HIV Drug Resistance Training Module 1 Introduction to




































- Slides: 68

HIV Drug Resistance Training Module 1: Introduction to HIV Drug Resistance 1

Topics Impact of HIV Drug Resistance Factors that Influence Development of Drug Resistance § How to Minimize Drug Resistance § How to Respond to Detection of Drug Resistance § Drug Resistance Assays § § 2

Objectives § § § Understand importance of measuring drug resistance for large-scale treatment programs. Identify factors that influence drug resistance. Understand the methods available for the measuring HIV drug resistance. Identify strategies for minimizing development of drug resistance. Identify strategies for responding to detection of moderate to high levels of drug resistant HIV. 3

impact of drug resistance Why is it important to measure drug resistance? How does it impact the success of large-scale treatment programs? 4

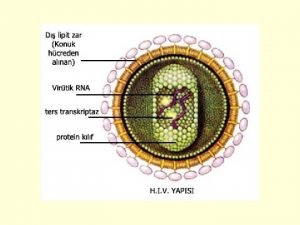
What is Antiviral Drug Resistance? § § Drugs no longer block virus replication Cause: – Mutations in the viral genome § One or more: – Specific for an antiviral drug OR – Affecting related drugs (cross-resistance) § How much resistance? Which drugs? – Depends on type and number of mutations 5

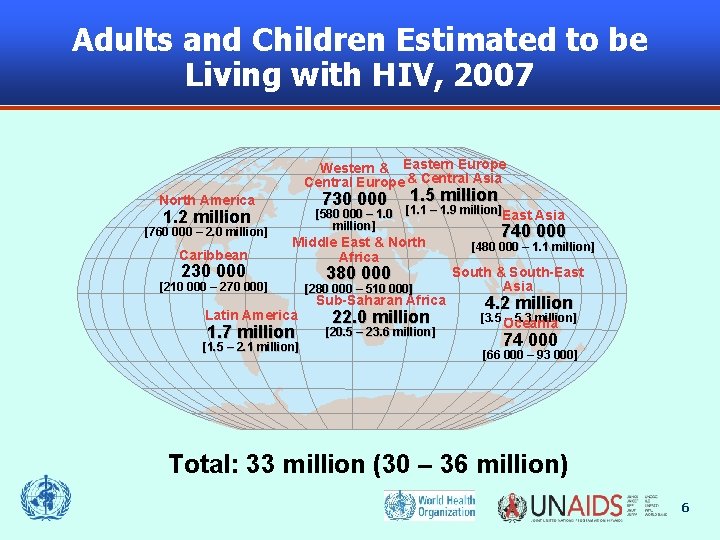
Adults and Children Estimated to be Living with HIV, 2007 Western & Eastern Europe Central Europe & Central Asia 730 000 North America 1. 2 million [760 000 – 2. 0 million] Caribbean 230 000 [580 000 – 1. 0 million] 1. 5 million [1. 1 – 1. 9 million]East Asia Middle East & North Africa [210 000 – 270 000] Latin America 1. 7 million [1. 5 – 2. 1 million] 380 000 [280 000 – 510 000] Sub-Saharan Africa 22. 0 million [20. 5 – 23. 6 million] 740 000 [480 000 – 1. 1 million] South & South-East Asia 4. 2 million [3. 5 Oceania – 5. 3 million] 74 000 [66 000 – 93 000] Total: 33 million (30 – 36 million) 6

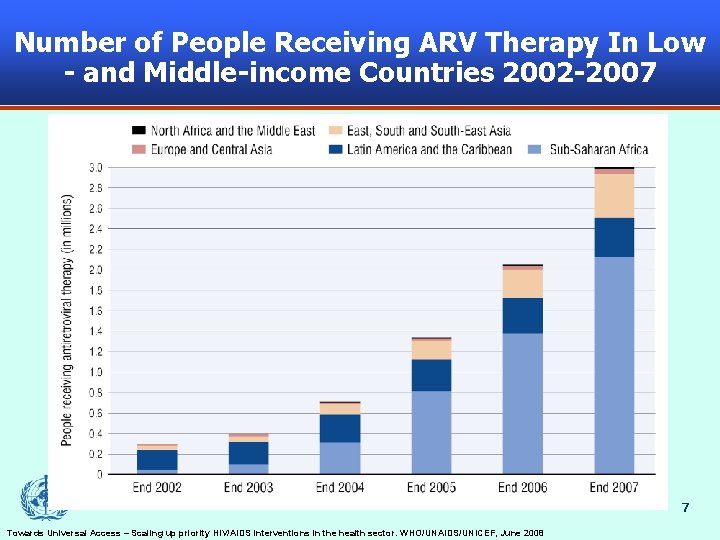
Number of People Receiving ARV Therapy In Low - and Middle-income Countries 2002 -2007 7 Towards Universal Access – Scaling up priority HIV/AIDS interventions in the health sector. WHO/UNAIDS/UNICEF, June 2008

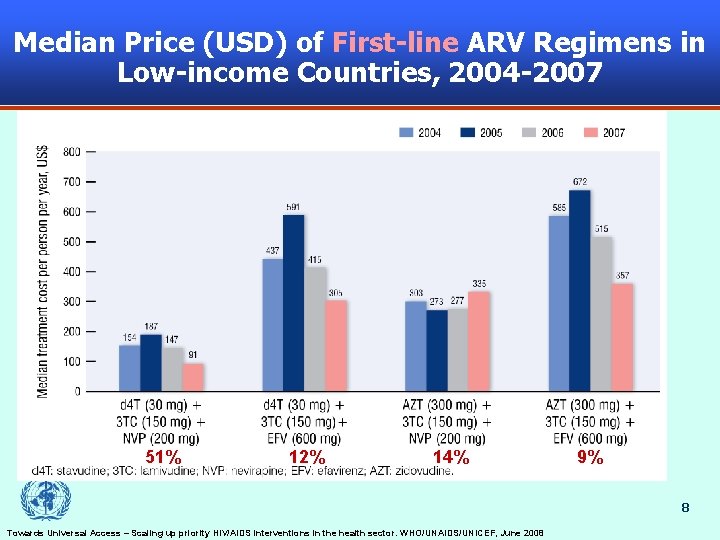
Median Price (USD) of First-line ARV Regimens in Low-income Countries, 2004 -2007 51% 12% 14% 9% 8 Towards Universal Access – Scaling up priority HIV/AIDS interventions in the health sector. WHO/UNAIDS/UNICEF, June 2008

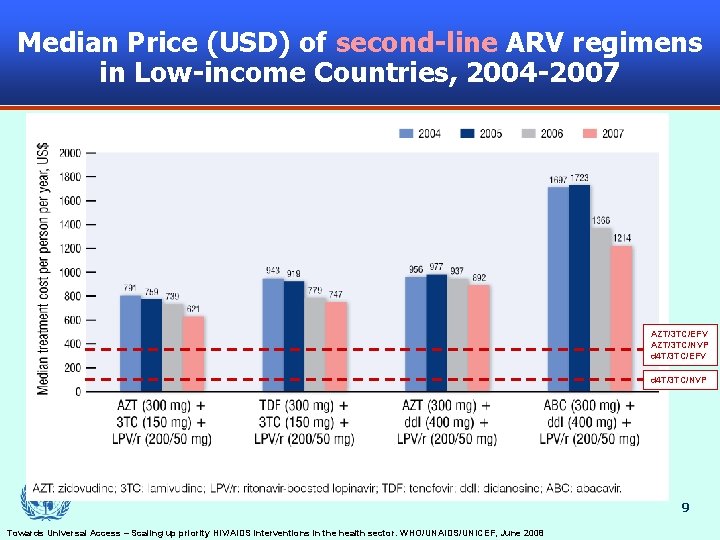
Median Price (USD) of second-line ARV regimens in Low-income Countries, 2004 -2007 AZT/3 TC/EFV AZT/3 TC/NVP d 4 T/3 TC/EFV d 4 T/3 TC/NVP 9 Towards Universal Access – Scaling up priority HIV/AIDS interventions in the health sector. WHO/UNAIDS/UNICEF, June 2008

HIV Drug Resistance is Inevitable § HIV DR is an inevitable consequence of ART, influenced by: – – – – § Ability of regimens to suppress replication completely Adherence and tolerability of regimens "Genetic barrier" to resistance Relative fitness of resistant variant(s) Pharmacokinetics (IQ) Availability/continuity of drug supply Removal of barriers to access to care Therefore, efforts to minimize HIVDR should be focused on these factors 10

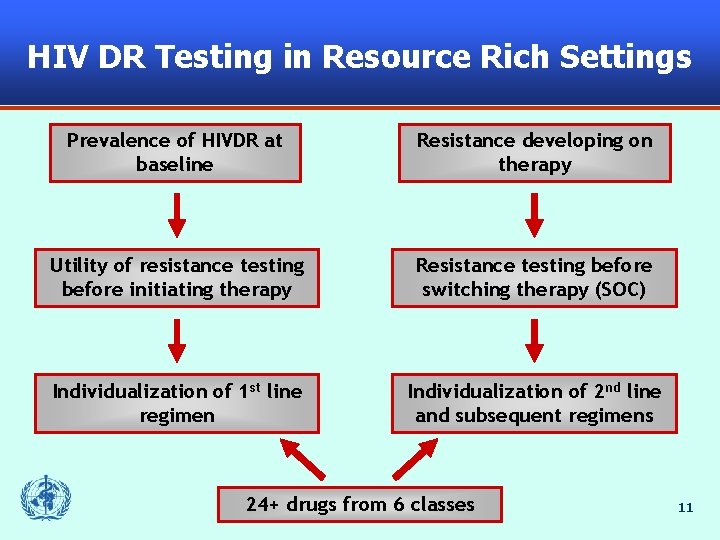
HIV DR Testing in Resource Rich Settings Prevalence of HIVDR at baseline Resistance developing on therapy Utility of resistance testing before initiating therapy Resistance testing before switching therapy (SOC) Individualization of 1 st line regimen Individualization of 2 nd line and subsequent regimens 24+ drugs from 6 classes 11

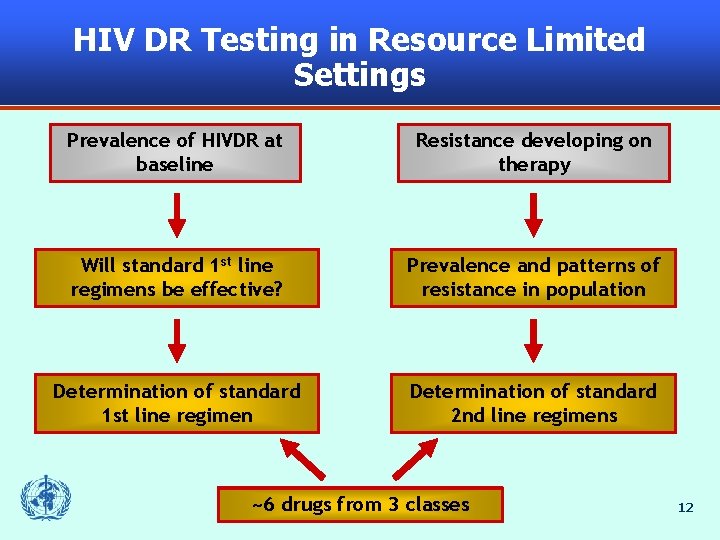
HIV DR Testing in Resource Limited Settings Prevalence of HIVDR at baseline Resistance developing on therapy Utility Willof standard resistance 1 st line testing before regimens initiating be effective? therapy Prevalence Resistance and testing patterns before of resistance switching therapy in population (SOC) Individualization Determination ofof standard 1 st line regimen Individualization Determination ofof standard 2 nd line and 2 nd subsequent line regimens 24+ drugs from 36 classes ~6 drugs 12

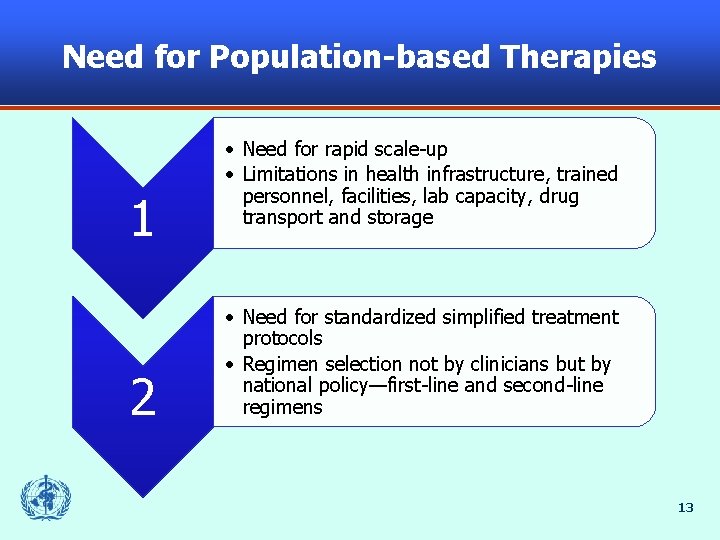
Need for Population-based Therapies 1 2 • Need for rapid scale-up • Limitations in health infrastructure, trained personnel, facilities, lab capacity, drug transport and storage • Need for standardized simplified treatment protocols • Regimen selection not by clinicians but by national policy—first-line and second-line regimens 13

Need to Maintain Effectiveness 1 2 • Limited number of regimens available • Need to minimize drug resistance 14

Drug Resistance and HIV… § evolves rapidly within human body § has a high replication rate § has a high mutation rate § Resistant strains can emerge within days if drug pressure is not sufficient to suppress replication. § Resistant strains persist indefinitely and can reemerge if same drugs are stopped and restarted (even if they are not detected by standard resistance assays). 15

Review § § Why is it important to measure drug resistance? How does it impact the success of large-scale treatment programs? 16

factors that influence development of drug resistance What regimens influence drug resistance? What patient factors influence drug resistance? What public health approaches influence drug resistance? 17

In Which Conditions is DR More Likely? § § § Treatment with <3 drugs Inappropriate selection of drugs Adding one drug to a failing regimen Interruption of treatment (even for a few days) Prolonging a failing regimen 18

In Which Conditions is DR Less Likely? Medication Factors All patients treated with 3 or more drugs Use of appropriate drug regimens Can reliably suppress HIV replication to levels of <50 copies/ml § Use of fixed-dose combinations to support adherence § § § 19

In Which Conditions is DR Less Likely? Systems Factors § § § Limited number of regimens Trained personnel, low turnover Supervision and monitoring Adequate lab services Drug supply and delivery systems 20

In Which Conditions is DR Less Likely? Patient Factors Adherence to treatment regimen Avoiding interruption of treatment, even if only a few days § Regular follow-up (going to clinic) § Staying on uninterrupted first-line ART as long as possible § § CONTINUITY! 21

Programmatic Factors Affecting Patient Compliance Cost of treatment to patient Distance patient must travel to get treatment Supply interruptions Availability of second-line regimens for patients whose first-line regimens fail § Timing of use of second-line regimens § § 22

Discussion § § § What regimens influence drug resistance? What patient factors influence drug resistance? What public health approaches influence drug resistance? 23

Reflection § § What regimens do we use in our country? What systematic and programmatic challenges do we face? 24

how to minimize drug resistance What can countries do to minimize or suppress drug resistance? 25

Elements of a National HIVDR Prevention and Assessment Strategy A. B. C. D. E. F. G. H. Development of a national HIVDR Working Group, five year plan and budget Regular assessment of HIVDR early warning indicators (EWI) from all ART sites (or representative sites) Surveys to monitor HIVDR and associated factors in sentinel ART sites HIVDR transmission threshold surveys in geographic areas where ART has been widespread for > 3 years HIVDR database development Designation of an in-country or regional WHO-accredited HIVDR genotyping laboratory HIVDR minimization activities review and support Preparation of annual HIVDR report and recommendations; use of data for ART and planning 26 Bennett, Bertagnolio, Sutherland Gilks, Antiviral Therapy 13 Suppl 2: 1– 13, 2008

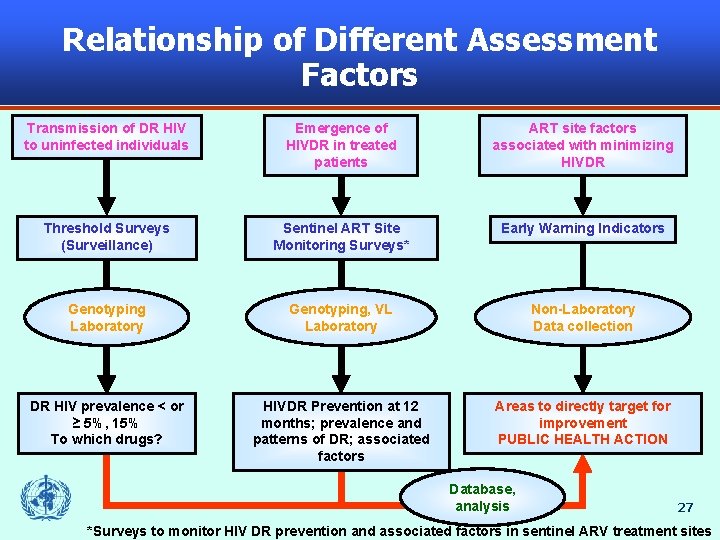
Relationship of Different Assessment Factors Transmission of DR HIV to uninfected individuals Emergence of HIVDR in treated patients ART site factors associated with minimizing HIVDR Threshold Surveys (Surveillance) Sentinel ART Site Monitoring Surveys* Early Warning Indicators Genotyping Laboratory Genotyping, VL Laboratory Non-Laboratory Data collection DR HIV prevalence < or ≥ 5%, 15% To which drugs? HIVDR Prevention at 12 months; prevalence and patterns of DR; associated factors Areas to directly target for improvement PUBLIC HEALTH ACTION Database, analysis 27 *Surveys to monitor HIV DR prevention and associated factors in sentinel ARV treatment sites

General WHO Strategies (1 of 2) § § § Strengthen existing programmes that minimize HIVDR Form national HIV drug resistance working group Monitor Early Warning Indicators Survey to monitor HIVDR prevention and associated factors in sentinel ART sites Watch for transmitted HIVDR in recently infected individuals 28

General Strategies (2 of 2) Designate one or more genotyping labs for HIVDR surveillance and monitoring § Maintain a national database to hold the HIV sequences collected through surveillance, monitoring, and research projects § Produce an annual report summarizing results from all the above § 29

Strengthen Existing Programmes That Minimize HIVDR § § § Support for adherence and follow-up Removal of barriers to ART access Drug supply continuity at the individual, ART site, and national levels 30

Form National HIV Drug Resistance Working Group § § § Develop HIVDR prevention, surveillance and monitoring plan Make evidence-based recommendations for HIVDR prevention Includes epidemiologists, clinicians, researchers, and laboratorians Collect indicators and implement surveys Develop evaluation strategies 31

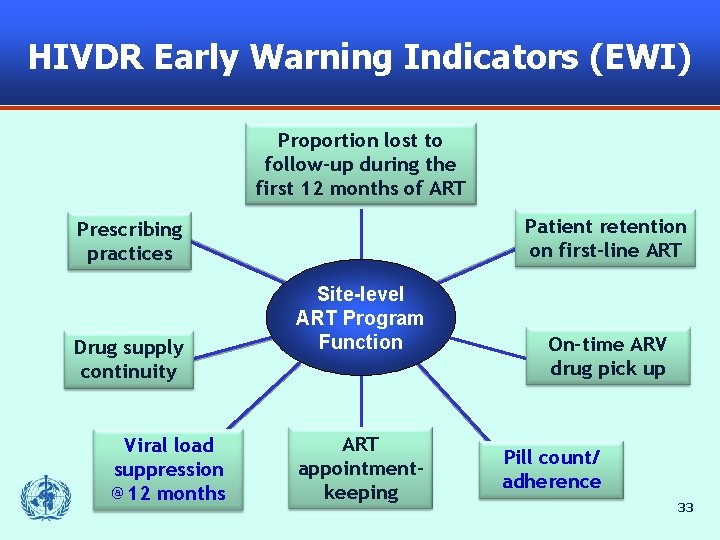
Monitor Early Warning Indicators § § Routine collection of medical and pharmacy records Monitor factors associated with HIVDR prevention or emergence – Extent to which prescribing practices meet national and international guidelines – % of patients still on first-line; % lost to follow-up – % patients with timely medication pick up and clinical follow-up – Drug supply continuity at site – Adherence and viral load, if feasible 32

HIVDR Early Warning Indicators (EWI) Proportion lost to follow-up during the first 12 months of ART Patient retention on first-line ART Prescribing practices Drug supply continuity Viral load suppression @12 months Site-level ART Program Function ART appointmentkeeping On-time ARV drug pick up Pill count/ adherence 33

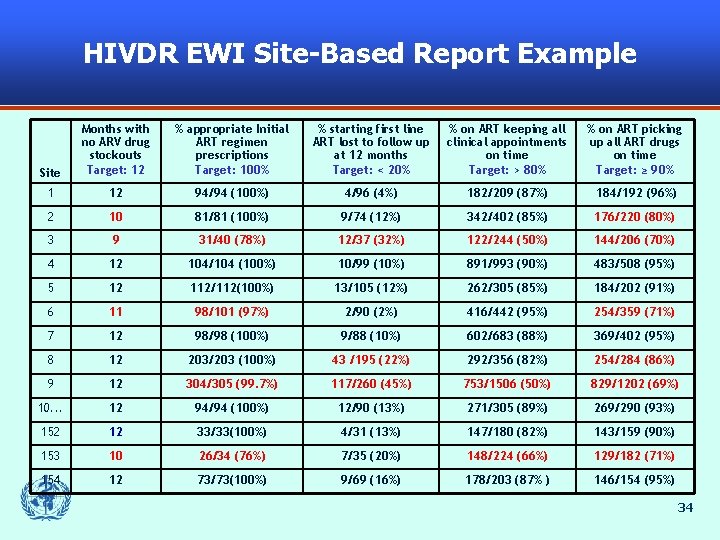
HIVDR EWI Site-Based Report Example Site Months with no ARV drug stockouts Target: 12 % appropriate Initial ART regimen prescriptions Target: 100% % starting first line ART lost to follow up at 12 months Target: < 20% % on ART keeping all clinical appointments on time Target: > 80% % on ART picking up all ART drugs on time Target: ≥ 90% 1 12 94/94 (100%) 4/96 (4%) 182/209 (87%) 184/192 (96%) 2 10 81/81 (100%) 9/74 (12%) 342/402 (85%) 176/220 (80%) 3 9 31/40 (78%) 12/37 (32%) 122/244 (50%) 144/206 (70%) 4 12 104/104 (100%) 10/99 (10%) 891/993 (90%) 483/508 (95%) 5 12 112/112(100%) 13/105 (12%) 262/305 (85%) 184/202 (91%) 6 11 98/101 (97%) 2/90 (2%) 416/442 (95%) 254/359 (71%) 7 12 98/98 (100%) 9/88 (10%) 602/683 (88%) 369/402 (95%) 8 12 203/203 (100%) 43 /195 (22%) 292/356 (82%) 254/284 (86%) 9 12 304/305 (99. 7%) 117/260 (45%) 753/1506 (50%) 829/1202 (69%) 10. . . 12 94/94 (100%) 12/90 (13%) 271/305 (89%) 269/290 (93%) 152 12 33/33(100%) 4/31 (13%) 147/180 (82%) 143/159 (90%) 153 10 26/34 (76%) 7/35 (20%) 148/224 (66%) 129/182 (71%) 154 12 73/73(100%) 9/69 (16%) 178/203 (87% ) 146/154 (95%) 34

For More Information § See Bennett, Bertagnolio, Sutherland Gilks (2008). The World Health Organization’s global strategy for prevention and assessment of HIV drug resistance. Antiviral Therapy 13 Suppl 2: 113. 35

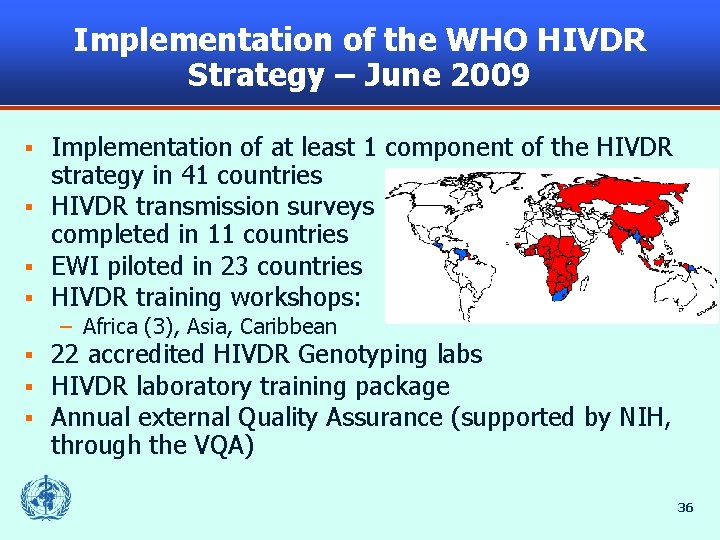
Implementation of the WHO HIVDR Strategy – June 2009 Implementation of at least 1 component of the HIVDR strategy in 41 countries § HIVDR transmission surveys completed in 11 countries § EWI piloted in 23 countries § HIVDR training workshops: § – Africa (3), Asia, Caribbean § § § 22 accredited HIVDR Genotyping labs HIVDR laboratory training package Annual external Quality Assurance (supported by NIH, through the VQA) 36

Other § Designate one or more genotyping labs for HIVDR surveillance and monitoring – The WHO offers accreditation, support, and training through the WHO Global HIVDR laboratory network Maintain a national database to hold the HIV sequences collected through surveillance, monitoring, and research projects § Produce an annual report summarizing results from all the above § 37

Discussion § What can countries do to minimize drug resistance? 38

Reflection § What are our highest-priority “next steps” for minimizing drug resistance? 39

how to respond to detection of drug resistance What can be done if widespread drug resistance is found? 40

Response to Detection of Transmitted Drug Resistance If the prevalence of transmitted resistance to important drugs or drug classes is 5 -15% or > 15%, surveys should be repeated in the original areas annually and extended to additional areas § Consider changing standard 1 st line regimen § Monitor Early Warning Indicators for clues about what can be improved at a programmatic level § 41

Response to Results from Monitoring Surveys If the patterns of resistance indicate reduced susceptibility to 2 nd line drugs, surveys should be repeated in the original areas annually and extended to additional areas. § Consider changing standard 2 nd line regimen. § Monitor Early Warning Indicators for clues about what can be improved at a programmatic level. § 42

hiv drug resistance assays What are the different types of resistance assays? What are the advantages and limitations of these assays? What results can we expect from these tests? What if the results from one type of test are inconsistent with results from another? 43

Clinical Use of Resistance Data Resistance tests are most accurate in assessing resistance to current regimen § Absence of resistance to previously used drug does not rule out reservoirs of resistant virus that might emerge after re-initiation of that drug § If resistance to given drug has ever been detected, that drug should probably not be used again, even if current test results suggest viral susceptibility § 45

Conditions for Drug Resistance Testing: Individual Patient Management § When to use: – When there are treatment options – To indicate drug exposure – While patient is on therapy or before starting for the 1 st time § What to consider: – – Treatment history, other reasons for therapy failure Lab-to-lab variability of results and interpretations Requires expert advice for optimal use Only test single agents, not combinations 46

Conditions for Drug Resistance Testing: Public Health Applications § When to use: – When making decisions for national treatment guidelines • 1 st line regimens (based on results of surveillance for transmitted drug resistant HIV) • 2 nd line regimens (based on patterns of resistance in treated patients) – To monitor program effectiveness minimizing HIVDR § What to consider: – Early Warning Indicators, programme and site factors – Treatment history, other reasons for therapy failure – Lab-to-lab variability of results and interpretations 47

Limitations of Resistance Testing: Only possible in case of a detectable viral load Lack of consistent quality control (see ENVA) Current assays do not pick up “minority species; ” information is given on predominant strain § Assays have mostly been studied in “late” failures; what is their value in early failures? § Susceptibility is not equal to activity (clinical efficacy) § Clinical validation: more data necessary § § § 48

Reflection What benefits do I hope to see through DR testing in our country? § What are my concerns right now about this type of testing? § 49

Types of Resistance Assays Genotypic Testing: Prediction of phenotype based on sequence § Phenotypic Testing: Measure of susceptibility to specific drugs § – Recombinant Assays: RT/PCR portion of patient virus and transfer into a vector • Several different versions commercialized, automated and regulated – PBMC Assay: Culture virus from patient • Largely replaced by recombinant assays due to difficulties in reproducibility and throughput 50

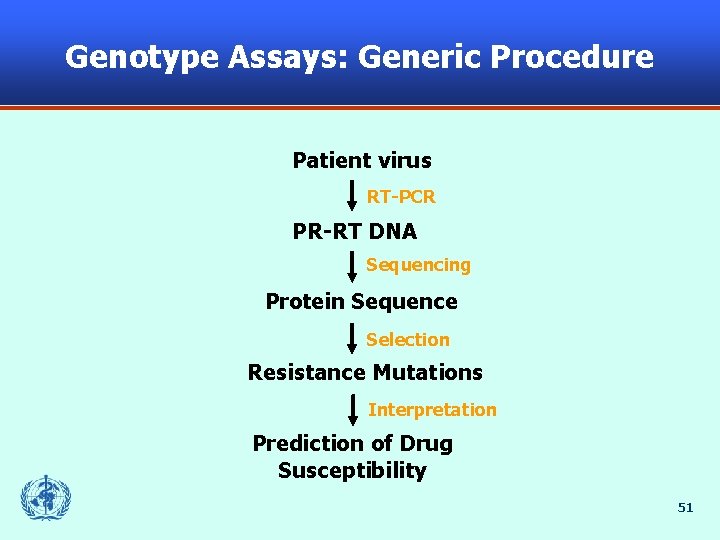
Genotype Assays: Generic Procedure Patient virus RT-PCR PR-RT DNA Sequencing Protein Sequence Selection Resistance Mutations Interpretation Prediction of Drug Susceptibility 51

Sources and Types of Genotype Assays § Reference Labs (North America and Europe) – Send blood sample in, receive results on report • Lab. Corp, Quest, AML, Specialty, Mayo, etc. • Monogram Biosciences, Virco § Commercial kits – Can be performed on site (in laboratory) • Tru. Gene (Siemens) • Viro. Seq (Abbott/Celera) § In-house assays – "home brew" assay performed at one site (clinical, hospital or research laboratory) – Must be validated and approved if used for patient management 52

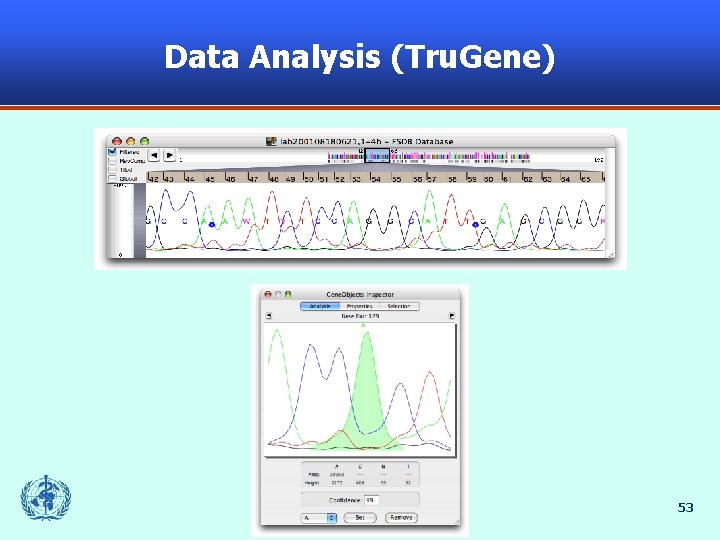
Data Analysis (Tru. Gene) 53

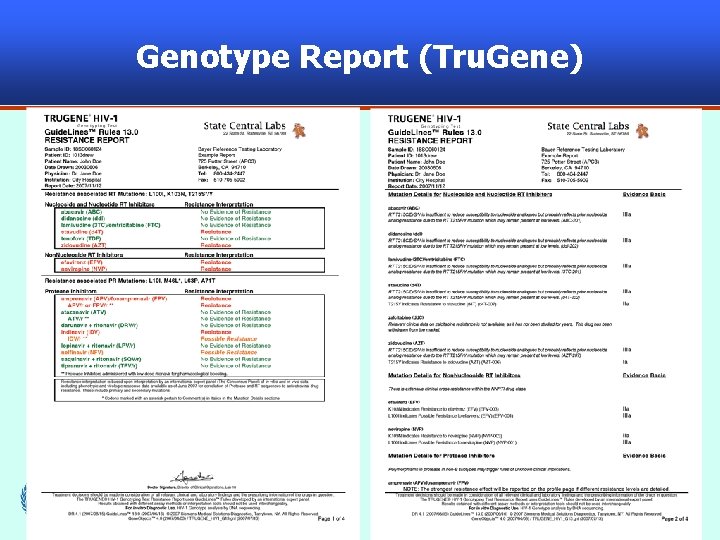
Genotype Report (Tru. Gene) 54

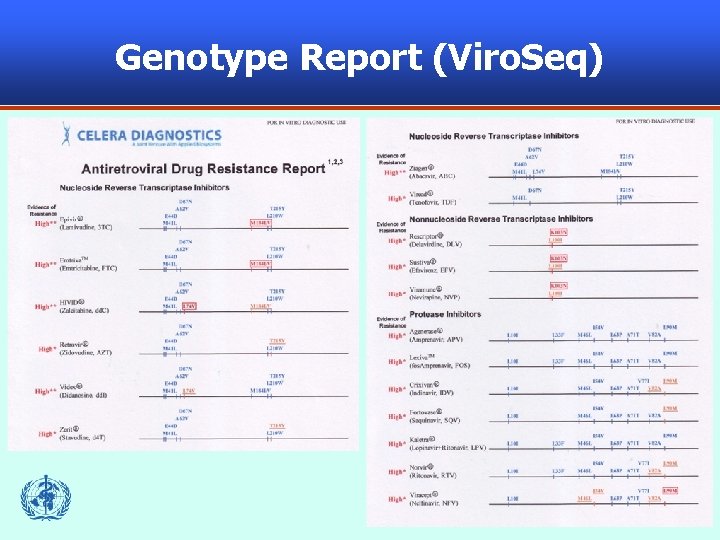
Genotype Report (Viro. Seq) 55

Genotyping: Pros and Cons Advantages Can be performed rapidly (days) § Relatively inexpensive ($100 - $400) § Available in many labs § Disadvantages § § § Does not directly measure susceptibility Sometimes difficult to interpret results Not all patterns of resistance mutations are known (esp. for new drugs and combinations) Generally qualitative Subjectivity in mixture detection 56

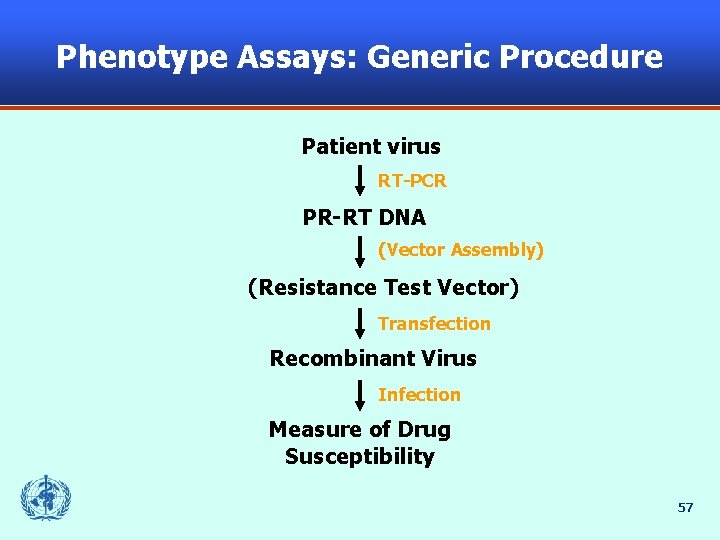
Phenotype Assays: Generic Procedure Patient virus RT-PCR PR-RT DNA (Vector Assembly) (Resistance Test Vector) Transfection Recombinant Virus Infection Measure of Drug Susceptibility 57

Commercially Available Phenotypic Assays § § All are recombinant virus phenotypic assays Antivirogram (Virco) – Homologous recombination used to generate vector – Replication occurs over multiple cycles § Pheno. Sense (Monogram) – Vector generated directly by digestion and ligation – Replication limited to single cycle – High precision and reproducibility § Phenoscript (VIRalliance) – Homologous recombination used to generate vector – Uses VSV-G protein for virus entry – Replication limited to single cycle 58

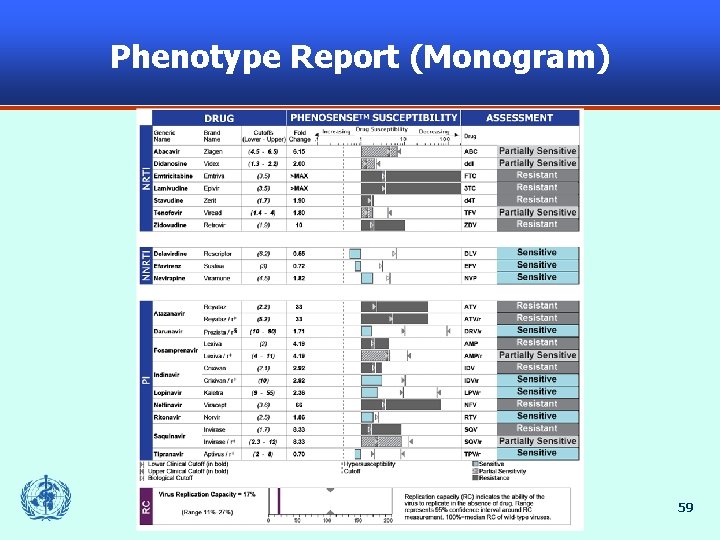
Phenotype Report (Monogram) 59

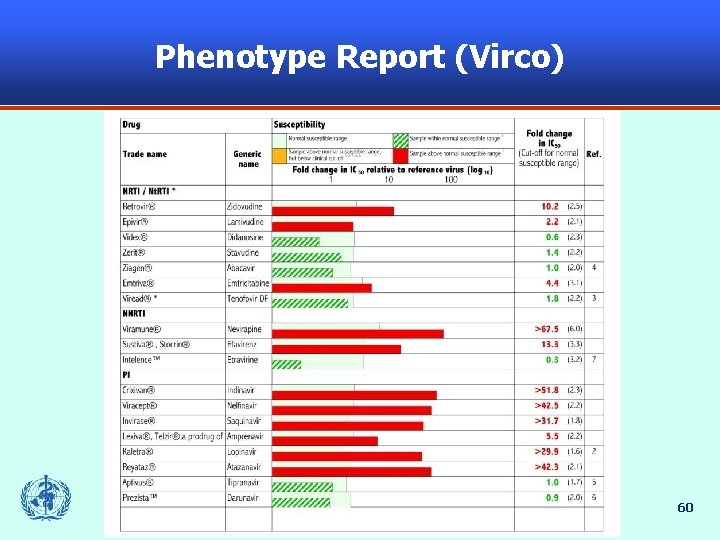
Phenotype Report (Virco) 60

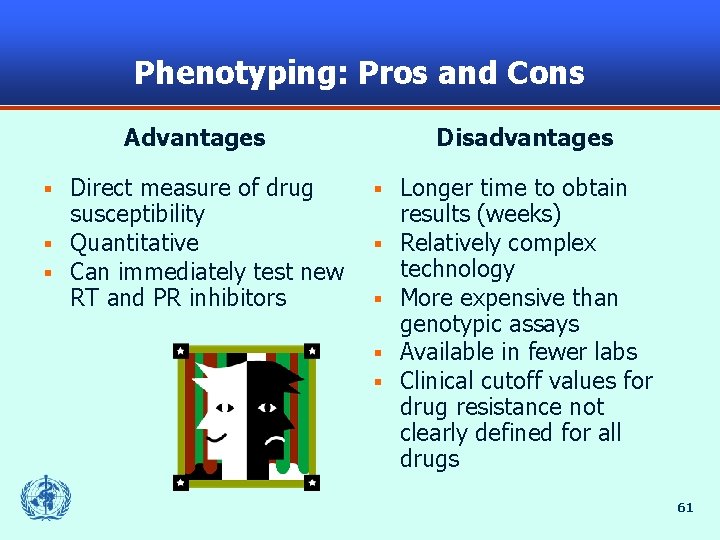
Phenotyping: Pros and Cons Advantages Direct measure of drug susceptibility § Quantitative § Can immediately test new RT and PR inhibitors § Disadvantages § § § Longer time to obtain results (weeks) Relatively complex technology More expensive than genotypic assays Available in fewer labs Clinical cutoff values for drug resistance not clearly defined for all drugs 61

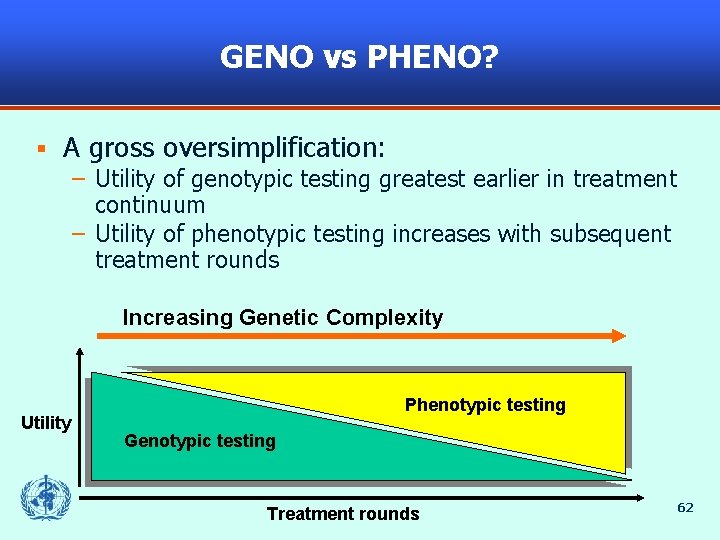
GENO vs PHENO? § A gross oversimplification: – Utility of genotypic testing greatest earlier in treatment continuum – Utility of phenotypic testing increases with subsequent treatment rounds Increasing Genetic Complexity Utility Phenotypic testing Genotypic testing Treatment rounds 62

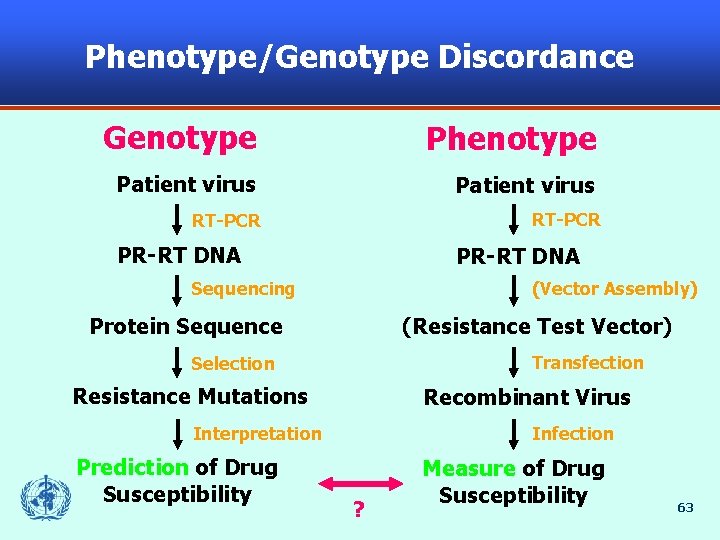
Phenotype/Genotype Discordance Genotype Phenotype Patient virus RT-PCR PR-RT DNA (Vector Assembly) Sequencing Protein Sequence (Resistance Test Vector) Transfection Selection Resistance Mutations Recombinant Virus Infection Interpretation Prediction of Drug Susceptibility ? Measure of Drug Susceptibility 63

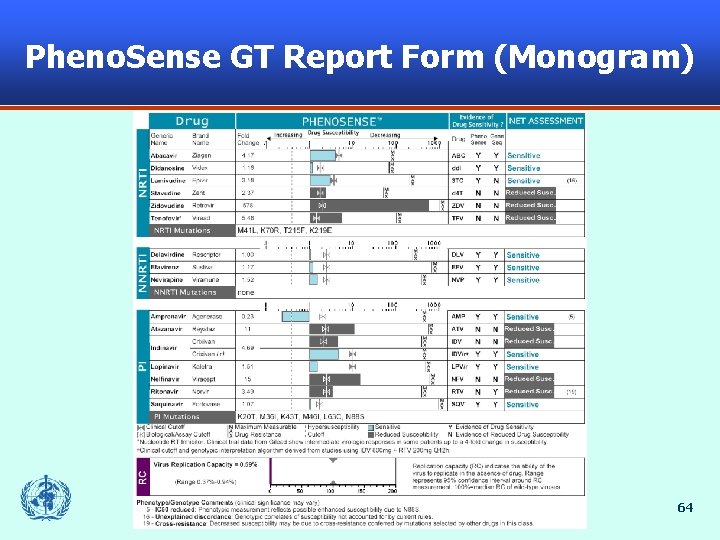
Pheno. Sense GT Report Form (Monogram) 64

3 Types of Phenotype-Genotype Discordance § Genotype predicts resistance, not reflected in phenotype ("PT-S, GT-R"); mixtures of resistant and sensitive viruses are present in the specimen § Genotype predicts resistance, not reflected in phenotype ("PT-S, GT-R"); not explained by mixtures § Genotype predicts susceptibility, but phenotype detects reduced susceptibility or resistance ("PTR, GT-S") Parkin et al. JAIDS 2002 65

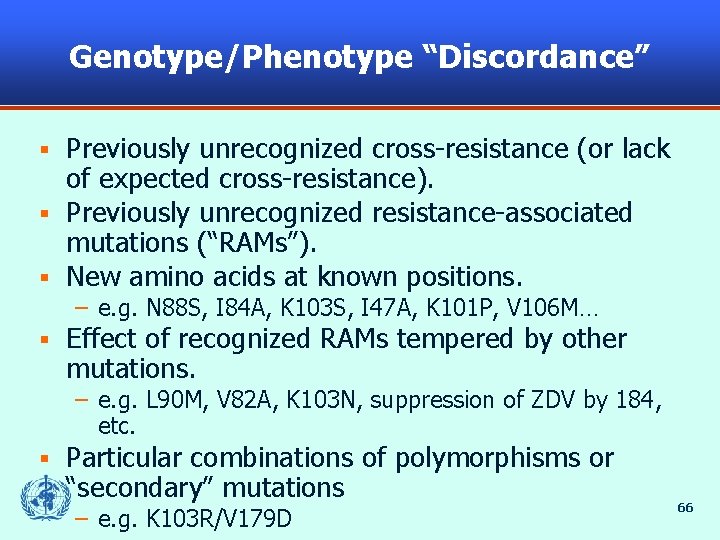
Genotype/Phenotype “Discordance” Previously unrecognized cross-resistance (or lack of expected cross-resistance). § Previously unrecognized resistance-associated mutations (“RAMs”). § New amino acids at known positions. § – e. g. N 88 S, I 84 A, K 103 S, I 47 A, K 101 P, V 106 M… § Effect of recognized RAMs tempered by other mutations. – e. g. L 90 M, V 82 A, K 103 N, suppression of ZDV by 184, etc. § Particular combinations of polymorphisms or “secondary” mutations – e. g. K 103 R/V 179 D 66

Discussion What are the different types of resistance assays? What are the advantages and limitations of these assays? § What results can we expect from these tests? § What if the results from one type of test are inconsistent with results from another? § § 67

Reflection What type of DR assay is most appropriate for our situation? § What results can we expect? § What factors should we keep in mind about this type of assay? § 68

Summary Impact of HIV Drug Resistance Factors that Influence Development of Drug Resistance ü How to Minimize Drug Resistance ü How to Respond to Detection of Drug Resistance ü Drug Resistance Assays ü ü 69
 Drug resistance training
Drug resistance training Molecular lab setup and workflow
Molecular lab setup and workflow Filtration introduction
Filtration introduction Friction and air resistance
Friction and air resistance Natural selection and drug resistance
Natural selection and drug resistance Natural selection and drug resistance
Natural selection and drug resistance Deliberate adulteration examples
Deliberate adulteration examples C device module module 1
C device module module 1 Define resistance exercise
Define resistance exercise Peer pressure resistance training
Peer pressure resistance training Drug impairment training
Drug impairment training Drug and alcohol safety training
Drug and alcohol safety training Corporate training materials
Corporate training materials Sbcc training module
Sbcc training module Ostp test
Ostp test Hbyc e module
Hbyc e module Word processing packages
Word processing packages Module 5 computer concepts exam answers
Module 5 computer concepts exam answers Module 11 computer concepts exam
Module 11 computer concepts exam 8 step training model example
8 step training model example Training module template
Training module template Training module template
Training module template Kaizen training module
Kaizen training module Amway schedule
Amway schedule Motivation training module
Motivation training module Mary fertakis training module
Mary fertakis training module Gmp training program
Gmp training program Idsp training module
Idsp training module Quang trung
Quang trung Where did hiv come from
Where did hiv come from Test wiedzy o aids z odpowiedziami
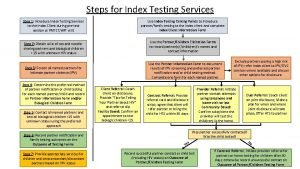
Test wiedzy o aids z odpowiedziami Hiv index testing steps
Hiv index testing steps Program nasional akreditasi rumah sakit
Program nasional akreditasi rumah sakit Phdp in hiv
Phdp in hiv Kuchecheudzwa
Kuchecheudzwa Window period hiv
Window period hiv Why do the bodys antibodies fail to protect people from hiv
Why do the bodys antibodies fail to protect people from hiv Iris syndrome
Iris syndrome Hiv
Hiv Hiv risk factors
Hiv risk factors Asante hiv-1 rapid recency assay
Asante hiv-1 rapid recency assay Fiebig hiv
Fiebig hiv Hiv case-based surveillance in ethiopia
Hiv case-based surveillance in ethiopia Triệu chứng nhiễm hiv
Triệu chứng nhiễm hiv A bacterial std that usually affects mucous membranes
A bacterial std that usually affects mucous membranes Chapter 24 sexually transmitted diseases and hiv/aids
Chapter 24 sexually transmitted diseases and hiv/aids Basic hiv course
Basic hiv course Stakeholders in hiv prevention
Stakeholders in hiv prevention Hiv treatments
Hiv treatments Causative organism of hiv/aids
Causative organism of hiv/aids What does hiv stand
What does hiv stand What does hiv stand
What does hiv stand Hiv test window period
Hiv test window period What does hiv diarrhea look like
What does hiv diarrhea look like Seks oralny a hiv
Seks oralny a hiv Hiv adalah
Hiv adalah Hiv lifecycle
Hiv lifecycle 4.jenerasyon vidas hıv duo ultra testi güvenilir mi
4.jenerasyon vidas hıv duo ultra testi güvenilir mi Hiv lifecycle
Hiv lifecycle Hiv
Hiv Recognize developmental disorders of the dentition
Recognize developmental disorders of the dentition Incubation period of hiv
Incubation period of hiv Ciclo replicativo
Ciclo replicativo Vidas hiv duo ultra package insert
Vidas hiv duo ultra package insert Profilaksis pasca pajanan hiv
Profilaksis pasca pajanan hiv Igéje szól igéje hív
Igéje szól igéje hív Tabela perímetro cefálico por idade
Tabela perímetro cefálico por idade Cytomegalavirus
Cytomegalavirus Most complex characters evolve
Most complex characters evolve