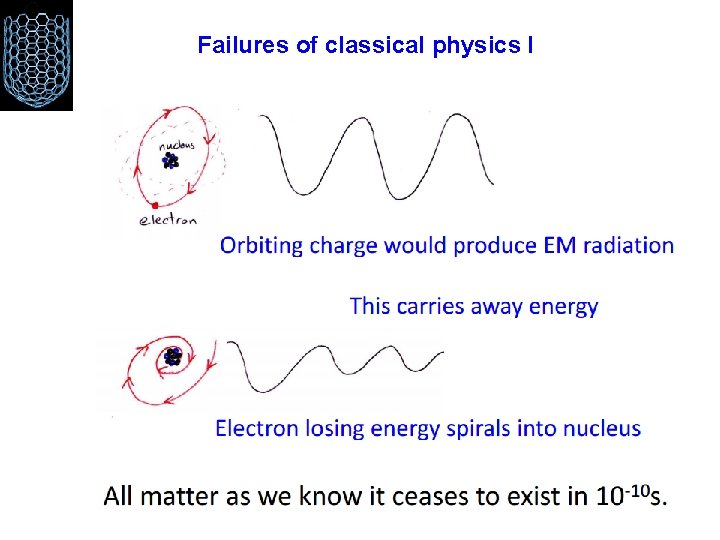
Failures of classical physics I

Failures of classical physics II

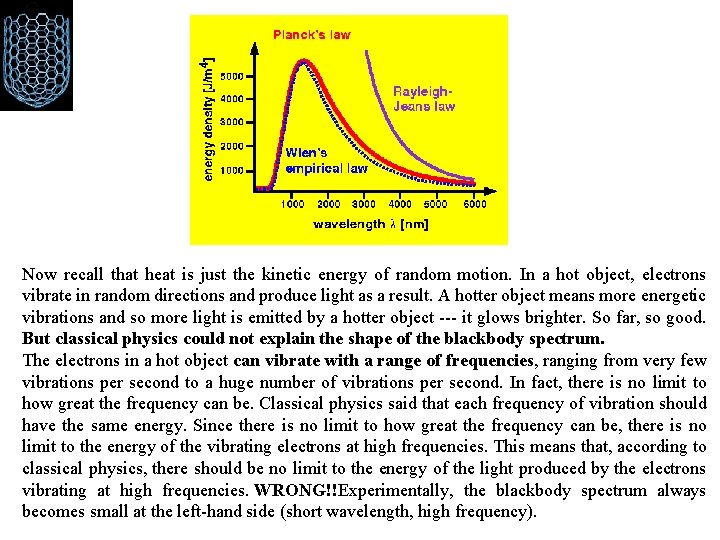
Failures of Classical mechanics ØBlack Body Radiation: WHAT IS BLACK BODY ? A black body is a theoretical object that absorbs 100% of the radiation that hits it. Therefore it reflects no radiation and appears perfectly black. It is also a perfect emitter of radiation. At a particular temperature the black body would emit the maximum amount of energy possible for that temperature. Any object with a temperature above absolute zero emits light at all wavelengths. If the object is perfectly black (so it doesn't reflect any light), then the light that comes from it is called blackbody radiation.

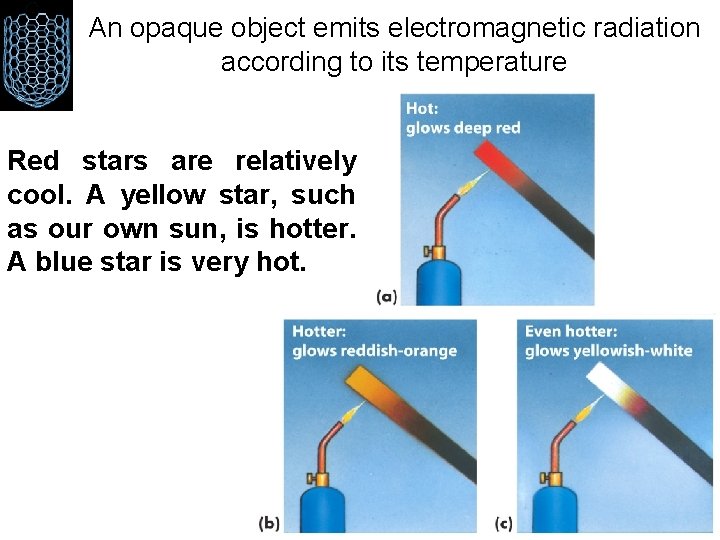
An opaque object emits electromagnetic radiation according to its temperature Red stars are relatively cool. A yellow star, such as our own sun, is hotter. A blue star is very hot.

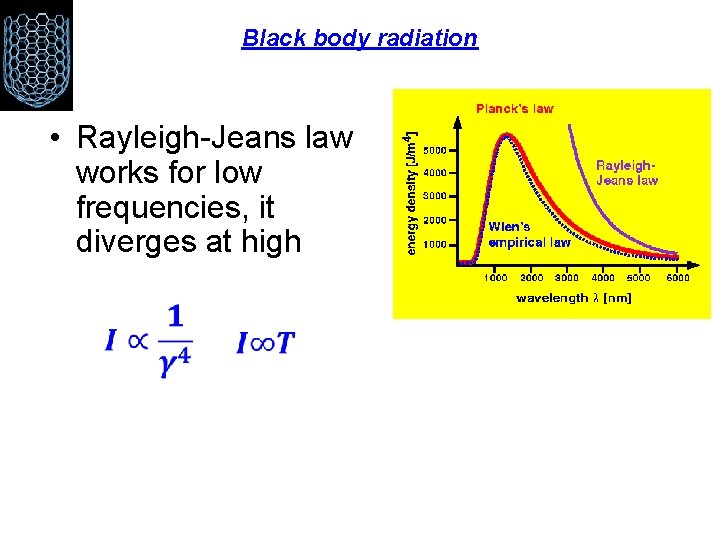
Black body radiation • Rayleigh-Jeans law works for low frequencies, it diverges at high

Now recall that heat is just the kinetic energy of random motion. In a hot object, electrons vibrate in random directions and produce light as a result. A hotter object means more energetic vibrations and so more light is emitted by a hotter object --- it glows brighter. So far, so good. But classical physics could not explain the shape of the blackbody spectrum. The electrons in a hot object can vibrate with a range of frequencies, ranging from very few vibrations per second to a huge number of vibrations per second. In fact, there is no limit to how great the frequency can be. Classical physics said that each frequency of vibration should have the same energy. Since there is no limit to how great the frequency can be, there is no limit to the energy of the vibrating electrons at high frequencies. This means that, according to classical physics, there should be no limit to the energy of the light produced by the electrons vibrating at high frequencies. WRONG!!Experimentally, the blackbody spectrum always becomes small at the left-hand side (short wavelength, high frequency).

Planck’s Theory Planck said that energy is not shared equally by electrons that vibrate with different frequencies. Planck said that energy comes in clumps. He called a clump of energy a quantum energy of a quantum = (a calibration constant) x (frequency of vibration) E = hf where h, =6 x 10 -34, very tiny! Planck won the Nobel Prize in Physics in 1918 Beginning of quantum era……………

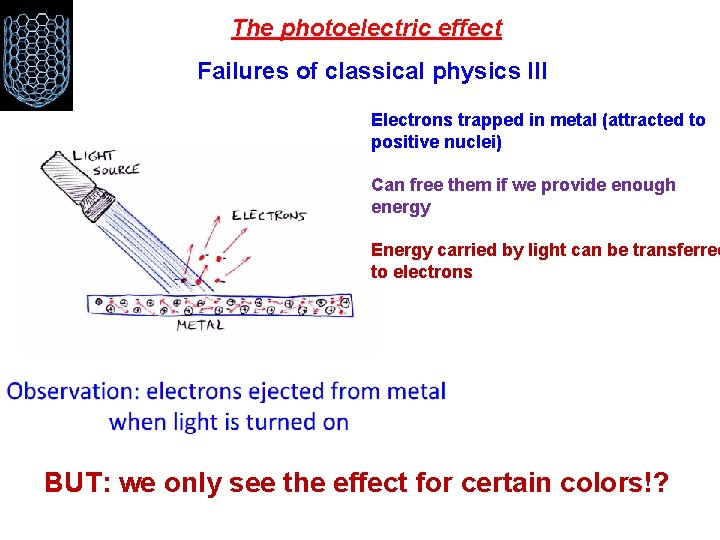
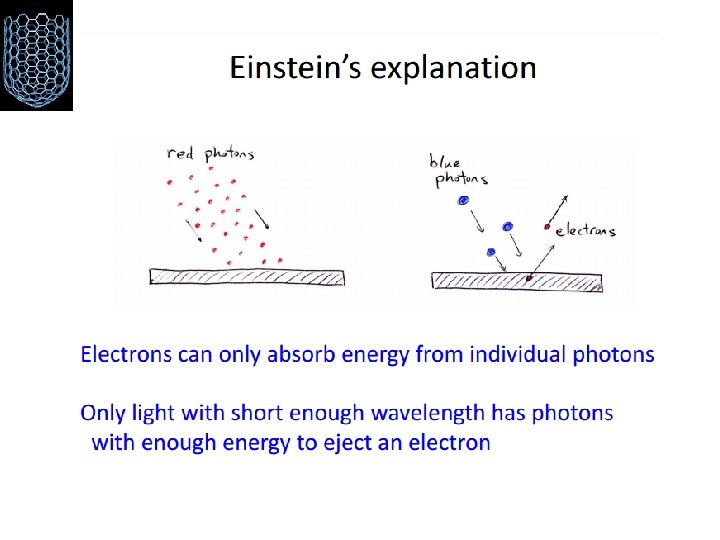
The photoelectric effect Failures of classical physics III Electrons trapped in metal (attracted to positive nuclei) Can free them if we provide enough energy Energy carried by light can be transferred to electrons BUT: we only see the effect for certain colors!?

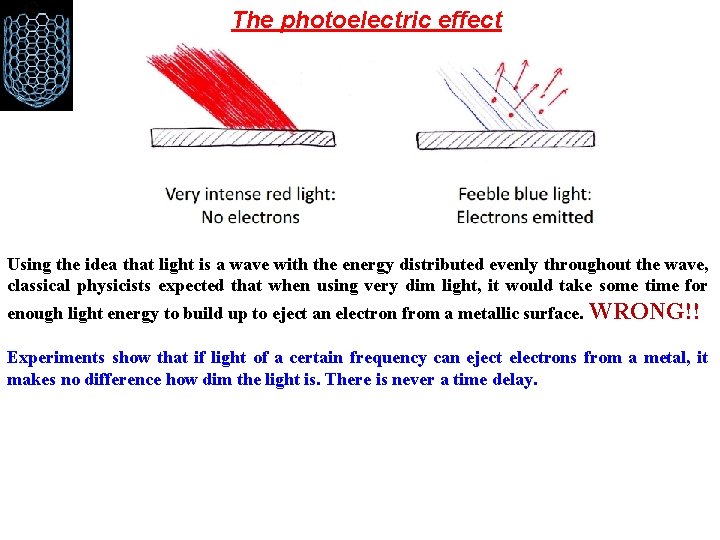
The photoelectric effect Using the idea that light is a wave with the energy distributed evenly throughout the wave, classical physicists expected that when using very dim light, it would take some time for enough light energy to build up to eject an electron from a metallic surface. WRONG!! Experiments show that if light of a certain frequency can eject electrons from a metal, it makes no difference how dim the light is. There is never a time delay.

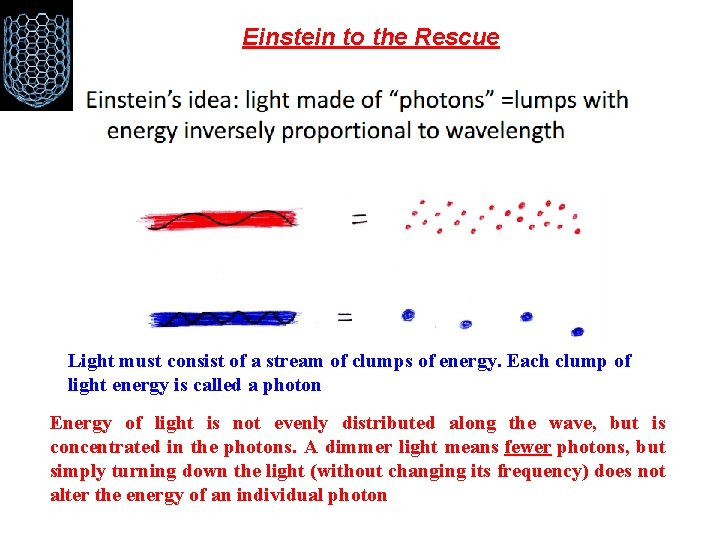
Einstein to the Rescue Light must consist of a stream of clumps of energy. Each clump of light energy is called a photon Energy of light is not evenly distributed along the wave, but is concentrated in the photons. A dimmer light means fewer photons, but simply turning down the light (without changing its frequency) does not alter the energy of an individual photon



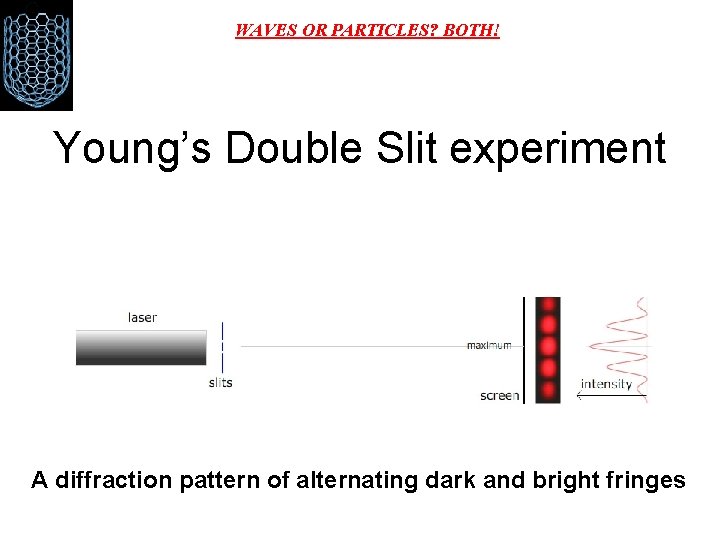
WAVES OR PARTICLES? BOTH! Young’s Double Slit experiment A diffraction pattern of alternating dark and bright fringes

WAVES OR PARTICLES? BOTH! Light acts like a wave if you want to know how it propagates, how it travels from one place to another. Light acts like particles (photons) if you want to know how light interacts with matter. We say that light exhibits a wave-particle duality. It can behave like either waves or particles (but not both at the same time), depending on the situation.

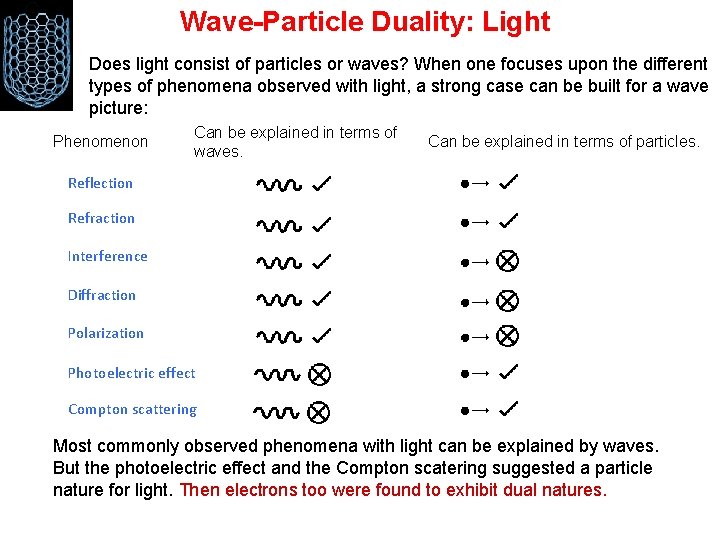
Wave-Particle Duality: Light Does light consist of particles or waves? When one focuses upon the different types of phenomena observed with light, a strong case can be built for a wave picture: Phenomenon Can be explained in terms of waves. Can be explained in terms of particles. Reflection Refraction Interference Diffraction Polarization Photoelectric effect Compton scattering Most commonly observed phenomena with light can be explained by waves. But the photoelectric effect and the Compton scatering suggested a particle nature for light. Then electrons too were found to exhibit dual natures.

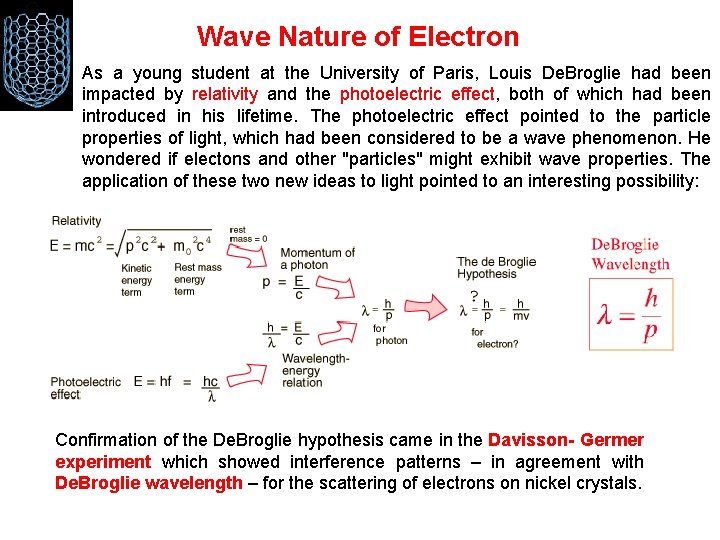
Wave Nature of Electron As a young student at the University of Paris, Louis De. Broglie had been impacted by relativity and the photoelectric effect, both of which had been introduced in his lifetime. The photoelectric effect pointed to the particle properties of light, which had been considered to be a wave phenomenon. He wondered if electons and other "particles" might exhibit wave properties. The application of these two new ideas to light pointed to an interesting possibility: Confirmation of the De. Broglie hypothesis came in the Davisson- Germer experiment which showed interference patterns – in agreement with De. Broglie wavelength – for the scattering of electrons on nickel crystals.

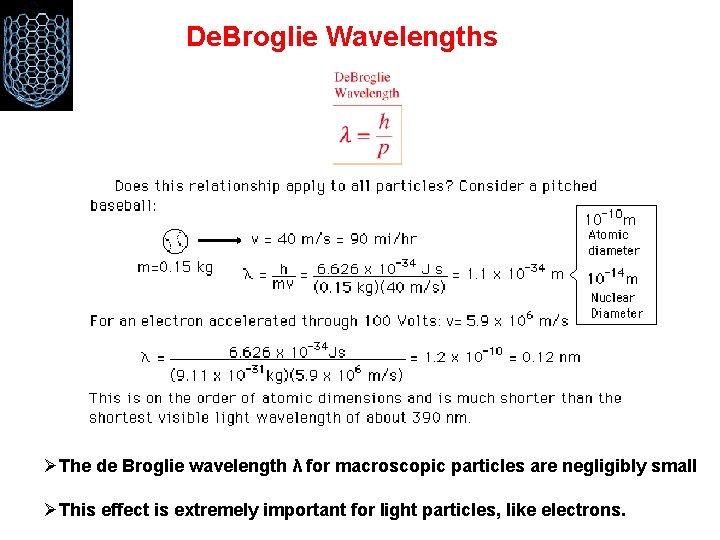
De. Broglie Wavelengths ØThe de Broglie wavelength λ for macroscopic particles are negligibly small ØThis effect is extremely important for light particles, like electrons.

Wave or Particle (Size ? ? ) • Objects that are large in the absolute sense have the property that the wavelengths associated with them are completely negligible compared to their size. Therefore, large particles only manifest their particle nature, they never manifest their wave nature.

Electro-magnetic waves may behave sometimes like particles (photons). Particles may behave sometimes like waves (de Broglie waves with λ = h/p). The energy of waves is quantised in such a way that E = hf The extremely small size of Planck’s constant = h = 6. 626 × 10− 34 J. s sets the scale for quantum effects

Wavefunction The question is, how do we proceed to construct a dynamical equation for quantum objects which are represented by wave functions ? The wave that in quantum mechanics replaces the classical concept of particle trajectory is called a wavefunction, ψ (“psi”). Because Schrodinger’s wave equation determines the mechanical behavior of quantum particles, the new physics that results is known as Quantum Mechanics.

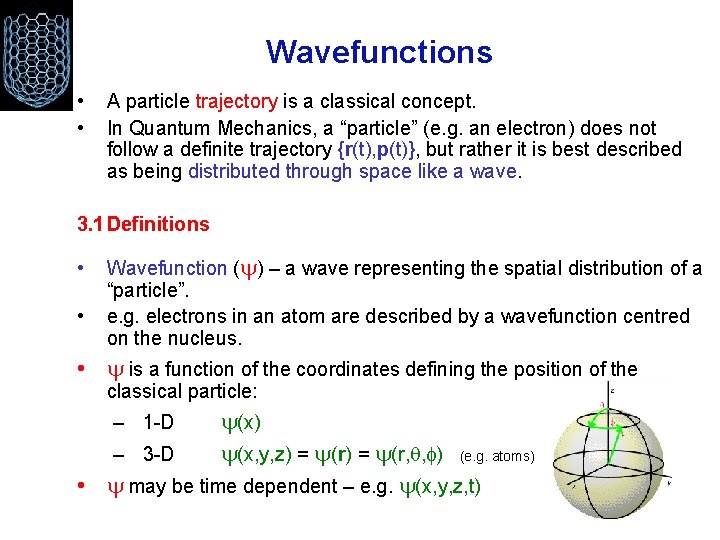
Wavefunctions • • A particle trajectory is a classical concept. In Quantum Mechanics, a “particle” (e. g. an electron) does not follow a definite trajectory {r(t), p(t)}, but rather it is best described as being distributed through space like a wave. 3. 1 Definitions • • Wavefunction ( ) – a wave representing the spatial distribution of a “particle”. e. g. electrons in an atom are described by a wavefunction centred on the nucleus. • is a function of the coordinates defining the position of the classical particle: (x) – 3 -D (x, y, z) = (r, , ) (e. g. atoms) • may be time dependent – e. g. (x, y, z, t) – 1 -D

The Importance of • completely defines the system (e. g. electron in an atom or molecule). • If is known, we can determine any observable property (e. g. energy, vibrational frequencies, …) of the system. • QM provides the tools to determine computationally, to interpret and to use to determine properties of the system.

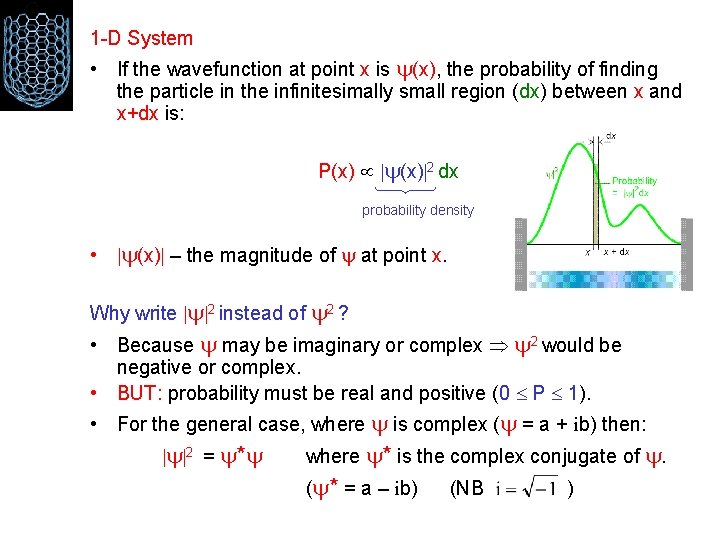
1 -D System • If the wavefunction at point x is (x), the probability of finding the particle in the infinitesimally small region (dx) between x and x+dx is: P(x) 2 dx probability density • (x) – the magnitude of at point x. Why write 2 instead of 2 ? • Because may be imaginary or complex 2 would be negative or complex. • BUT: probability must be real and positive (0 P 1). • For the general case, where is complex ( = a + ib) then: 2 = * where * is the complex conjugate of . ( * = a – ib) (NB )

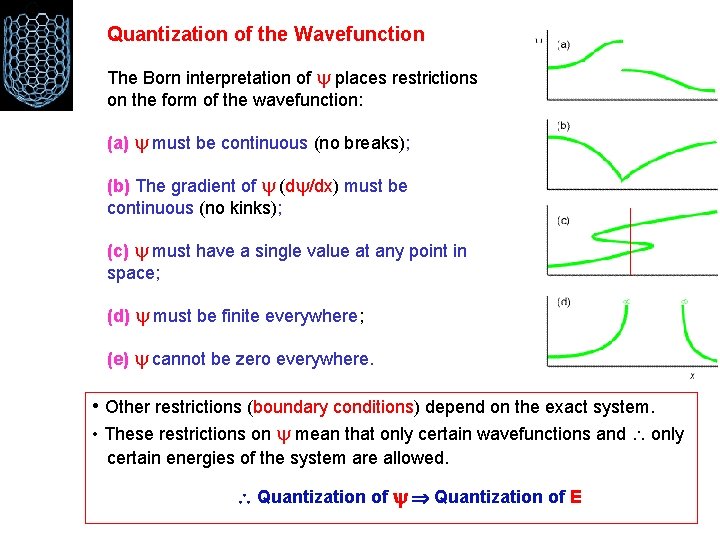
Quantization of the Wavefunction The Born interpretation of places restrictions on the form of the wavefunction: (a) must be continuous (no breaks); (b) The gradient of (d /dx) must be continuous (no kinks); (c) must have a single value at any point in space; (d) must be finite everywhere; (e) cannot be zero everywhere. • Other restrictions (boundary conditions) depend on the exact system. • These restrictions on mean that only certain wavefunctions and only certain energies of the system are allowed. Quantization of E

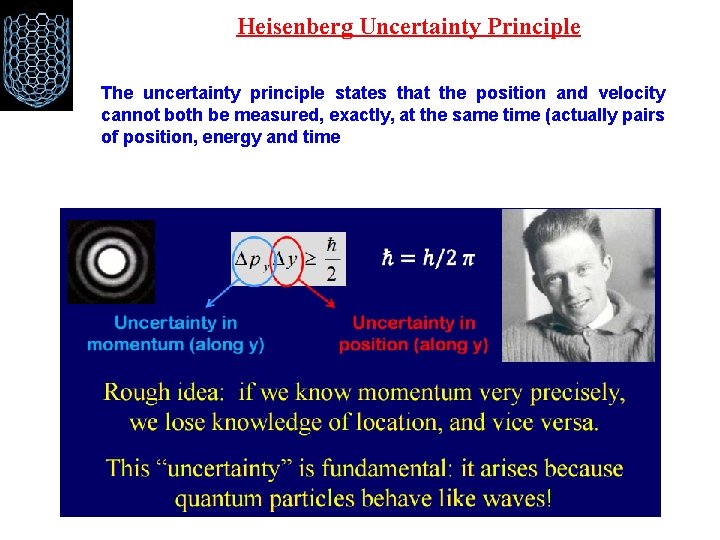
Heisenberg Uncertainty Principle To measure something, we have to interact with it somehow (Suppose we use photons to probe the electrons position and momentum) If we use a very energetic photon, i. e. , one that has lots of momentum, it is possible to get a very good estimate of the electron's position because the photon's direction will be relatively unaltered by scattering off of the electron. However, it will give it an abrupt kick and vastly disturb our knowledge of its momentum. On the other hand, a low energy, or low momentum photon, will not disturb the electrons momentum very much, but because of its low momentum, it will also have a long wavelength, and this will make it bend around the electron so that the electron's position will be poorly known. This is how it works.

Heisenberg Uncertainty Principle The uncertainty principle states that the position and velocity cannot both be measured, exactly, at the same time (actually pairs of position, energy and time