Dermatological Toxicities of ART HAIVN Harvard Medical School






















- Slides: 40

Dermatological Toxicities of ART HAIVN Harvard Medical School AIDS Initiative in Vietnam 1

Learning Objectives By the end of this session, participants should be able to: n Explain how to grade dermatological toxicity n Describe the clinical manifestation of rash and explain how to manage rash caused by: • NNRTI • Cotrimoxazole • Abacavir n Explain the management of a patient with Stevens-Johnson Syndrome 2

Differential Diagnosis of Rash in PLHIV Drug toxicity or allergy • ARVs • Cotrimoxazole • Other drugs Allergic reactions to: • Foods • Contact dermatitis Systemic infection • • Penicilliosis Syphilis Viral infection (i. e. Dengue) Scabies • Eczema Other • Eosinophilic folliculitis dermatological • Papulo-Pruritic Eruption (PPE) diseases 3

Grading Rash 4

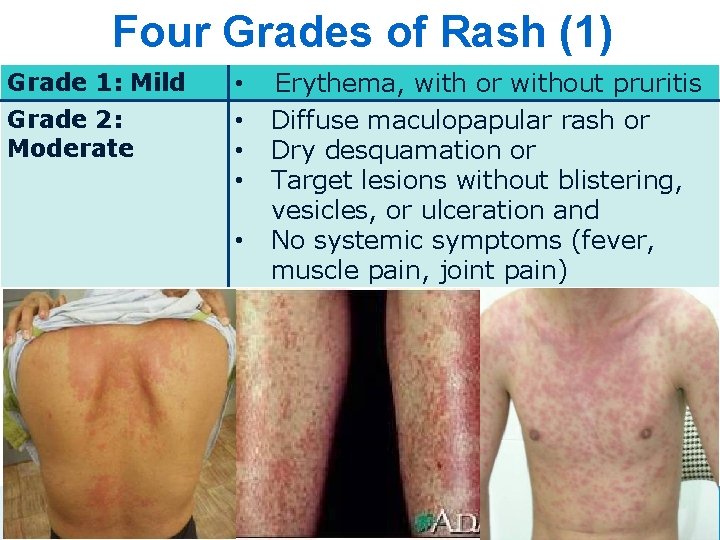
Four Grades of Rash (1) Grade 1: Mild Grade 2: Moderate • • • 5 Erythema, with or without pruritis Diffuse maculopapular rash or Dry desquamation or Target lesions without blistering, vesicles, or ulceration and No systemic symptoms (fever, muscle pain, joint pain) 5

Four Grades of Rash (2) Grade 3: Severe § Vesiculation § Moist desquamation § Ulceration § Systemic symptoms • Fever • Blistering • Muscle and/or joint pain, edema • Elevated transaminases

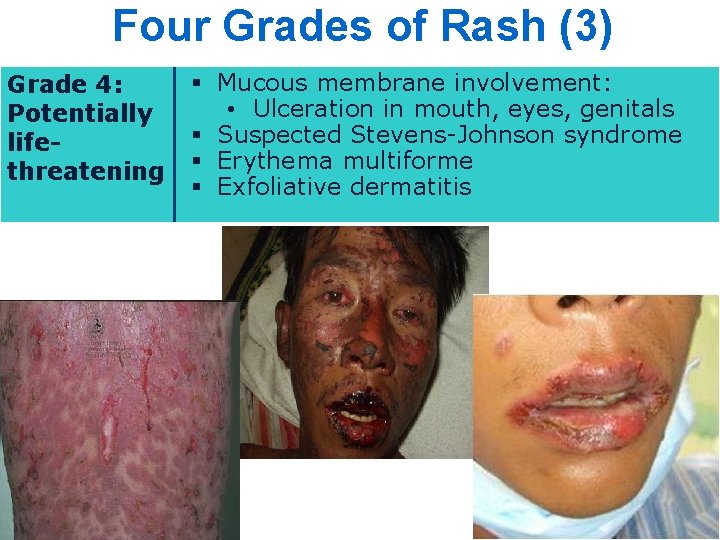
Four Grades of Rash (3) Grade 4: Potentially lifethreatening § Mucous membrane involvement: • Ulceration in mouth, eyes, genitals § Suspected Stevens-Johnson syndrome § Erythema multiforme § Exfoliative dermatitis

Evaluating the Possible Causes of Rash 8

Evaluating the Rash (1) – How to Find the Etiology? n Take a thorough history of the rash and concomitant symptoms: • ask about other possible allergens • find out where and when exactly the rash started on the body n n Get a good medication history Do a thorough medical and laboratory evaluation to exclude other etiologies 9

Evaluating the Rash (2) – Is Rash Caused by ARV? n n Did the patient recently start an ARV likely to cause rash? Does the patient has a known history of allergies to other medications that he/she is taking? Is the treatment of other causes of rash not helpful? Are evaluations of other causes of rash negative? 10

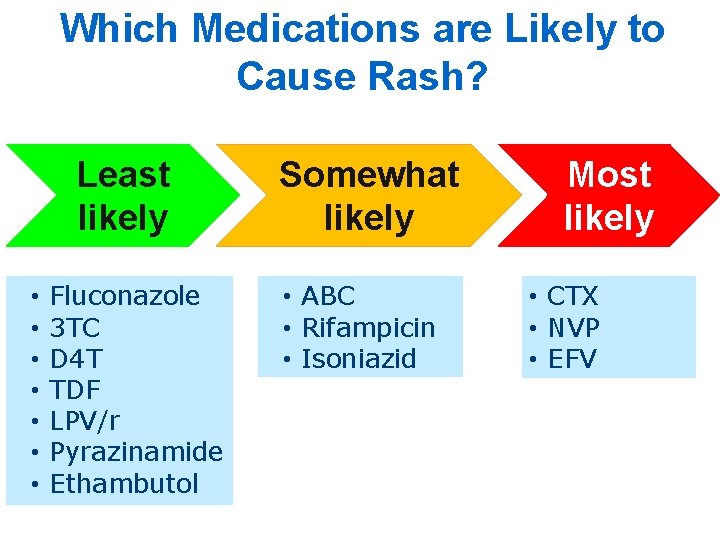
Which Medications are Likely to Cause Rash? Least likely • • Fluconazole 3 TC D 4 T TDF LPV/r Pyrazinamide Ethambutol Somewhat likely • ABC • Rifampicin • Isoniazid Most likely • CTX • NVP • EFV

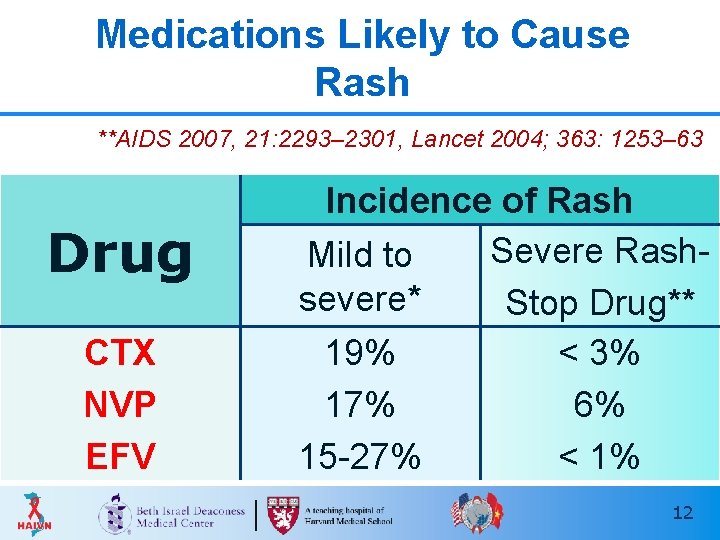
Medications Likely to Cause Rash t 2007; **AIDS 2007, 21: 2293– 2301, Lancet 2004; 363: 1253– 63 Drug CTX NVP EFV Incidence of Rash Severe Rash. Mild to severe* Stop Drug** 19% < 3% 17% 6% 15 -27% < 1% 12

NNRTI Rash 13

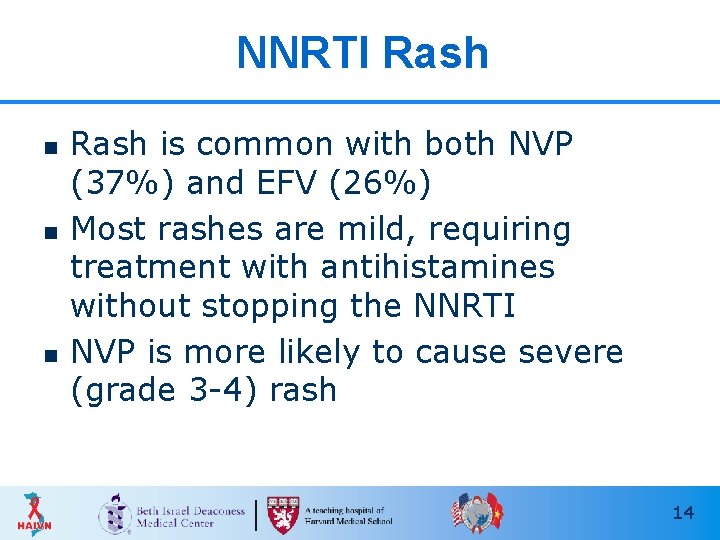
NNRTI Rash n n n Rash is common with both NVP (37%) and EFV (26%) Most rashes are mild, requiring treatment with antihistamines without stopping the NNRTI NVP is more likely to cause severe (grade 3 -4) rash 14

Management of NNRTI Rash: Stage 1 - 2 n n n Continue ARV; Give antihistamines Delay escalating dose of NVP Closely monitor for development of systemic symptoms, worsening rash, LFT elevations: • Stop CTX if this was started around same time as ARV and allergy can’t be ruled out • Stop or change ARV if rash progresses to stage 3 or 4 15

Management of NNRTI Rash: Stage 3 (1) n n n If NNRTI (e. g. NVP) is the most likely cause, stop it and continue the 2 NRTI drugs for up to 7 days Stop CTX if this was started around the same time as ARV, and allergy to it is also possible Give antihistamines 16

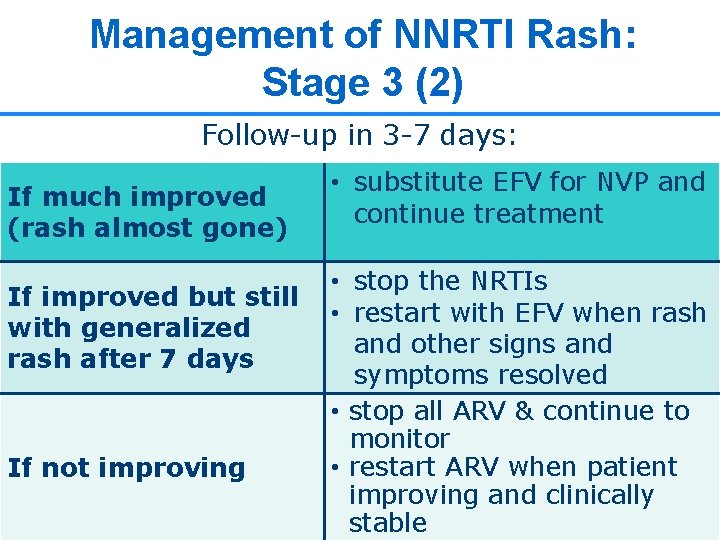
Management of NNRTI Rash: Stage 3 (2) Follow-up in 3 -7 days: If much improved (rash almost gone) If improved but still with generalized rash after 7 days If not improving • substitute EFV for NVP and continue treatment • stop the NRTIs • restart with EFV when rash and other signs and symptoms resolved • stop all ARV & continue to monitor • restart ARV when patient improving and clinically 17 17 stable

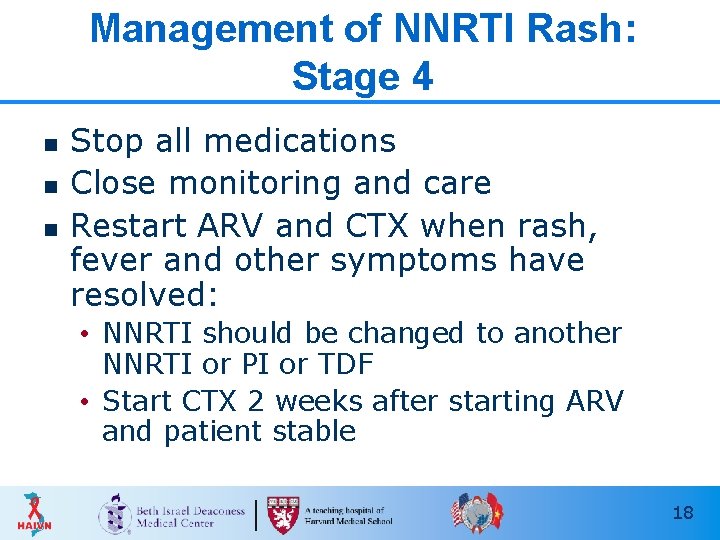
Management of NNRTI Rash: Stage 4 n n n Stop all medications Close monitoring and care Restart ARV and CTX when rash, fever and other symptoms have resolved: • NNRTI should be changed to another NNRTI or PI or TDF • Start CTX 2 weeks after starting ARV and patient stable 18

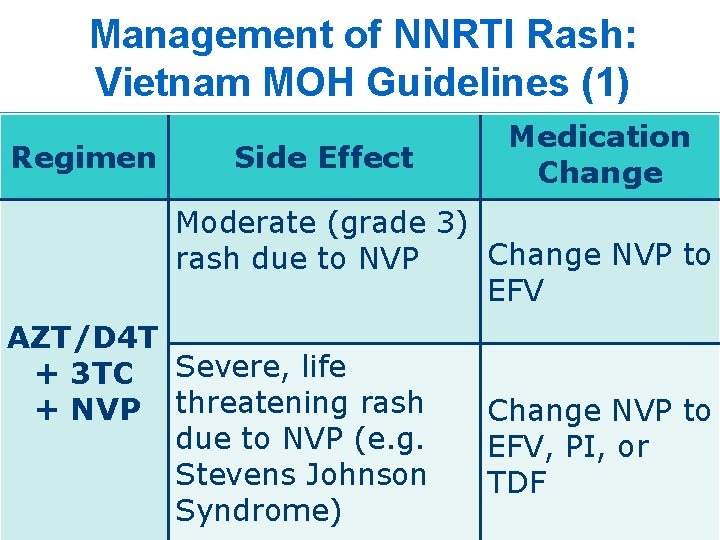
Management of NNRTI Rash: Vietnam MOH Guidelines (1) Regimen Side Effect Medication Change Moderate (grade 3) Change NVP to rash due to NVP EFV AZT/D 4 T + 3 TC Severe, life + NVP threatening rash due to NVP (e. g. Stevens Johnson Syndrome) Change NVP to EFV, PI, or TDF 19

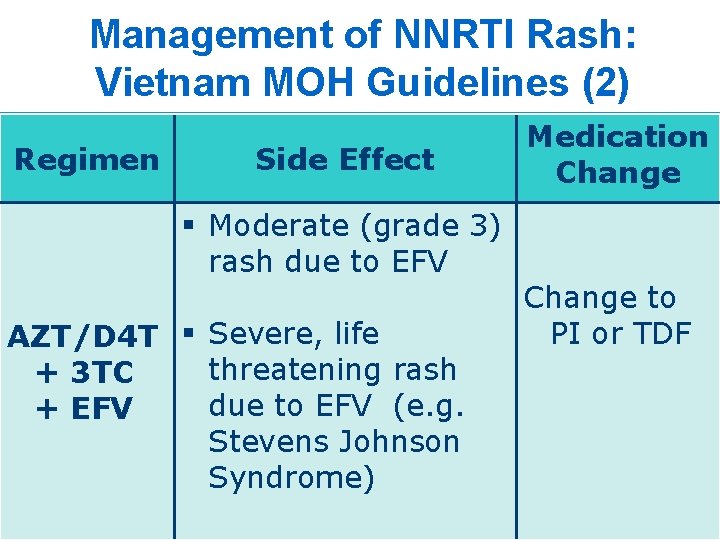
Management of NNRTI Rash: Vietnam MOH Guidelines (2) Regimen Side Effect § Moderate (grade 3) rash due to EFV AZT/D 4 T § Severe, life threatening rash + 3 TC due to EFV (e. g. + EFV Stevens Johnson Syndrome) Medication Change to PI or TDF 20

Cotrimoxazole Rash 21

Cotrimoxazole Allergy n Clinically: • Maculopapular rash • Can have fever • Usually within first few weeks of treatment n Epidemiology • No studies in Asia • In Africa, about 2% had allergy to CTX* n Resolves when drug is stopped Lancet. 2004 Oct 16 -22; 364(9443): 1428 -34. 22

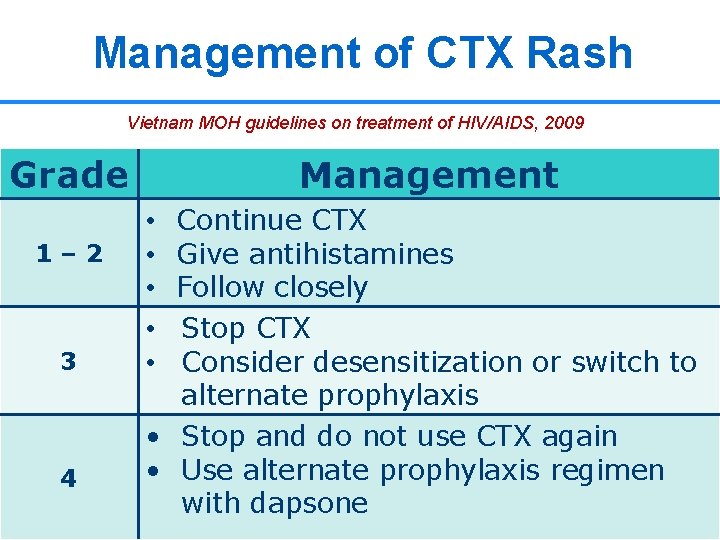
Management of CTX Rash Vietnam MOH guidelines on treatment of HIV/AIDS, 2009 Grade 1– 2 3 4 Management Continue CTX Give antihistamines Follow closely Stop CTX Consider desensitization or switch to alternate prophylaxis • Stop and do not use CTX again • Use alternate prophylaxis regimen 23 23 with dapsone • • •

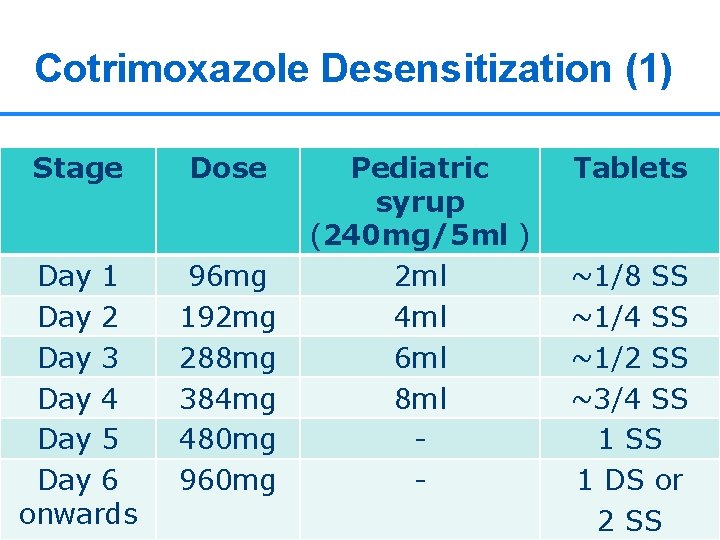
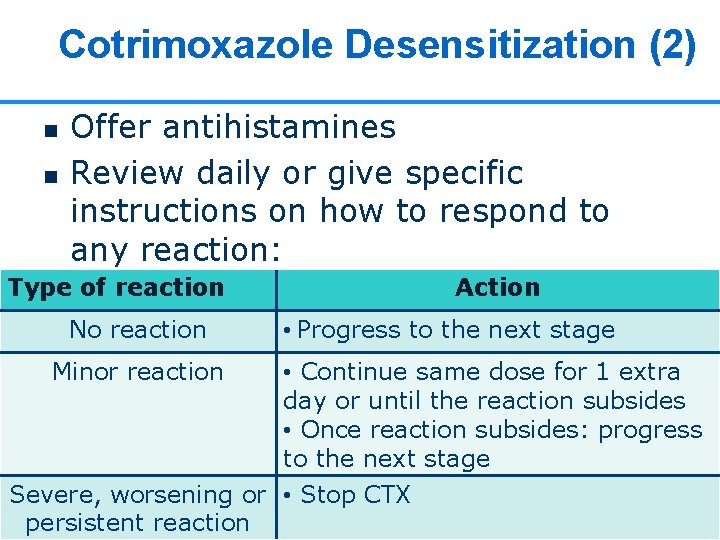
Cotrimoxazole Desensitization (1) WHO August 2006: Guidelines on co-trimoxazole prophylaxis Stage Dose Day 1 Day 2 Day 3 Day 4 Day 5 Day 6 onwards 96 mg 192 mg 288 mg 384 mg 480 mg 960 mg Pediatric syrup (240 mg/5 ml ) 2 ml 4 ml 6 ml 8 ml - Tablets ~1/8 SS ~1/4 SS ~1/2 SS ~3/4 SS 1 DS or 2 24 SS 24

Cotrimoxazole Desensitization (2) n n Offer antihistamines Review daily or give specific instructions on how to respond to any reaction: Type of reaction No reaction Minor reaction Action • Progress to the next stage • Continue same dose for 1 extra day or until the reaction subsides • Once reaction subsides: progress to the next stage Severe, worsening or • Stop CTX 25 25 persistent reaction

Abacavir Hypersensitivity Rash 26

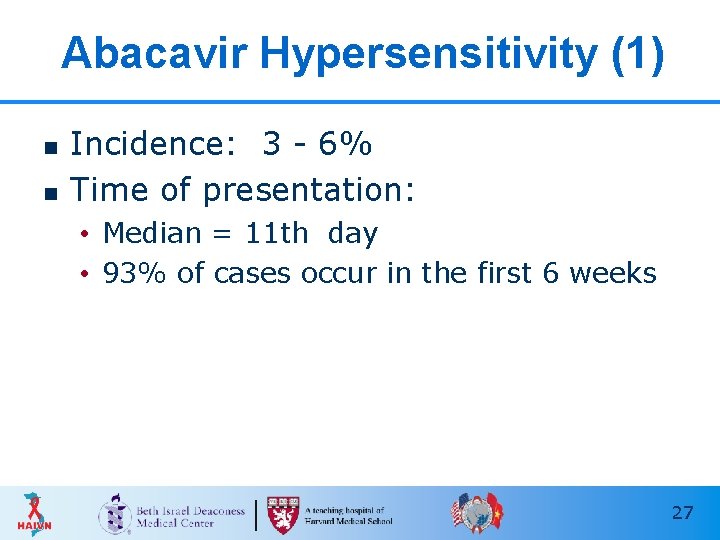
Abacavir Hypersensitivity (1) n n Incidence: 3 - 6% Time of presentation: • Median = 11 th day • 93% of cases occur in the first 6 weeks 27

Abacavir Hypersensitivity (2) n Clinical symptoms: • Most common: fever, maculopapular rash, fatigue • GI Symptoms: nausea, vomiting, diarrhea, abdominal pain • Respiratory symptoms: cough, shortness of breath 28

Abacavir Hypersensitivity (3)

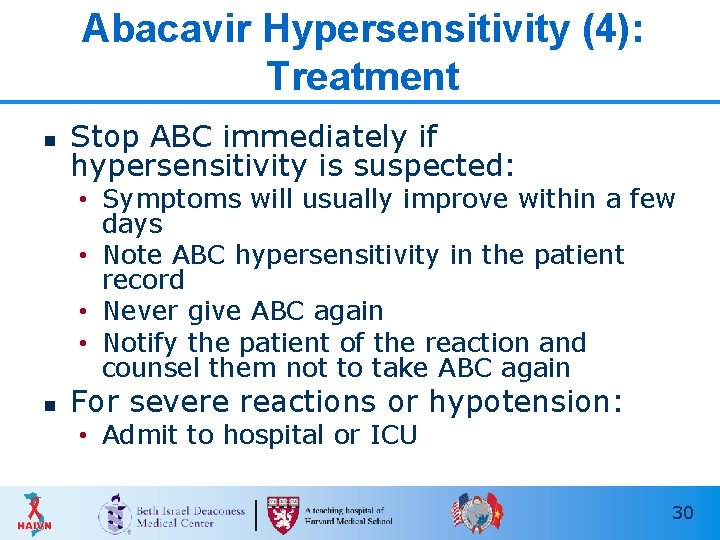
Abacavir Hypersensitivity (4): Treatment n Stop ABC immediately if hypersensitivity is suspected: • Symptoms will usually improve within a few days • Note ABC hypersensitivity in the patient record • Never give ABC again • Notify the patient of the reaction and counsel them not to take ABC again n For severe reactions or hypotension: • Admit to hospital or ICU 30

Stevens Johnson Syndrome (SJS) 31

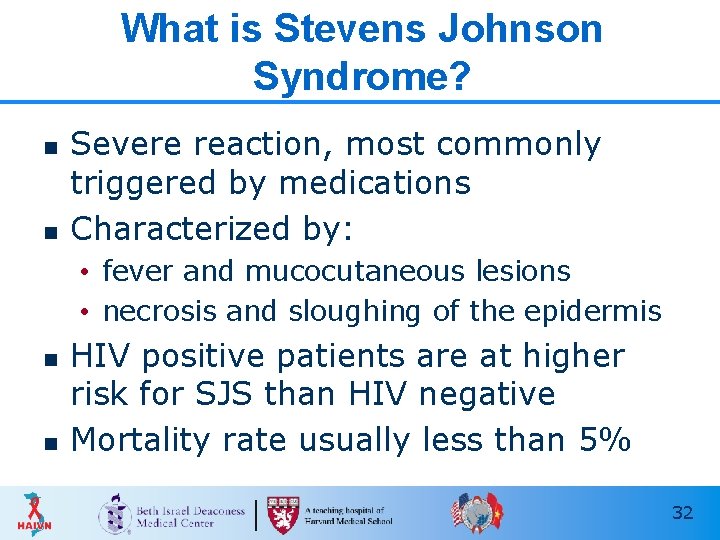
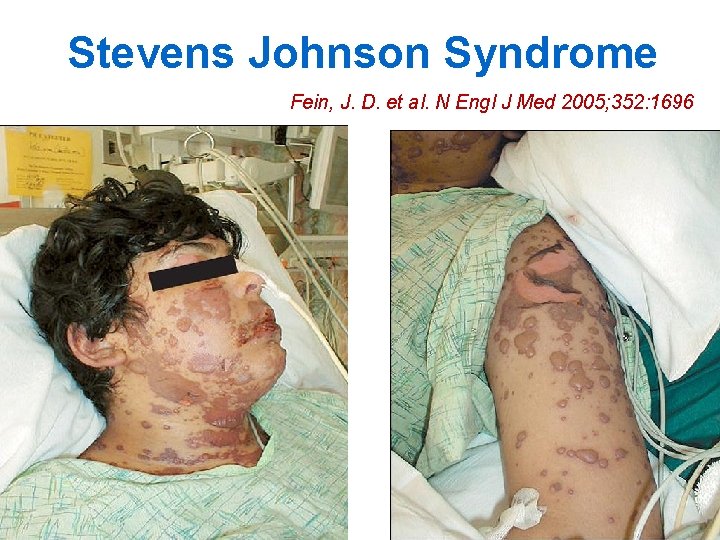
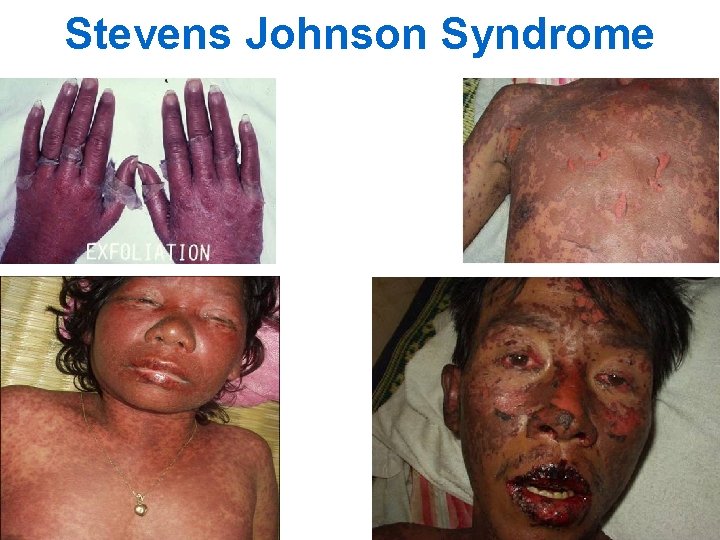
What is Stevens Johnson Syndrome? n n Severe reaction, most commonly triggered by medications Characterized by: • fever and mucocutaneous lesions • necrosis and sloughing of the epidermis n n HIV positive patients are at higher risk for SJS than HIV negative Mortality rate usually less than 5% 32

SJS: Skin Lesions (1) n n n Begins 1 -3 weeks after drug initiation Typically fever and flu-like symptoms occur 1 -3 days before rash onset Initial skin lesions: • Poorly defined macules with purpuric centers that coalesce to form blisters • Symmetrically distributed • Located on face and upper trunk • Lesions may burn or be painful 33

SJS: Skin Lesions (2) n Lesions then progress to epidermal detachment • Rash is most severe on 4 th day n Nikolsky's sign shows extensive epidermal detachment: • separation of the outer layer of the epidermis from the basal layer when lateral pressure is applied to the skin 34

Stevens Johnson Syndrome Fein, J. D. et al. N Engl J Med 2005; 352: 1696

Stevens Johnson Syndrome

Stevens Johnson Syndrome: Other Findings n Mucosal involvement • conjunctiva, oral cavity, genital mucosa • esophagus occasionally involved n Ophthalmologic involvement: • conjunctival lesions n Pulmonary involvement: • dyspnea, cough with sputum, hypoxemia • interstitial infiltrates, pulmonary edema, bronchiolitis obliterans 37

Stevens Johnson Syndrome: Management n n n Early recognition and immediate withdraw of any potential causative agent ICU transfer (burn unit) Topical antibacterial ointments or silver sulfadiazine Surgical debridement to remove necrotic epidermis Ophthalmologic care 38

Key Points n n Common drugs that cause rash in PLHIV include NNRTIs, CTX, and Abacavir Skin rashes are graded by severity using grades 1 - 4 • Grade 1 -2 may resolve with antihistamines and continuation of the drug • Grades 3 and 4 usually necessitate medication withdrawal or change n SJS is best managed by early recognition and withdrawal of causative drug 39

Thank you! Questions? 40
 Haivn
Haivn Haivn
Haivn What is receptive vaginal sex
What is receptive vaginal sex Haivn
Haivn Haivn
Haivn Haivn
Haivn Harvard medical school curriculum
Harvard medical school curriculum Harvard medical
Harvard medical Harvard extension course catalog
Harvard extension course catalog Study abroad harvard summer
Study abroad harvard summer Michael luca harvard business school
Michael luca harvard business school Hbsp cases
Hbsp cases Doctors license number
Doctors license number Greater baltimore medical center medical records
Greater baltimore medical center medical records Difference between medical report and medical certificate
Difference between medical report and medical certificate Torrance memorial outpatient lab
Torrance memorial outpatient lab Cartersville medical center medical records
Cartersville medical center medical records Servier medical art
Servier medical art Schisotoma
Schisotoma Servier medical art
Servier medical art Servier medical art
Servier medical art Servier medical art
Servier medical art Servier medical art brain
Servier medical art brain Server medical art
Server medical art Servier medical art
Servier medical art Service medical art
Service medical art Server medical art
Server medical art Servier medical art
Servier medical art Servier
Servier Servier medical art
Servier medical art Servier medical art
Servier medical art Server medical art
Server medical art Servier medical art
Servier medical art Servier medical art immunology
Servier medical art immunology Servier medical art
Servier medical art Servier medical art
Servier medical art Servier medical art
Servier medical art Swiss medical art
Swiss medical art Servier medical art
Servier medical art Creative commons
Creative commons A
A