Elastic Scattering HansJrgen Wollersheim Rutherford scattering discovery of

- Slides: 26

Elastic Scattering Hans-Jürgen Wollersheim

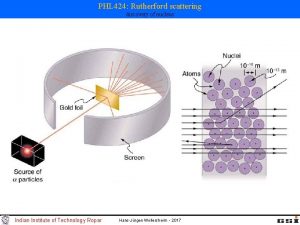
Rutherford scattering discovery of nucleus

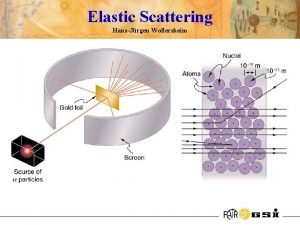
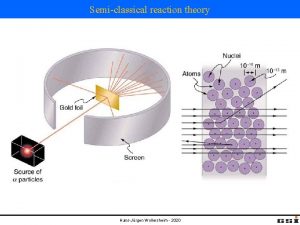
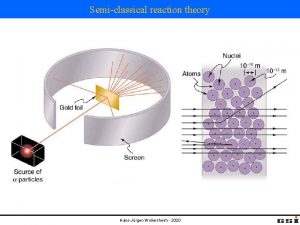
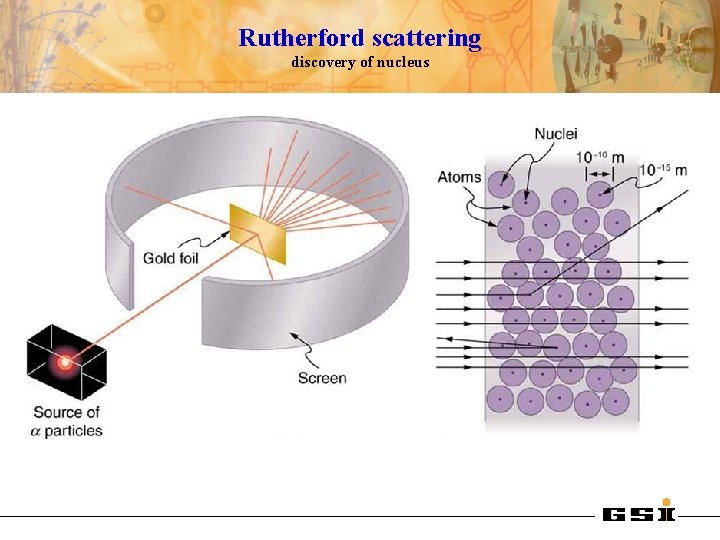
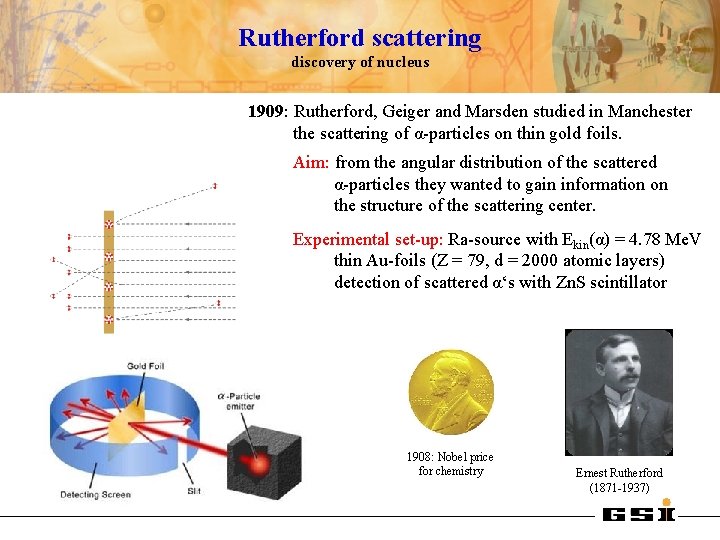
Rutherford scattering discovery of nucleus 1909: Rutherford, Geiger and Marsden studied in Manchester the scattering of α-particles on thin gold foils. Aim: from the angular distribution of the scattered α-particles they wanted to gain information on the structure of the scattering center. Experimental set-up: Ra-source with Ekin(α) = 4. 78 Me. V thin Au-foils (Z = 79, d = 2000 atomic layers) detection of scattered α‘s with Zn. S scintillator 1908: Nobel price for chemistry Ernest Rutherford (1871 -1937)

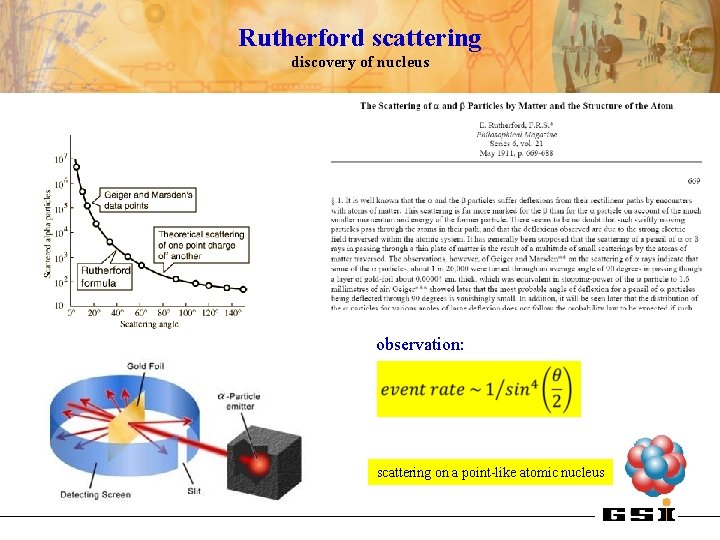
Rutherford scattering discovery of nucleus observation: scattering on a point-like atomic nucleus

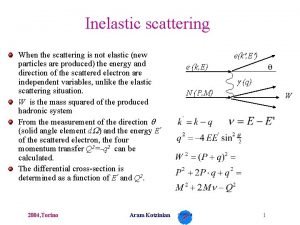
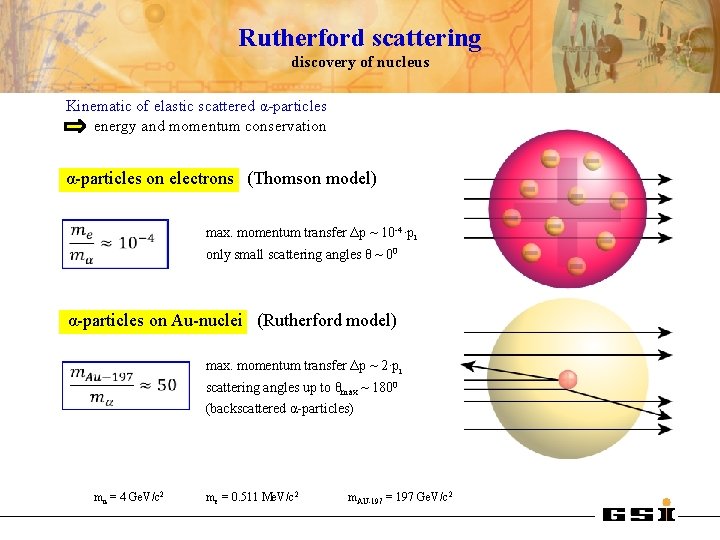
Rutherford scattering discovery of nucleus Kinematic of elastic scattered α-particles energy and momentum conservation α-particles on electrons (Thomson model) max. momentum transfer Δp ~ 10 -4·pi only small scattering angles θ ~ 00 α-particles on Au-nuclei (Rutherford model) max. momentum transfer Δp ~ 2·pi scattering angles up to θmax ~ 1800 (backscattered α-particles) mα = 4 Ge. V/c 2 me = 0. 511 Me. V/c 2 m. AU-197 = 197 Ge. V/c 2

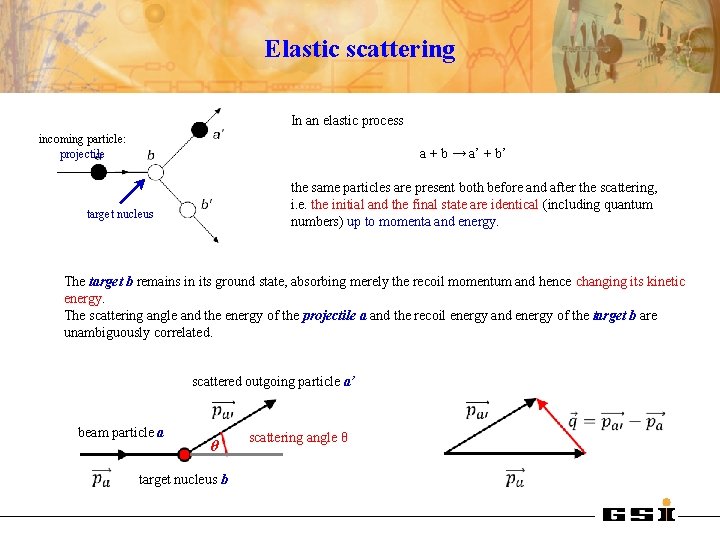
Elastic scattering In an elastic process incoming particle: projectile a + b → a’ + b’ the same particles are present both before and after the scattering, i. e. the initial and the final state are identical (including quantum numbers) up to momenta and energy. target nucleus The target b remains in its ground state, absorbing merely the recoil momentum and hence changing its kinetic energy. The scattering angle and the energy of the projectile a and the recoil energy and energy of the target b are unambiguously correlated. scattered outgoing particle a’ beam particle a θ target nucleus b scattering angle θ

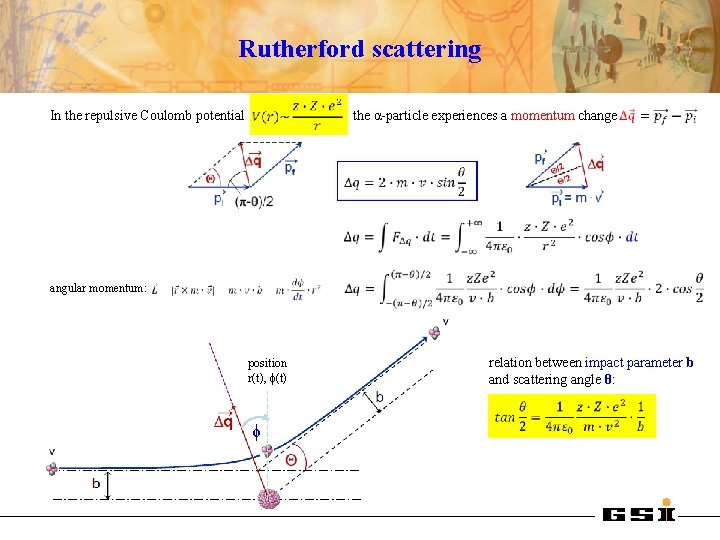
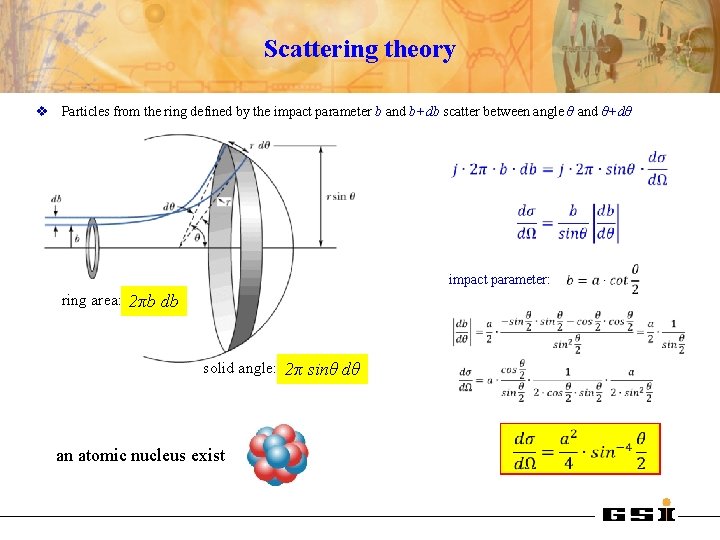
Rutherford scattering In the repulsive Coulomb potential the α-particle experiences a momentum change angular momentum: position r(t), ϕ(t) ϕ relation between impact parameter b and scattering angle θ:

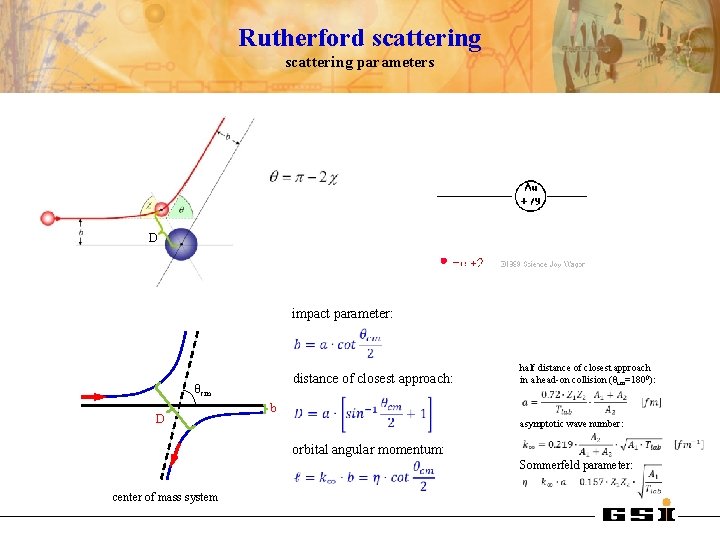
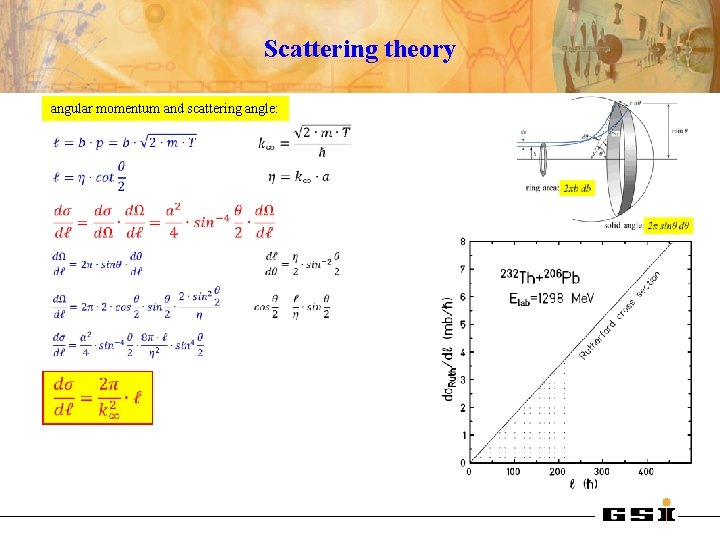
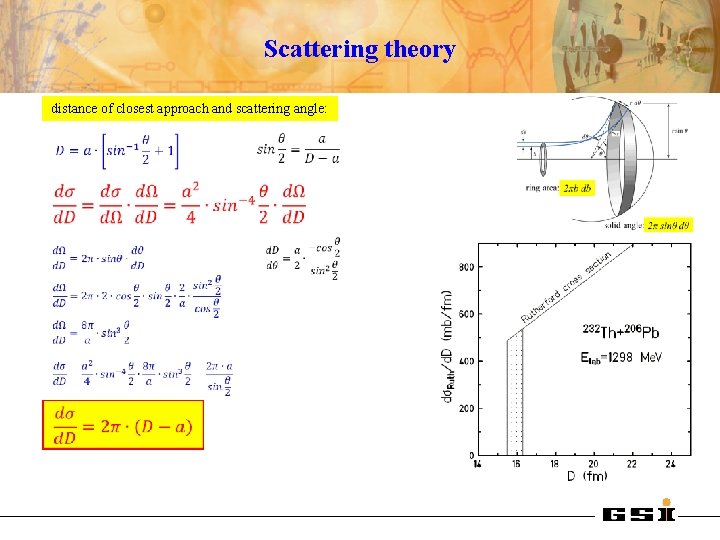
Rutherford scattering parameters D impact parameter: distance of closest approach: θcm D half distance of closest approach in a head-on collision (θcm=1800): b asymptotic wave number: orbital angular momentum: Sommerfeld parameter: center of mass system

Scattering theory v Particles from the ring defined by the impact parameter b and b+db scatter between angle θ and θ+dθ impact parameter: ring area: 2πb db solid angle: 2π sinθ dθ an atomic nucleus exist

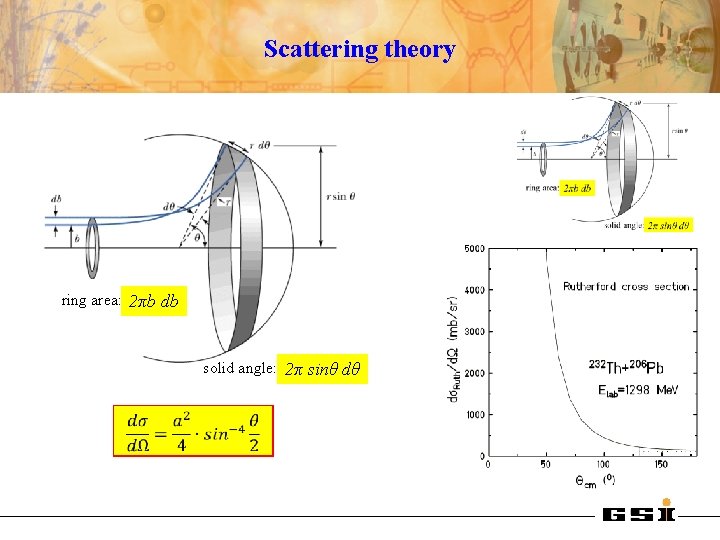
Scattering theory ring area: 2πb db solid angle: 2π sinθ dθ

Scattering theory angular momentum and scattering angle:

Scattering theory distance of closest approach and scattering angle:

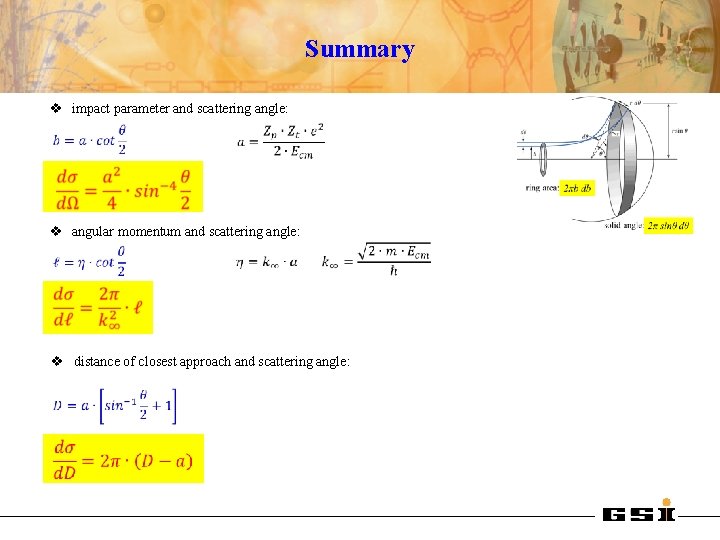
Summary v impact parameter and scattering angle: v angular momentum and scattering angle: v distance of closest approach and scattering angle:

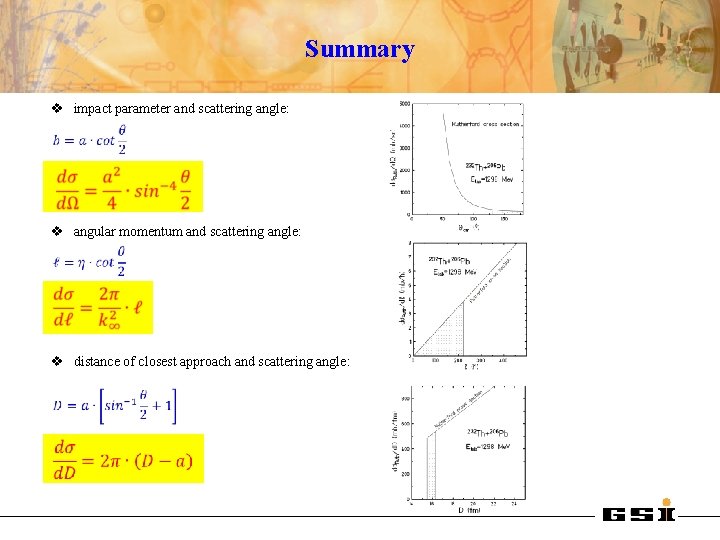
Summary v impact parameter and scattering angle: v angular momentum and scattering angle: v distance of closest approach and scattering angle:

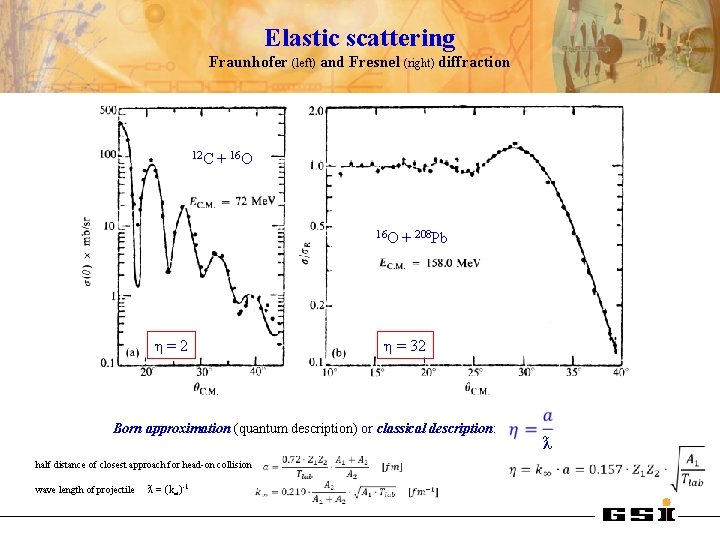
Elastic scattering Fraunhofer (left) and Fresnel (right) diffraction 12 C + 16 O η=2 + 208 Pb η = 32 Born approximation (quantum description) or classical description: half distance of closest approach for head-on collision wave length of projectile ƛ = (k∞)-1 ƛ

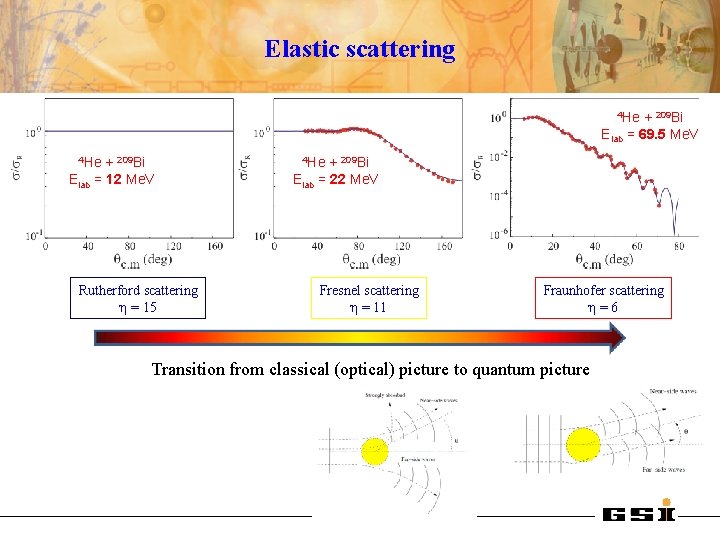
Elastic scattering 4 He + 209 Bi Elab = 69. 5 Me. V 4 He + 209 Bi Elab = 12 Me. V Rutherford scattering η = 15 4 He + 209 Bi Elab = 22 Me. V Fresnel scattering η = 11 Fraunhofer scattering η=6 Transition from classical (optical) picture to quantum picture

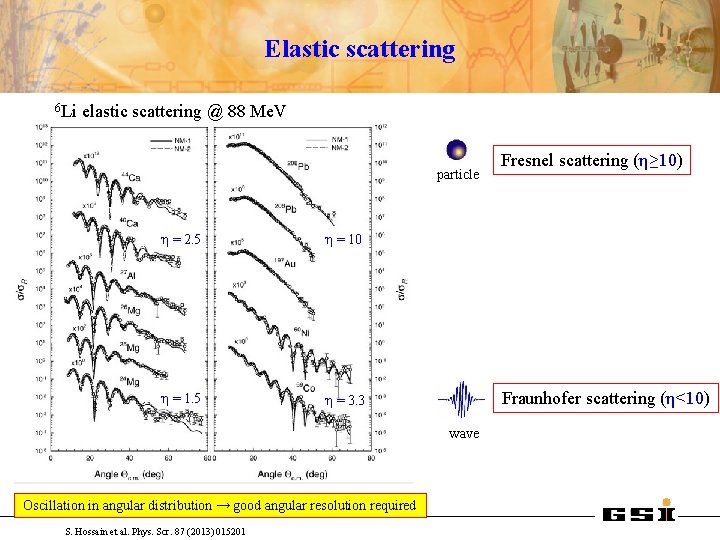
Elastic scattering 6 Li elastic scattering @ 88 Me. V particle η = 2. 5 η = 10 η = 1. 5 η = 3. 3 Fraunhofer scattering (η<10) wave Oscillation in angular distribution → good angular resolution required S. Hossain et al. Phys. Scr. 87 (2013) 015201 Fresnel scattering (η≥ 10)

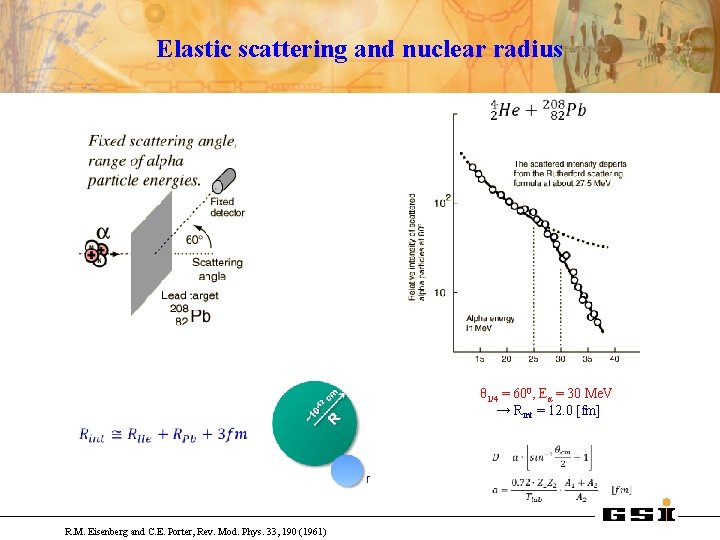
Elastic scattering and nuclear radius θ 1/4 = 600, Eα = 30 Me. V → Rint = 12. 0 [fm] R. M. Eisenberg and C. E. Porter, Rev. Mod. Phys. 33, 190 (1961)

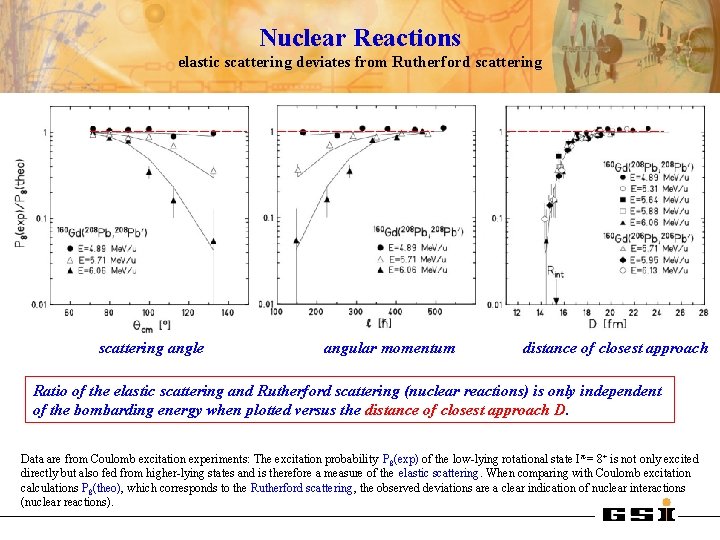
Nuclear Reactions elastic scattering deviates from Rutherford scattering angle angular momentum distance of closest approach Ratio of the elastic scattering and Rutherford scattering (nuclear reactions) is only independent of the bombarding energy when plotted versus the distance of closest approach D. Data are from Coulomb excitation experiments: The excitation probability P 8(exp) of the low-lying rotational state I π = 8+ is not only excited directly but also fed from higher-lying states and is therefore a measure of the elastic scattering. When comparing with Coulomb excitation calculations P 8(theo), which corresponds to the Rutherford scattering, the observed deviations are a clear indication of nuclear interactions (nuclear reactions).

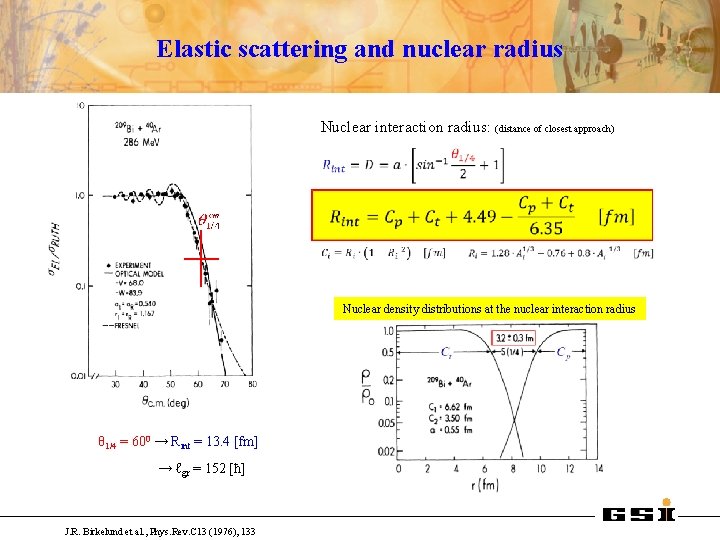
Elastic scattering and nuclear radius Nuclear interaction radius: (distance of closest approach) Nuclear density distributions at the nuclear interaction radius θ 1/4 = 600 → Rint = 13. 4 [fm] → ℓgr = 152 [ħ] J. R. Birkelund et al. , Phys. Rev. C 13 (1976), 133

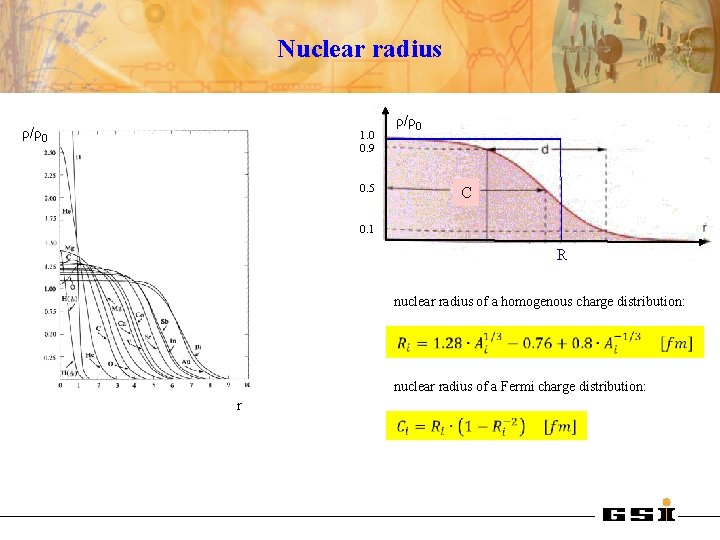
Nuclear radius ρ/ρ0 1. 0 0. 9 0. 5 ρ/ρ0 C 0. 1 R nuclear radius of a homogenous charge distribution: nuclear radius of a Fermi charge distribution: r

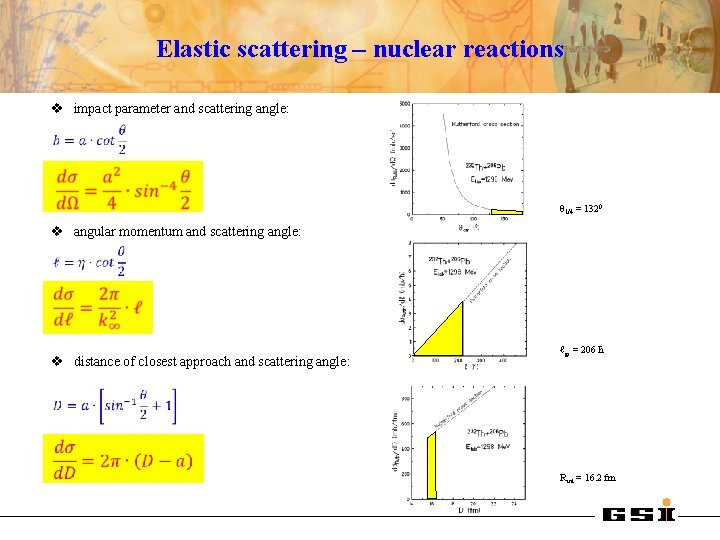
Elastic scattering – nuclear reactions v impact parameter and scattering angle: θ 1/4 = 1320 v angular momentum and scattering angle: v distance of closest approach and scattering angle: ℓgr = 206 ħ Rint = 16. 2 fm

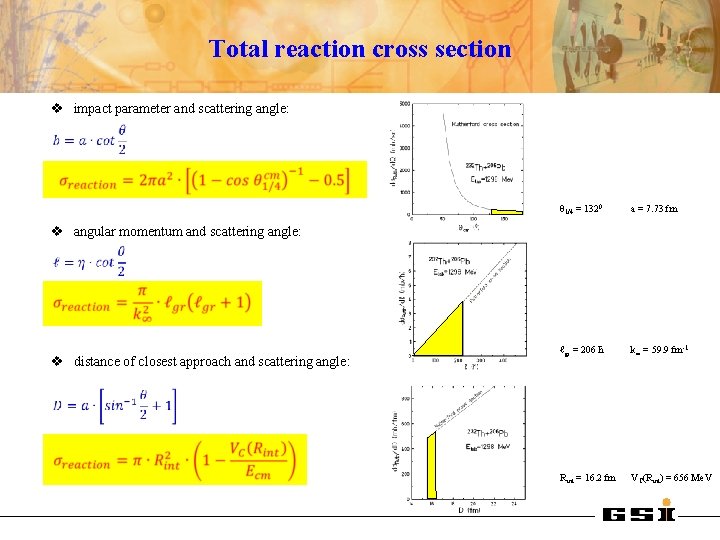
Total reaction cross section v impact parameter and scattering angle: θ 1/4 = 1320 a = 7. 73 fm ℓgr = 206 ħ k∞ = 59. 9 fm-1 Rint = 16. 2 fm VC(Rint) = 656 Me. V v angular momentum and scattering angle: v distance of closest approach and scattering angle:

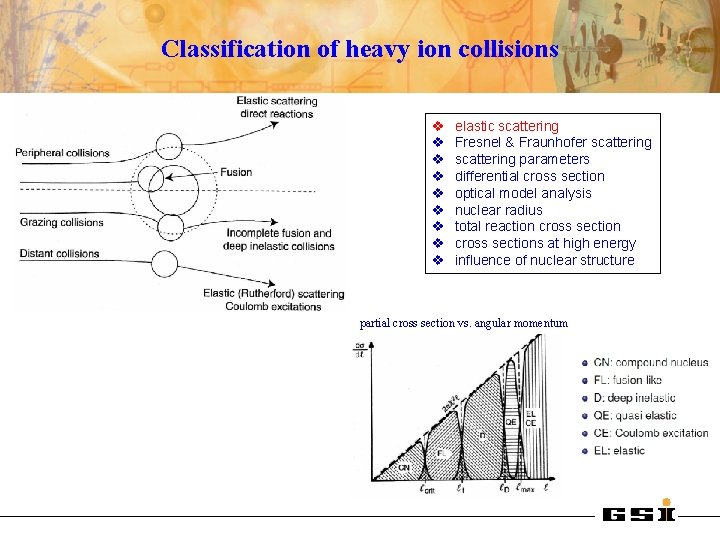
Classification of heavy ion collisions v v v v v elastic scattering Fresnel & Fraunhofer scattering parameters differential cross section optical model analysis nuclear radius total reaction cross sections at high energy influence of nuclear structure partial cross section vs. angular momentum

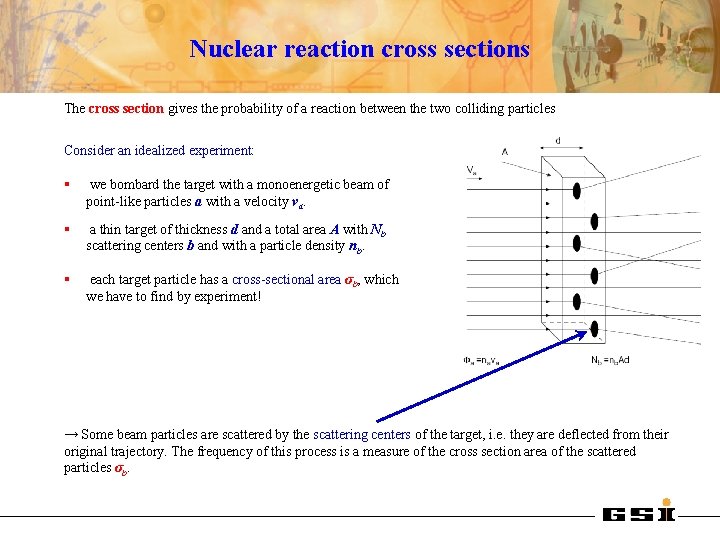
Nuclear reaction cross sections The cross section gives the probability of a reaction between the two colliding particles Consider an idealized experiment: § we bombard the target with a monoenergetic beam of point-like particles a with a velocity va. § a thin target of thickness d and a total area A with Nb scattering centers b and with a particle density nb. § each target particle has a cross-sectional area σb, which we have to find by experiment! → Some beam particles are scattered by the scattering centers of the target, i. e. they are deflected from their original trajectory. The frequency of this process is a measure of the cross section area of the scattered particles σb.

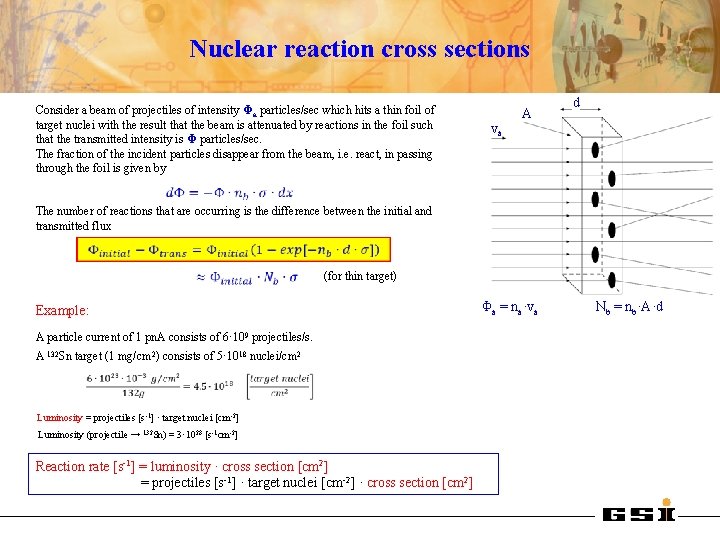
Nuclear reaction cross sections Consider a beam of projectiles of intensity Φa particles/sec which hits a thin foil of target nuclei with the result that the beam is attenuated by reactions in the foil such that the transmitted intensity is Φ particles/sec. The fraction of the incident particles disappear from the beam, i. e. react, in passing through the foil is given by va A d The number of reactions that are occurring is the difference between the initial and transmitted flux (for thin target) Example: A particle current of 1 pn. A consists of 6· 109 projectiles/s. A 132 Sn target (1 mg/cm 2) consists of 5· 1018 nuclei/cm 2 Luminosity = projectiles [s-1] · target nuclei [cm -2] Luminosity (projectile → 132 Sn) = 3· 1028 [s-1 cm-2] Reaction rate [s-1] = luminosity · cross section [cm 2] = projectiles [s-1] · target nuclei [cm-2] · cross section [cm 2] Φa = na·va Nb = nb·A·d
 Non elastic scattering
Non elastic scattering Rutherford scattering
Rutherford scattering Scattering di rutherford
Scattering di rutherford Lacunae in bone
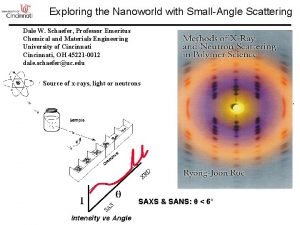
Lacunae in bone Small angle scattering
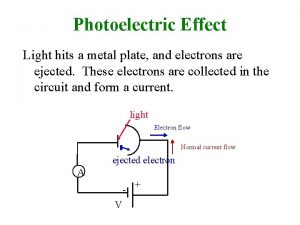
Small angle scattering Photoelectric effect สรุป
Photoelectric effect สรุป Scattering matrix
Scattering matrix Rayleigh vs mie
Rayleigh vs mie Double parton scattering
Double parton scattering Double scattering
Double scattering Rayleigh scattering formula
Rayleigh scattering formula Diffraction and scattering
Diffraction and scattering Scattering matrix
Scattering matrix Dynamic light scattering 원리
Dynamic light scattering 원리 Scattering of light in suspension
Scattering of light in suspension Compton effect
Compton effect Elastic energy
Elastic energy Pauli blocking of light scattering in degenerate fermions
Pauli blocking of light scattering in degenerate fermions Mie scattering vs rayleigh
Mie scattering vs rayleigh Scattering efficiency
Scattering efficiency Contoh refraksi gelombang
Contoh refraksi gelombang Horizontal
Horizontal Liquid crystal display
Liquid crystal display Light scattering
Light scattering Scattering in a central force field
Scattering in a central force field Mie plot
Mie plot Reciprocal network
Reciprocal network