Dimensional Reasoning DRILL 1 Is either of these




- Slides: 30

Dimensional Reasoning DRILL 1. Is either of these equations correct? 2. What is the common problem in the two examples below? Sign outside New Cuyama, CA 1998 Mars Polar Orbiter

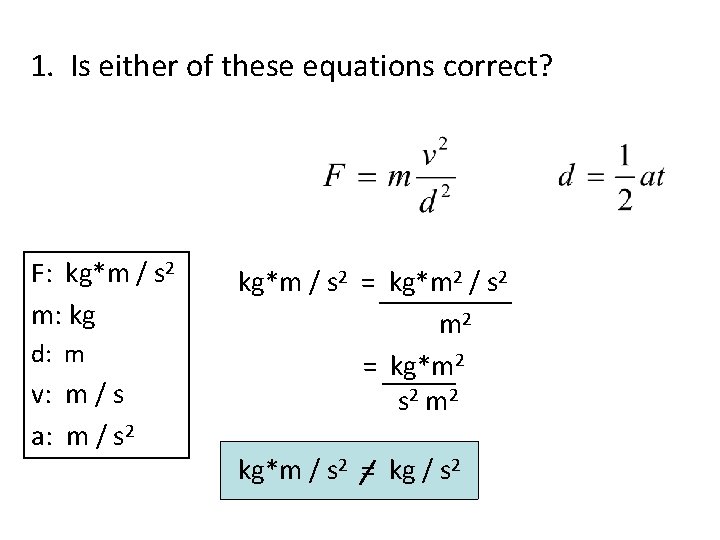
1. Is either of these equations correct? F: kg*m / s 2 m: kg d: m v: m / s a: m / s 2 kg*m / s 2 = kg*m 2 / s 2 m 2 = kg*m 2 s 2 m 2 kg*m / s 2 = kg / s 2

2. What is the common problem in the two images below? Pounds-force Newtons-force UNITS! $125 mil error: “Instead of passing about 150 km above the Martian atmosphere before entering orbit, the spacecraft actually passed about 60 km above the surface…This was far too close and the spacecraft burnt up due to friction with the atmosphere. ” – BBC News

Dimensional Reasoning Lecture Outline: 1. Units – base and derived 2. Units – quantitative considerations 3. Dimensions and Dimensional Analysis – fundamental rules and uses

Dimensional Reasoning Measurements consist of 2 properties: 1. a quality or dimension 2. a quantity expressed in terms of “units” Let’s look at #2 first: THE INTERNATIONAL SI SYSTEM OF MEASUREMENT IS COMPRISED OF 7 FUNDAMENTAL (OR BASE) QUANTITIES. THE ENGLISH SYSTEM, USED IN THE UNITED STATES, HAS SIMILARITIES AND THERE ARE CONVERSION FACTORS WHEN NECESSARY.

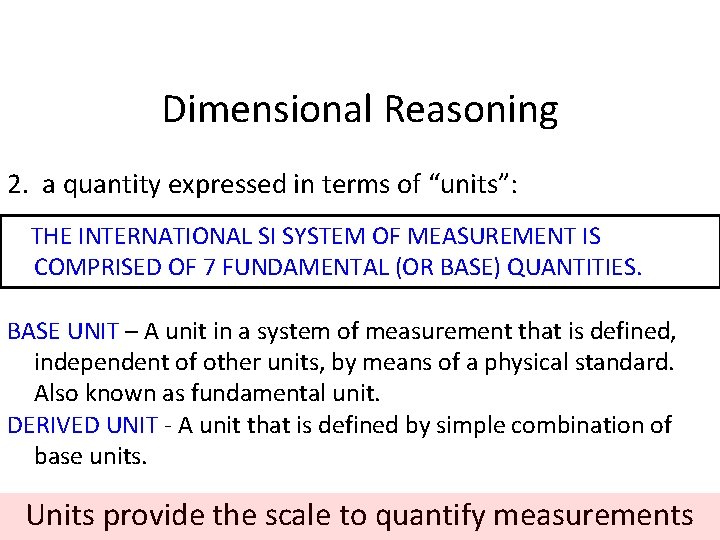
Dimensional Reasoning 2. a quantity expressed in terms of “units”: THE INTERNATIONAL SI SYSTEM OF MEASUREMENT IS COMPRISED OF 7 FUNDAMENTAL (OR BASE) QUANTITIES. BASE UNIT – A unit in a system of measurement that is defined, independent of other units, by means of a physical standard. Also known as fundamental unit. DERIVED UNIT - A unit that is defined by simple combination of base units. Units provide the scale to quantify measurements

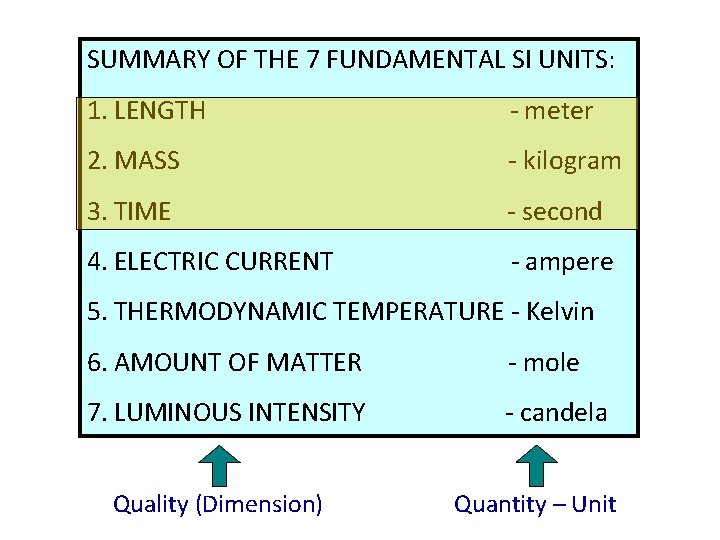
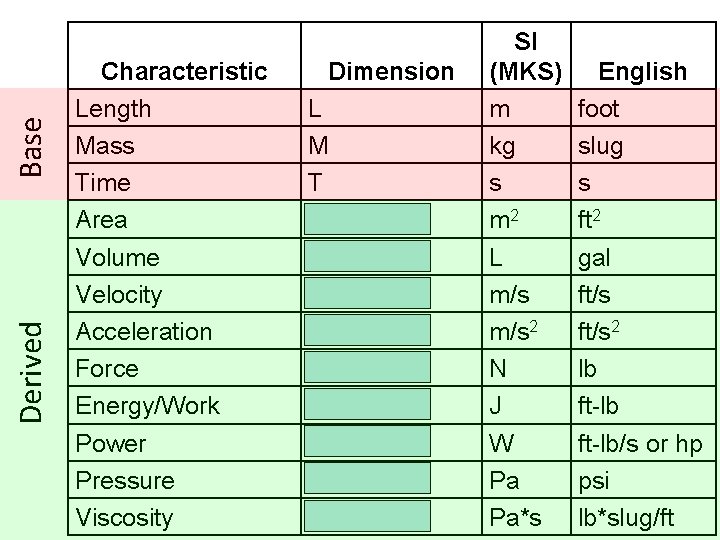
SUMMARY OF THE 7 FUNDAMENTAL SI UNITS: 1. LENGTH - meter 2. MASS - kilogram 3. TIME - second 4. ELECTRIC CURRENT - ampere 5. THERMODYNAMIC TEMPERATURE - Kelvin 6. AMOUNT OF MATTER - mole 7. LUMINOUS INTENSITY - candela Quality (Dimension) Quantity – Unit

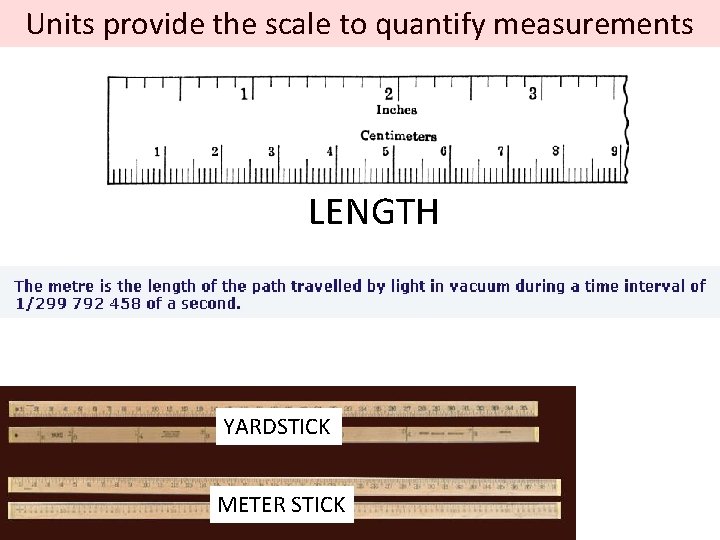
Units provide the scale to quantify measurements LENGTH YARDSTICK METER STICK

Units provide the scale to quantify measurements MASS

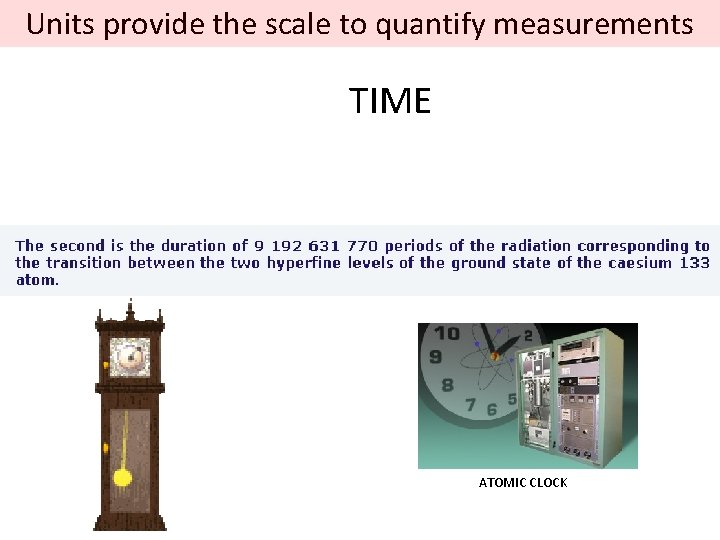
Units provide the scale to quantify measurements TIME ATOMIC CLOCK

Units provide the scale to quantify measurements ELECTRIC CURRENT

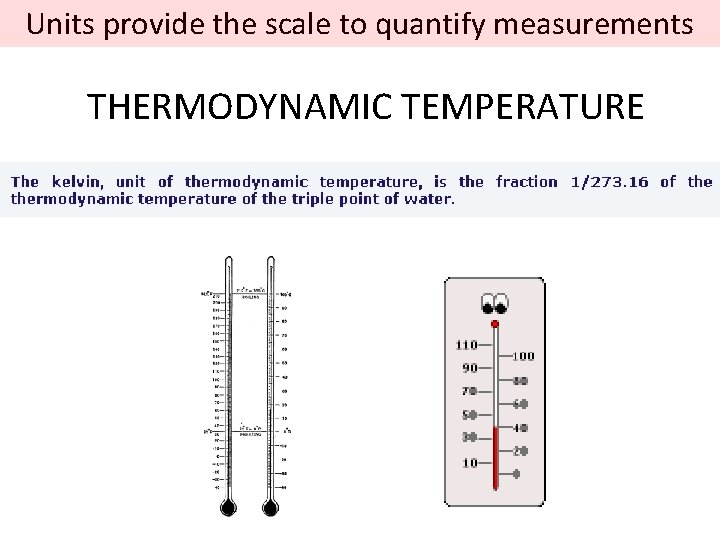
Units provide the scale to quantify measurements THERMODYNAMIC TEMPERATURE

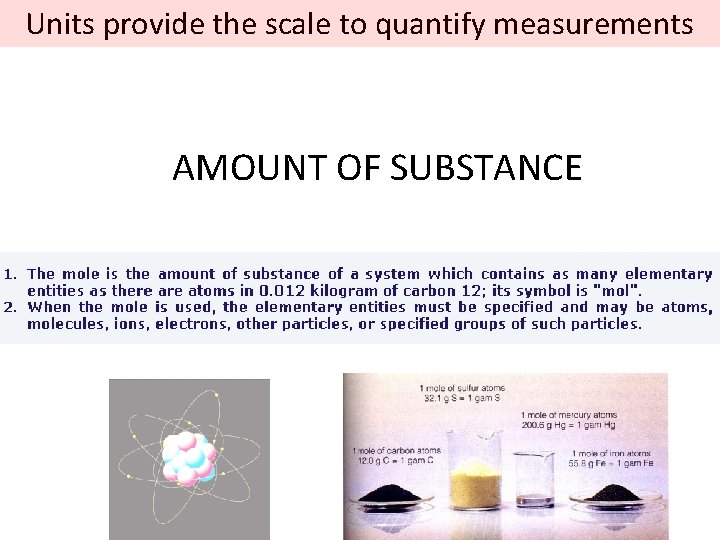
Units provide the scale to quantify measurements AMOUNT OF SUBSTANCE

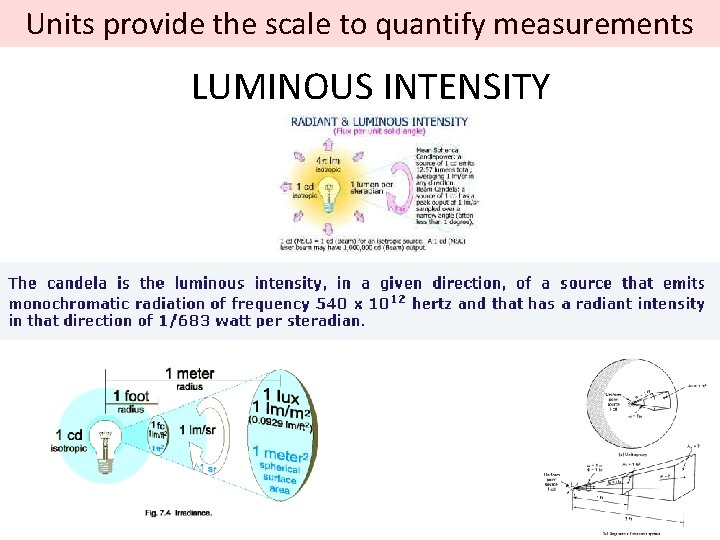
Units provide the scale to quantify measurements LUMINOUS INTENSITY

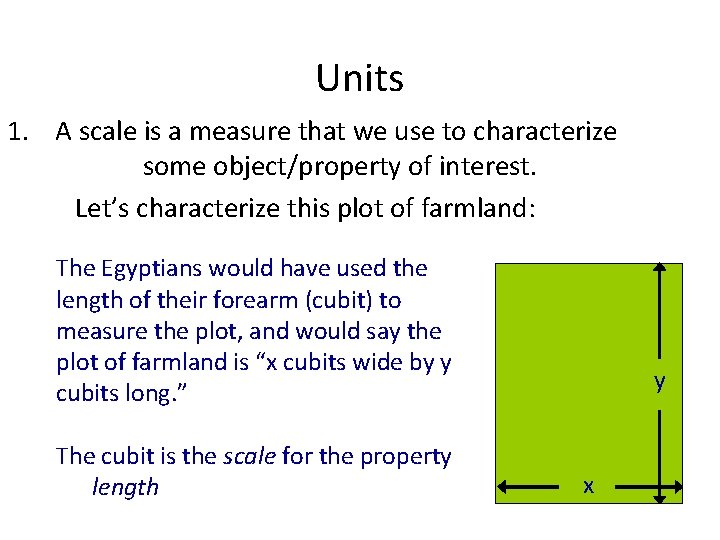
Units 1. A scale is a measure that we use to characterize some object/property of interest. Let’s characterize this plot of farmland: The Egyptians would have used the length of their forearm (cubit) to measure the plot, and would say the plot of farmland is “x cubits wide by y cubits long. ” The cubit is the scale for the property length y x

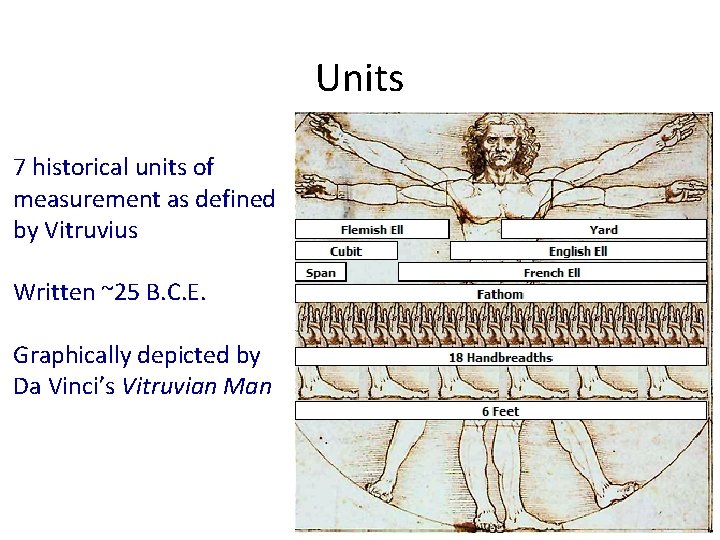
Units 7 historical units of measurement as defined by Vitruvius Written ~25 B. C. E. Graphically depicted by Da Vinci’s Vitruvian Man

Units 2. Each measurement must carry some unit of measurement (unless it is a dimensionless quantity). Numbers without units are meaningless. I am “ 72 tall” 72 what? Fingers, handbreadths, inches, centimeters? ?

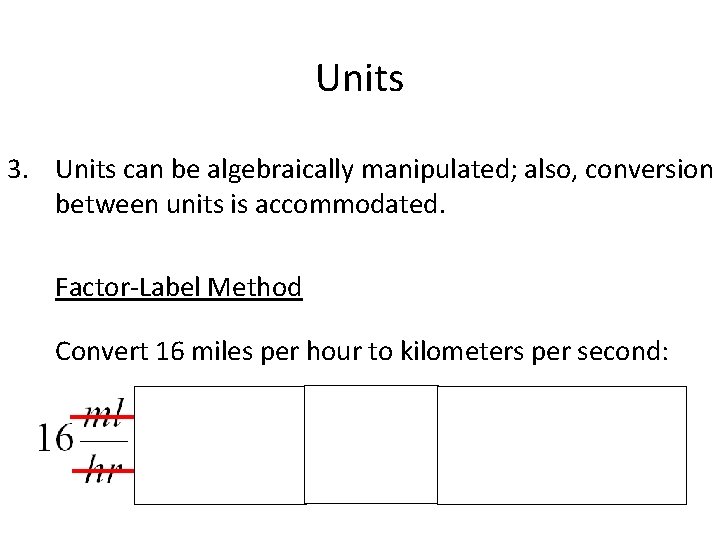
Units 3. Units can be algebraically manipulated; also, conversion between units is accommodated. Factor-Label Method Convert 16 miles per hour to kilometers per second:

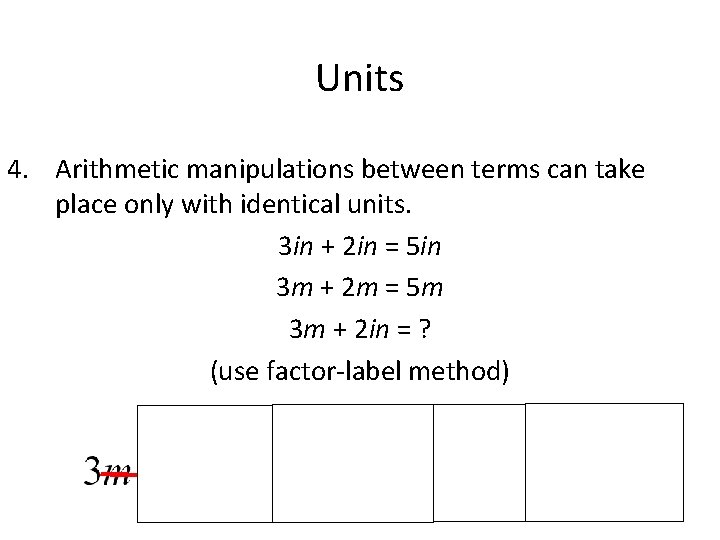
Units 4. Arithmetic manipulations between terms can take place only with identical units. 3 in + 2 in = 5 in 3 m + 2 m = 5 m 3 m + 2 in = ? (use factor-label method)

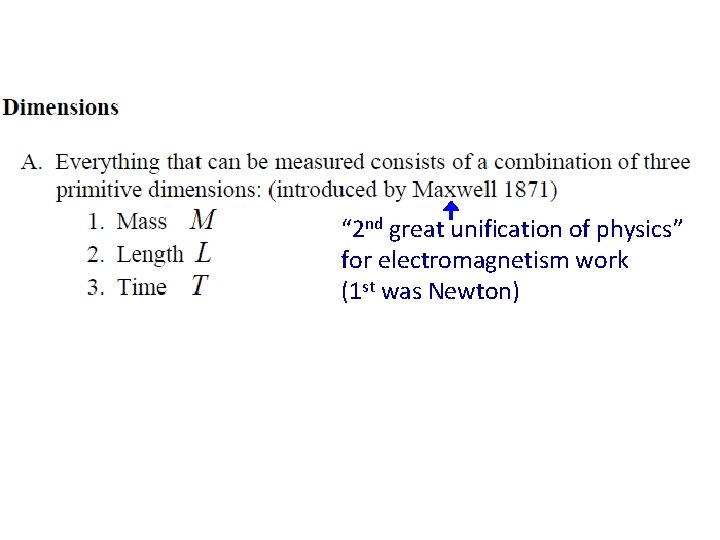
“ 2 nd great unification of physics” for electromagnetism work (1 st was Newton) Dimensions are intrinsic to the variables themselves

Base Derived Characteristic Length Mass Time Area Volume Velocity Acceleration Force Energy/Work Power Pressure Viscosity Dimension L M T L 2 L 3 LT-1 LT-2 ML 2 T-2 ML 2 T-3 ML-1 T-2 ML-1 T-1 SI (MKS) m kg s m 2 L m/s 2 N J W Pa Pa*s English foot slug s ft 2 gal ft/s 2 lb ft-lb/s or hp psi lb*slug/ft

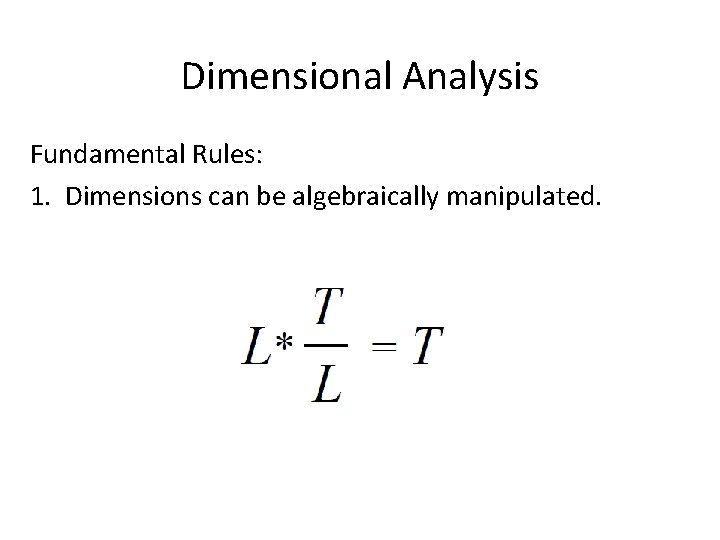
Dimensional Analysis Fundamental Rules: 1. Dimensions can be algebraically manipulated.

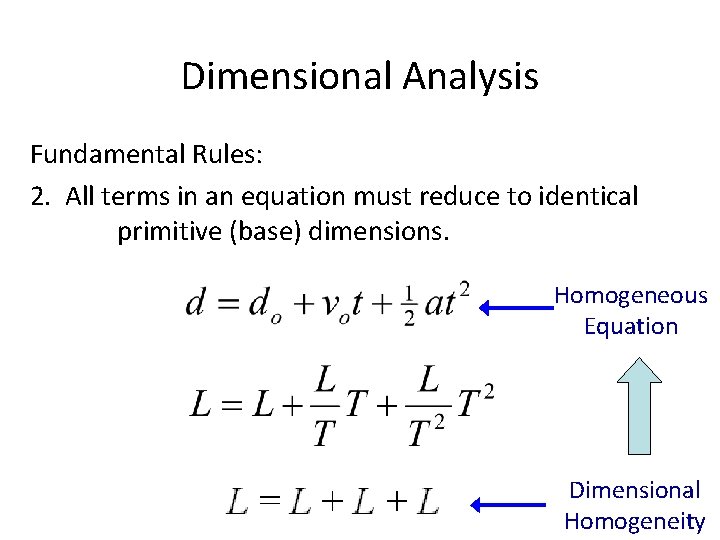
Dimensional Analysis Fundamental Rules: 2. All terms in an equation must reduce to identical primitive (base) dimensions. Homogeneous Equation Dimensional Homogeneity

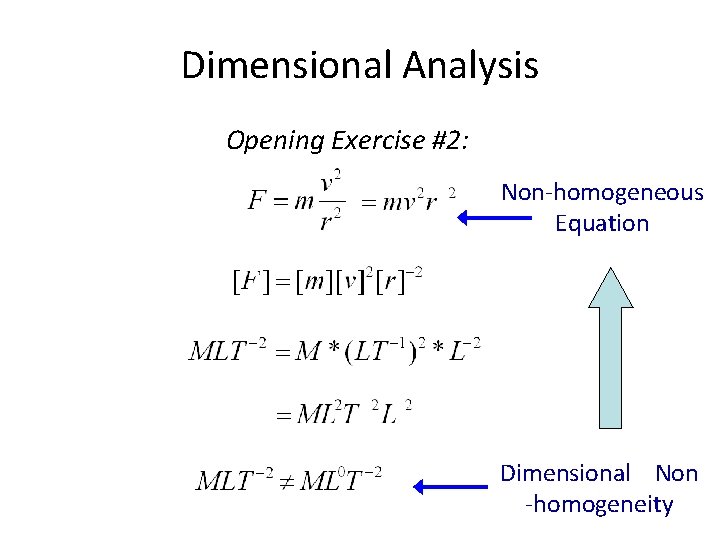
Dimensional Analysis Opening Exercise #2: Non-homogeneous Equation Dimensional Non -homogeneity

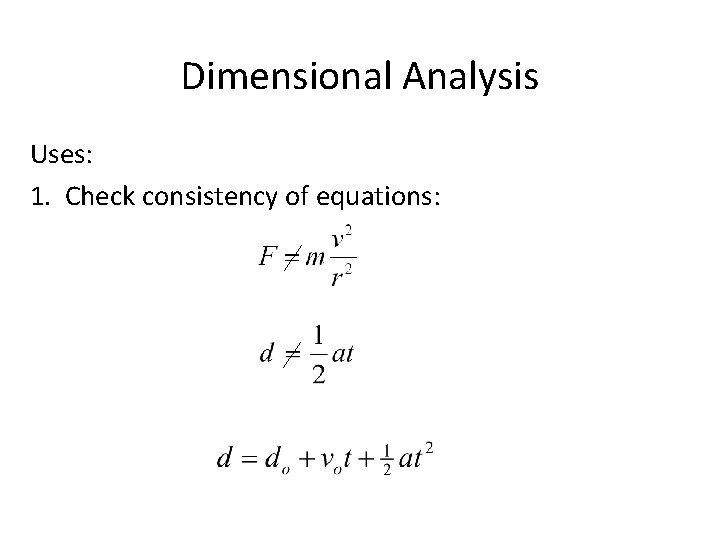
Dimensional Analysis Uses: 1. Check consistency of equations:

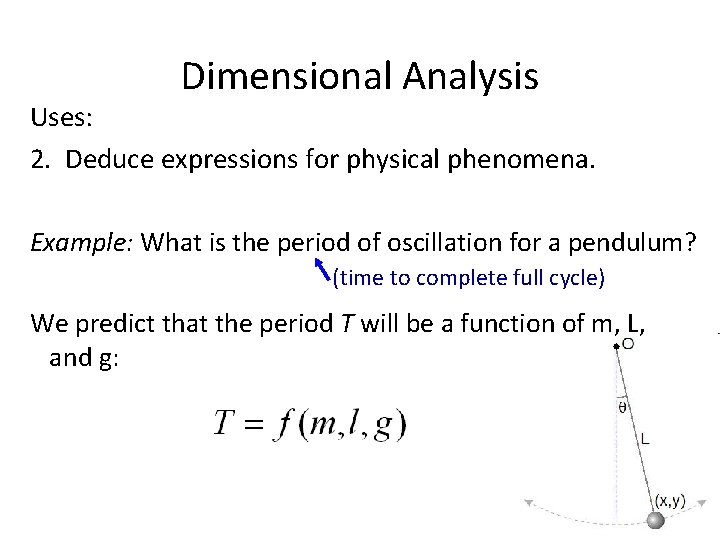
Dimensional Analysis Uses: 2. Deduce expressions for physical phenomena. Example: What is the period of oscillation for a pendulum? (time to complete full cycle) We predict that the period T will be a function of m, L, and g:

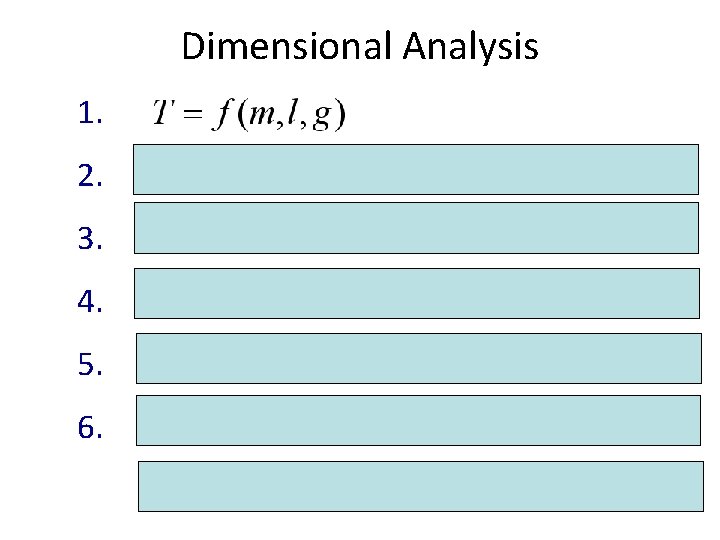
Dimensional Analysis 1. 2. 3. 4. 5. 6. power-law expression

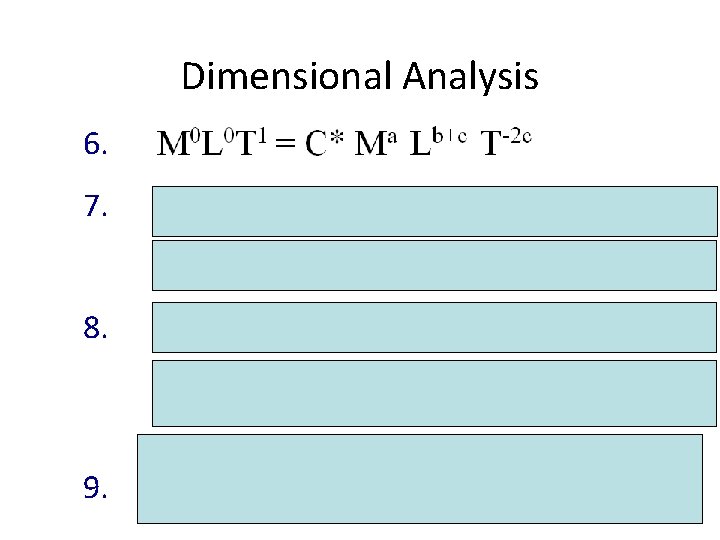
Dimensional Analysis 6. 7. 8. 9.

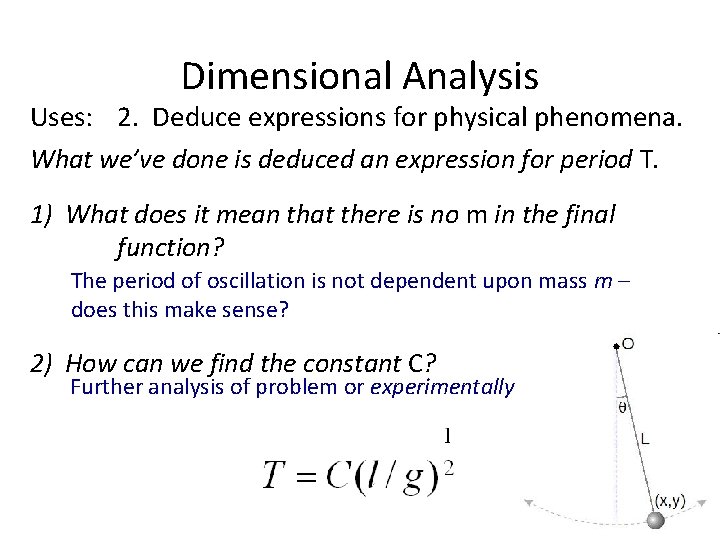
Dimensional Analysis Uses: 2. Deduce expressions for physical phenomena. What we’ve done is deduced an expression for period T. 1) What does it mean that there is no m in the final function? The period of oscillation is not dependent upon mass m – does this make sense? 2) How can we find the constant C? Further analysis of problem or experimentally

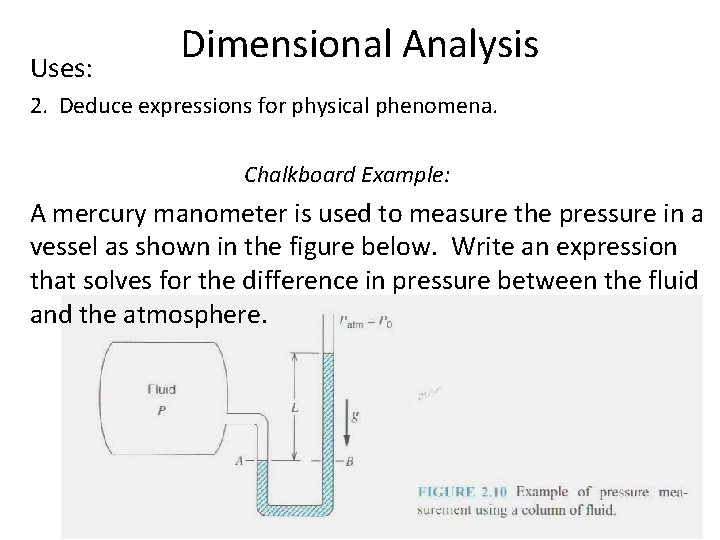
Uses: Dimensional Analysis 2. Deduce expressions for physical phenomena. Chalkboard Example: A mercury manometer is used to measure the pressure in a vessel as shown in the figure below. Write an expression that solves for the difference in pressure between the fluid and the atmosphere.