Drug resistance motifs HBV Phenotypic resistance Clinical resistance


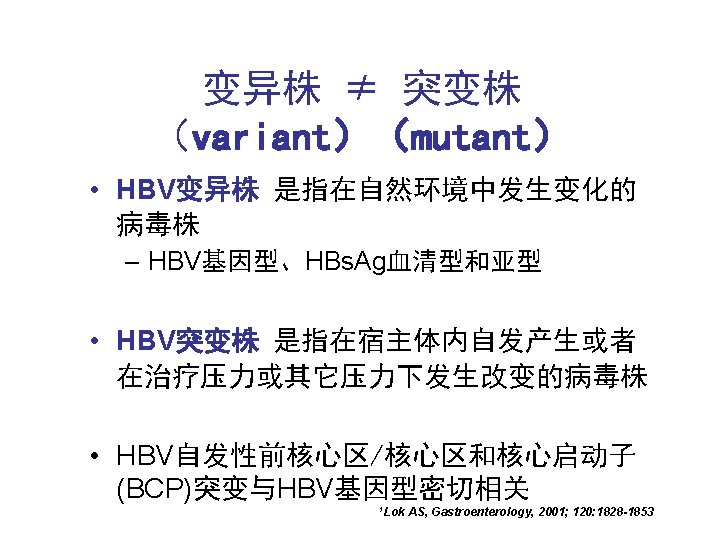


耐药分析 • 耐药基序 (Drug resistance motifs) • HBV表型耐药 (Phenotypic resistance) • 临床耐药 (Clinical resistance) 三者相关,但不完全等同
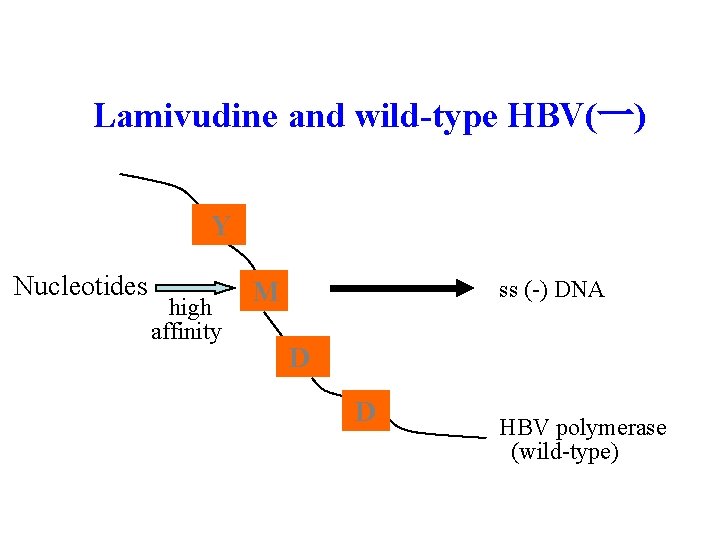
Lamivudine and wild-type HBV(一) Y Nucleotides high affinity ss (-) DNA M D D HBV polymerase (wild-type)
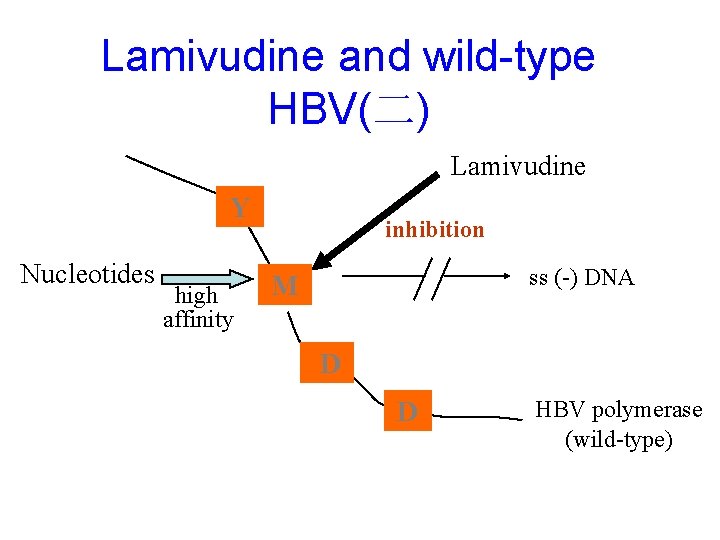
Lamivudine and wild-type HBV(二) Lamivudine Y Nucleotides high affinity inhibition ss (-) DNA M D D HBV polymerase (wild-type)
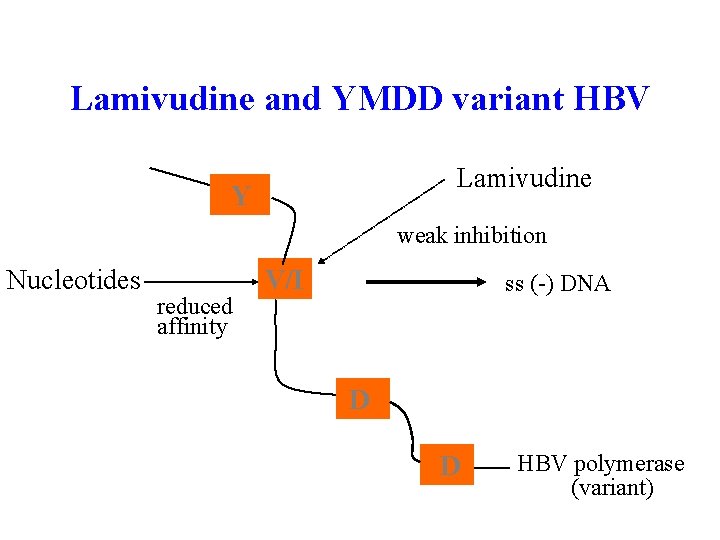
Lamivudine and YMDD variant HBV Lamivudine Y weak inhibition Nucleotides reduced affinity V/I ss (-) DNA D D HBV polymerase (variant)


耐药变异的检测方法 • Sequences analysis • Line probe assay • PCR-RFLP analysis (Restriction fragment length polymorphism) • Real-time PCR • Molecular beacons • DNA chip
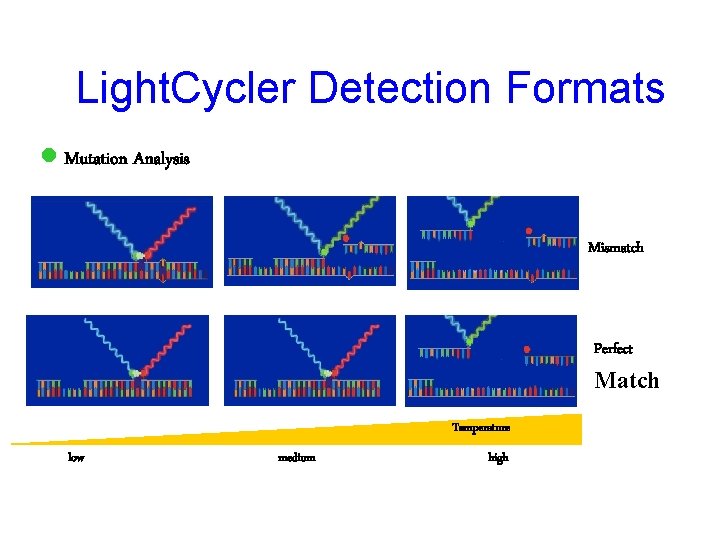
Light. Cycler Detection Formats l Mutation Analysis Mismatch Perfect Match Temperature low medium high
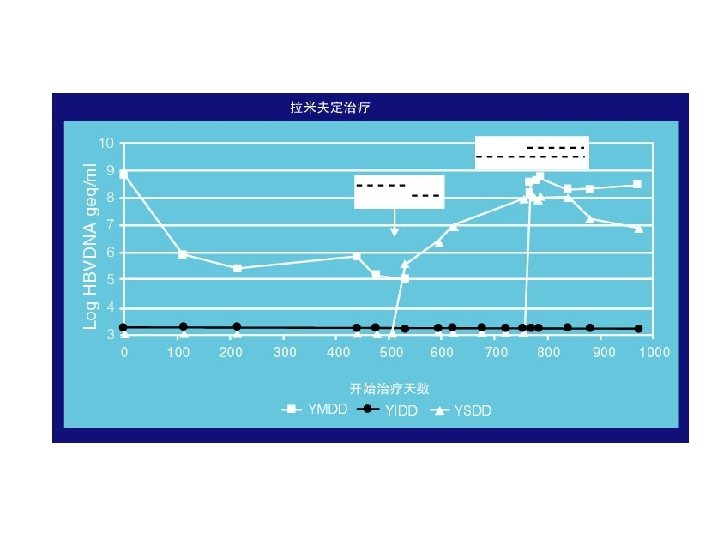
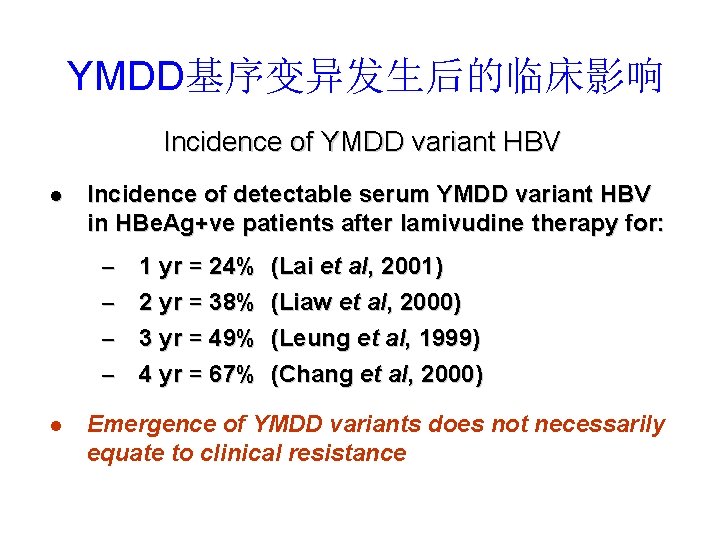
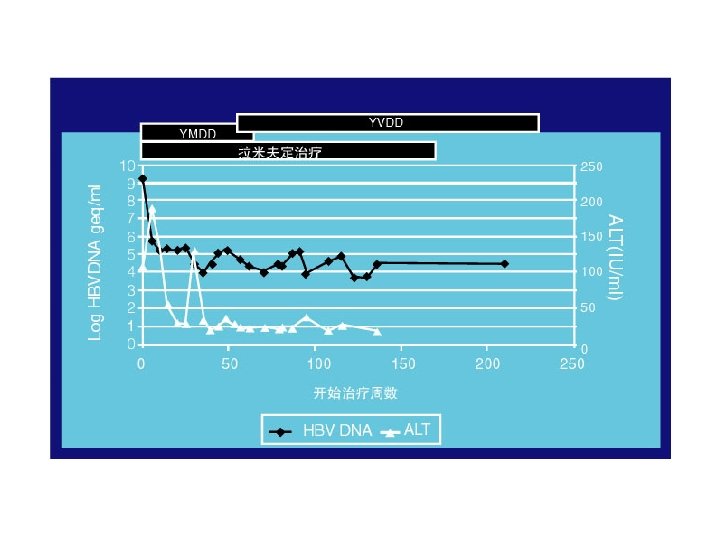
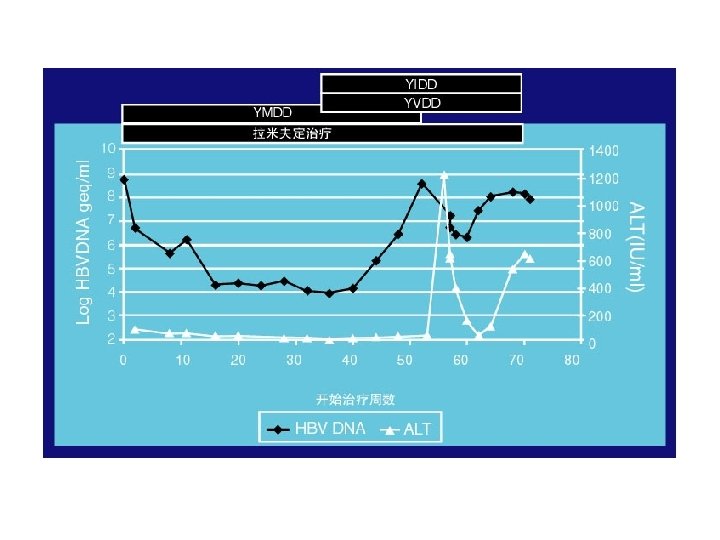
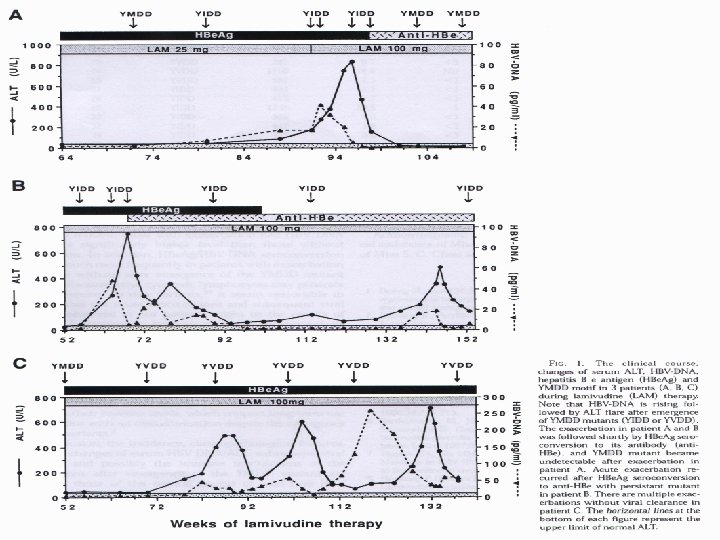
YMDD基序变异发生后的临床影响 Incidence of YMDD variant HBV l Incidence of detectable serum YMDD variant HBV in HBe. Ag+ve patients after lamivudine therapy for: – – l 1 yr = 24% (Lai et al, 2001) 2 yr = 38% (Liaw et al, 2000) 3 yr = 49% (Leung et al, 1999) 4 yr = 67% (Chang et al, 2000) Emergence of YMDD variants does not necessarily equate to clinical resistance

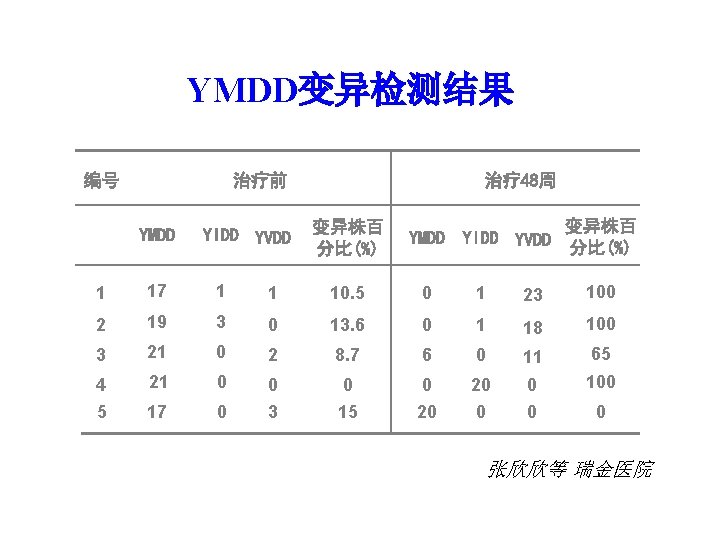
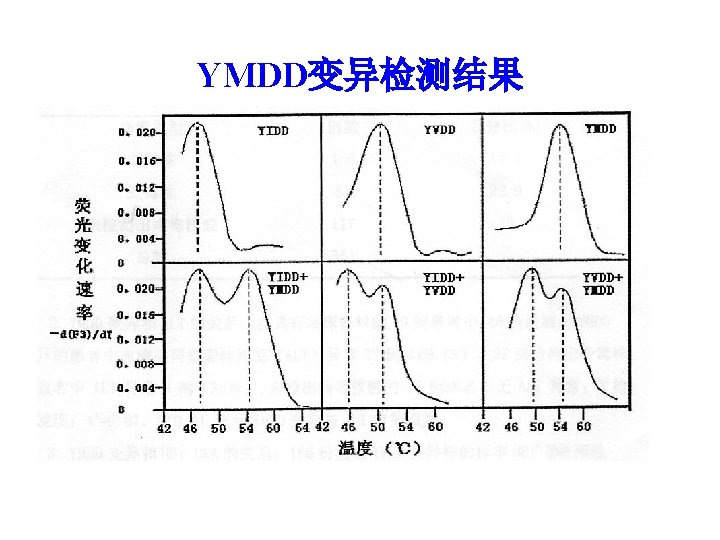
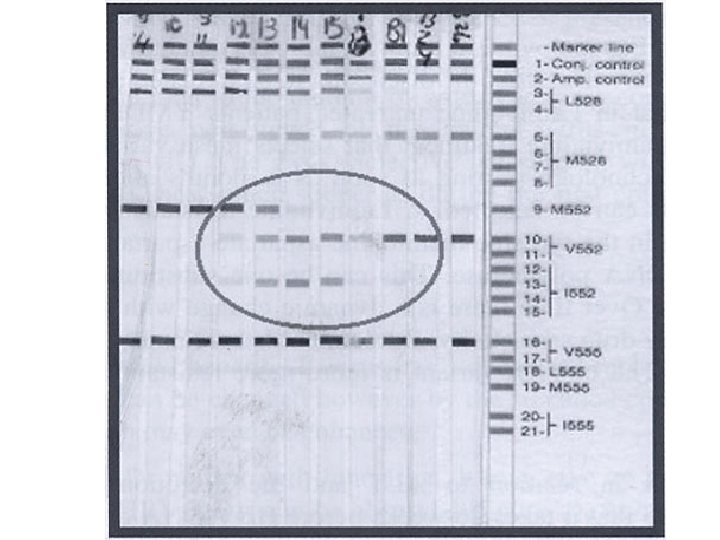
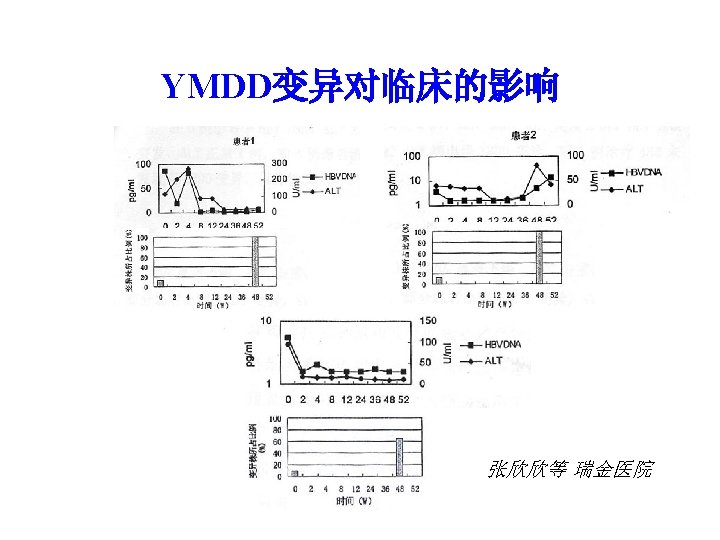
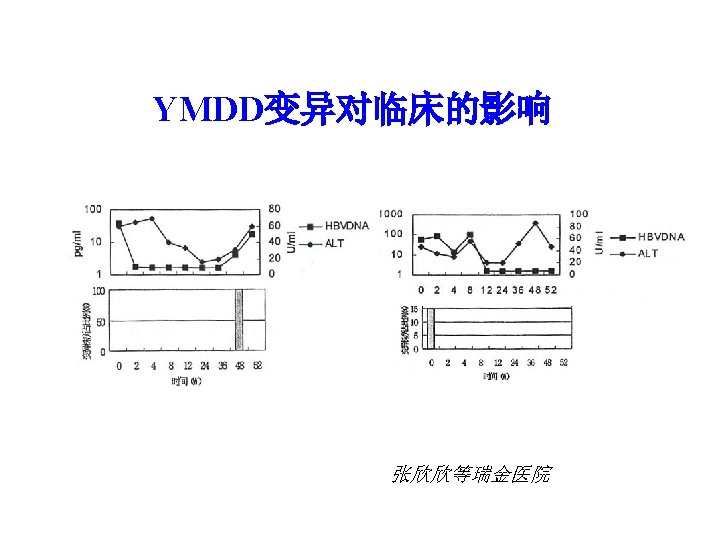
YMDD变异检测结果 编号 治疗前 YMDD YIDD YVDD 治疗 48周 变异株百 分比(%) YMDD YIDD YVDD 变异株百 分比(%) 1 17 1 1 10. 5 0 1 23 100 2 19 3 0 13. 6 0 1 18 100 3 21 0 2 8. 7 6 0 11 65 4 21 0 0 20 0 100 5 17 0 3 15 20 0 张欣欣等 瑞金医院
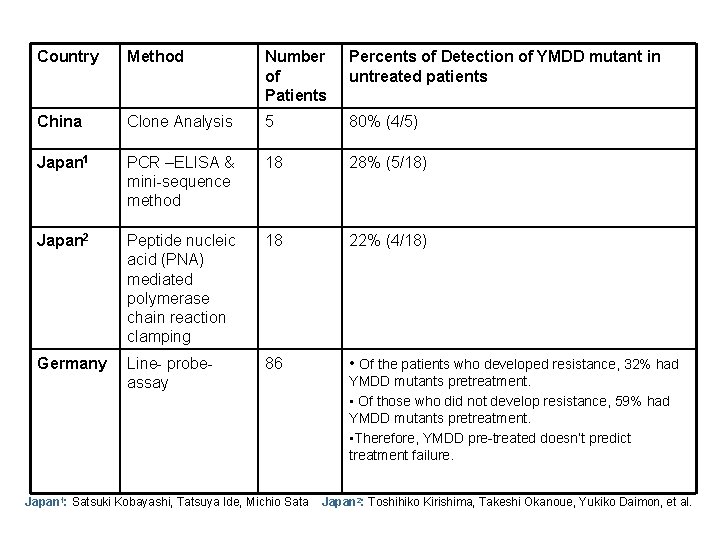
Country Method Number of Patients Percents of Detection of YMDD mutant in untreated patients China Clone Analysis 5 80% (4/5) Japan 1 PCR –ELISA & mini-sequence method 18 28% (5/18) Japan 2 Peptide nucleic acid (PNA) mediated polymerase chain reaction clamping 18 22% (4/18) Germany Line- probeassay 86 • Of the patients who developed resistance, 32% had Japan 1: Satsuki Kobayashi, Tatsuya Ide, Michio Sata YMDD mutants pretreatment. • Of those who did not develop resistance, 59% had YMDD mutants pretreatment. • Therefore, YMDD pre-treated doesn’t predict treatment failure. Japan 2: Toshihiko Kirishima, Takeshi Okanoue, Yukiko Daimon, et al.

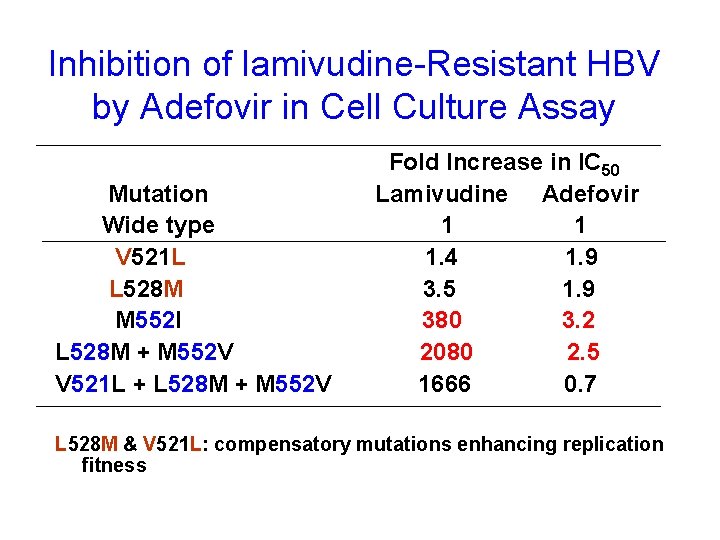
Inhibition of lamivudine-Resistant HBV by Adefovir in Cell Culture Assay Mutation Wide type V 521 L L 528 M M 552 I L 528 M + M 552 V V 521 L + L 528 M + M 552 V Fold Increase in IC 50 Lamivudine Adefovir 1 1 1. 4 1. 9 3. 5 1. 9 380 3. 2 2080 2. 5 1666 0. 7 L 528 M & V 521 L: compensatory mutations enhancing replication fitness
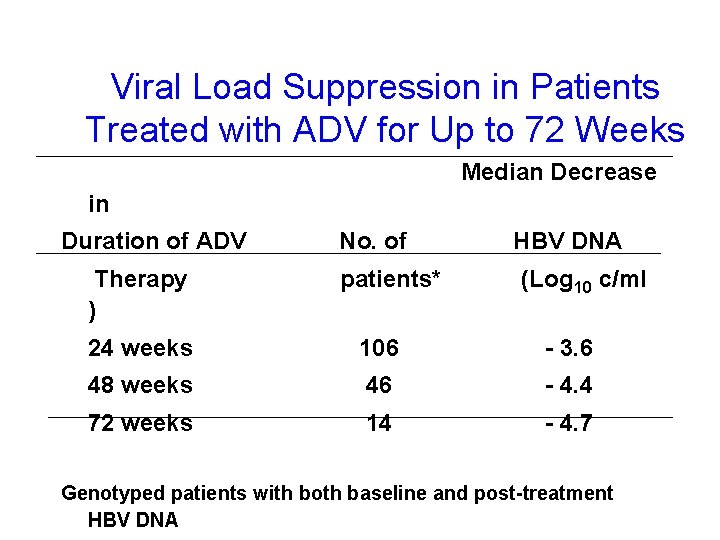
Viral Load Suppression in Patients Treated with ADV for Up to 72 Weeks Median Decrease in Duration of ADV Therapy ) No. of patients* HBV DNA (Log 10 c/ml 24 weeks 106 - 3. 6 48 weeks 46 - 4. 4 72 weeks 14 - 4. 7 Genotyped patients with both baseline and post-treatment HBV DNA

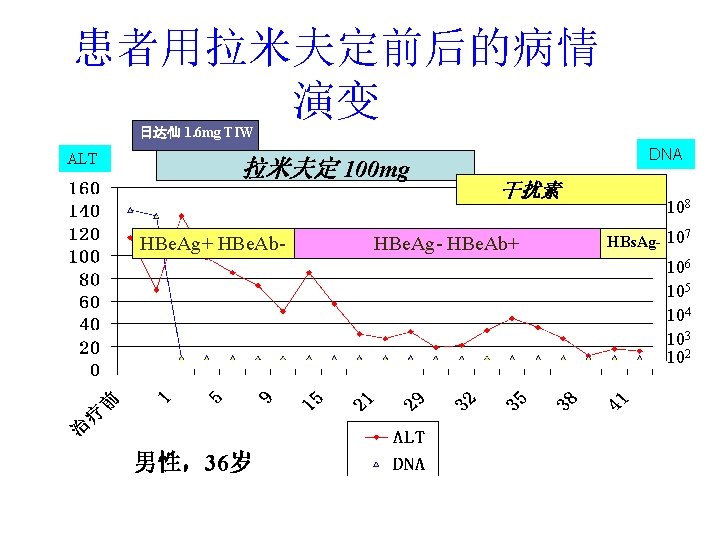
患者用拉米夫定前后的病情 演变 日达仙 1. 6 mg TIW ALT 拉米夫定 100 mg HBe. Ag+ HBe. Ab- DNA 干扰素 HBe. Ag- HBe. Ab+ 108 7 HBs. Ag- 10 106 105 104 103 102 男性,36岁


- Slides: 31