Immune Reconstitution Inflammatory Syndrome IRIS HAIVN Harvard Medical
























- Slides: 33

Immune Reconstitution Inflammatory Syndrome (IRIS) HAIVN Harvard Medical School AIDS Initiative in Vietnam

Outline of Presentation • • • Definition of IRIS Pathogenesis of IRIS Clinical presentation of IRIS due to TB, Hepatitis B, CMV Diagnosis of IRIS Management of IRIS

Learning objectives At the end of this presentation, the trainee will be able to describe: - The two forms of the Immune Reconstitution Inflammatory Syndrome (IRIS) - The etiological agents and syndromes of IRIS - The management of IRIS - Commonly encountered forms of IRIS

IRIS: Definition • IRIS = IRD: Immune Restoration Disease • IRIS: symptoms or signs consistent with an inflammatory and/or atypical presentation of OIs or tumors – Is not a side effect of ARV, – Occur after initiation, reintroduction, or change of ARV – In patients who have evidence of viral load suppression.

IRIS: Definition • IRIS is a pattern of diseases presenting soon after the initiation of ARV. • Typically, it occurs in the first 2 -12 weeks after starting ARV. • IRIS is a “paradoxical” overreaction against a foreign antigen (alive or dead) in patients starting ARV.

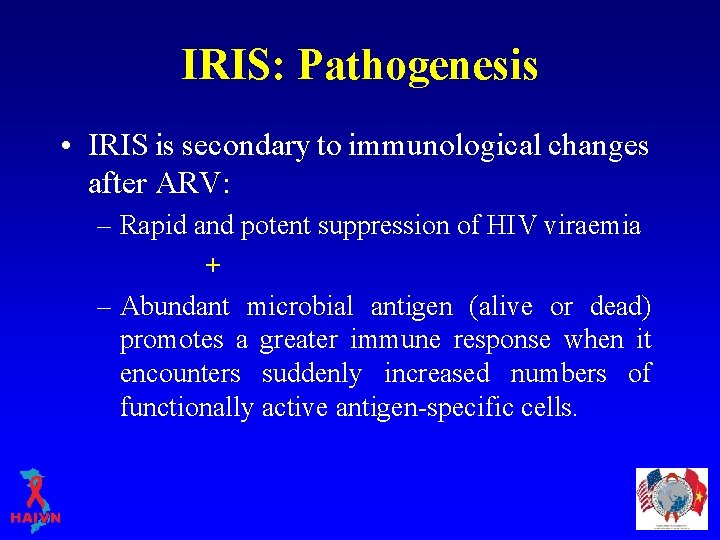
IRIS: Pathogenesis • IRIS is secondary to immunological changes after ARV: – Rapid and potent suppression of HIV viraemia + – Abundant microbial antigen (alive or dead) promotes a greater immune response when it encounters suddenly increased numbers of functionally active antigen-specific cells.

IRIS: Clinical presentations • IRIS in patients already receiving therapy for an OI at the time at which ARV is initiated. Clinical deterioration of the disease: “paradoxical reaction”. • IRIS may trigger the presentation of an OI that was sub-clinical prior ARV: “unmasking IRIS”.

IRIS: Clinical presentations • Inflammatory response that causes the unexpected worsening of the patient’s condition. • Often there is no detectable evidence of the underlying pathogen. • Mycobacterium Tuberculosis accounts for 1/3 of all IRIS events. • BCG vaccine: very common in infants

Common Pathogens All infections have been reported to cause IRIS • Mycobacteria: – TB and MAC, BCG in children • Fungal: – Cryptococcosis, PCP, histoplasmosis, Penicilliosis • Viral: – CMV, VZV, HBV, HCV • Parasites: – Strongyloides stercoralis, cryptosporidium

IRIS Risk factors • Low CD 4 count before starting ARV • Rapid reduction in HIV viral load with ARV • High pathogen load at the beginning of ARV, i. e. starting ARV therapy in the setting of an active OI • High number of prior OIs – Minor criteria: increase in CD 4 count.

IRIS TB • Worsening of signs and symptoms of TB in patients being started on anti-TB chemo-therapy • Described in the pre-HIV era but appear to be more frequent in HIV-infected patients • Occur in 7 to 36% of patients • More frequently when HAART is started within 2 months of beginning anti-TB treatment

IRIS TB • Factors complicating ARV and TB therapy: – Adherence to heightened pill burden – Overlapping drug toxicities – Drug/drug interactions

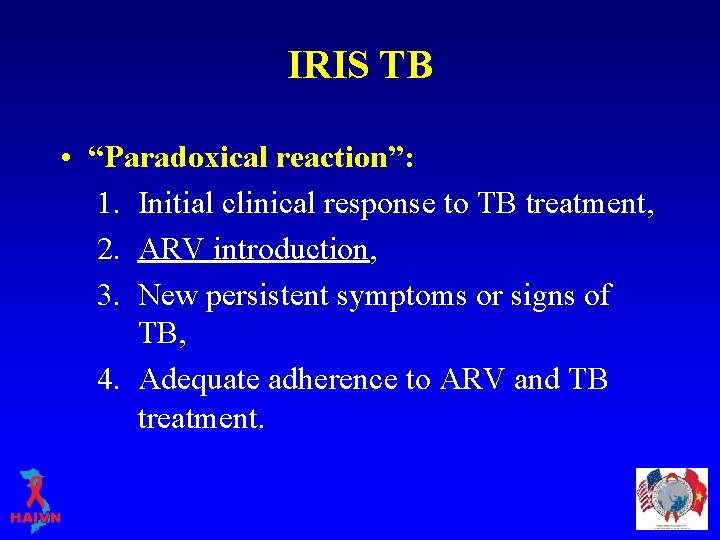
IRIS TB • “Paradoxical reaction”: 1. Initial clinical response to TB treatment, 2. ARV introduction, 3. New persistent symptoms or signs of TB, 4. Adequate adherence to ARV and TB treatment.

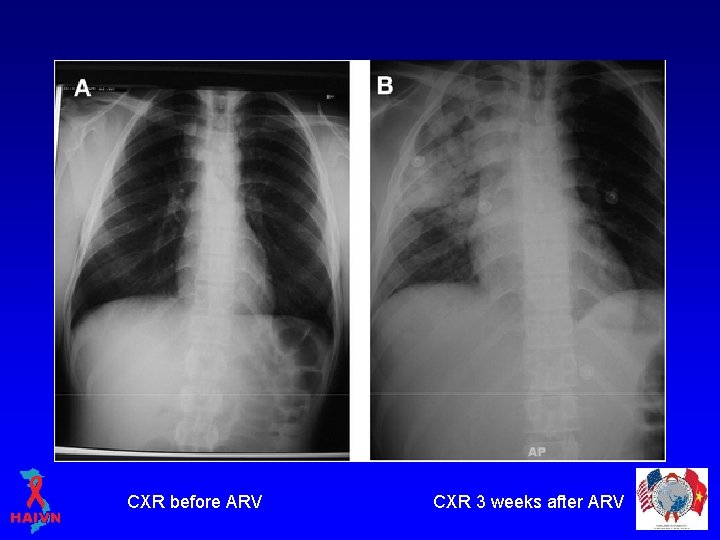
IRIS TB Diagnosis • “Paradoxical reaction”: – Chest X ray/Ultrasound: worsening or new lesions, – Good virological response, – Clear exclusion of other conditions: • • TB treatment failure, resistant TB others OI, Malabsortion, Drug reactions.

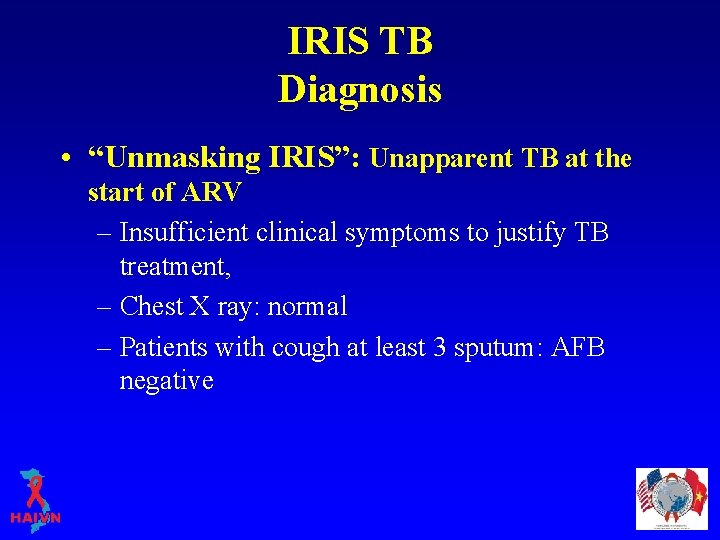
IRIS TB Diagnosis • “Unmasking IRIS”: Unapparent TB at the start of ARV – Insufficient clinical symptoms to justify TB treatment, – Chest X ray: normal – Patients with cough at least 3 sputum: AFB negative

IRIS TB Diagnosis • “Unmasking IRIS”: – TB appeared within the first 6 months of ARV – Good virological response to ARV – Adequate adherence to ARV

CXR before ARV CXR 3 weeks after ARV

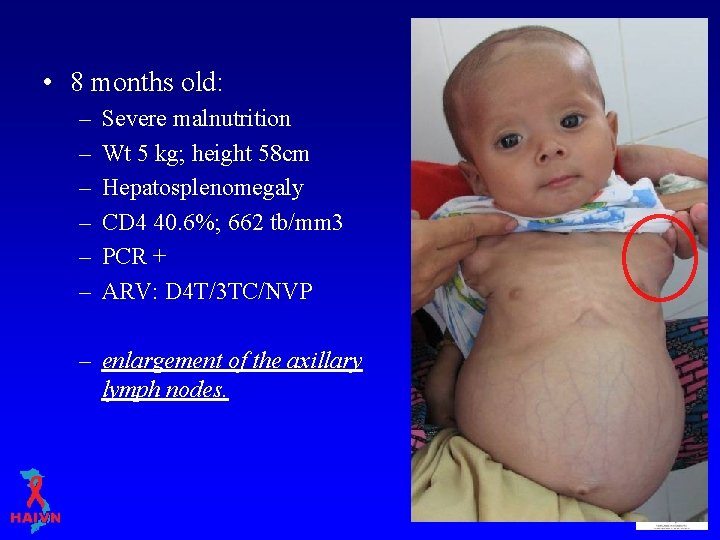
• 8 months old: – – – Severe malnutrition Wt 5 kg; height 58 cm Hepatosplenomegaly CD 4 40. 6%; 662 tb/mm 3 PCR + ARV: D 4 T/3 TC/NVP – enlargement of the axillary lymph nodes.

IRIS TB Differential diagnosis • For patients on TB treatment who develop IRIS after start ARV: “Paradoxical reaction” – Side effects of ARV • drug fevers – TB infection not responding to treatment • Resistant TB • Poor adherence to TB treatment – Other HIV or non-HIV related infections • IRIS is a diagnosis of exclusion !!!

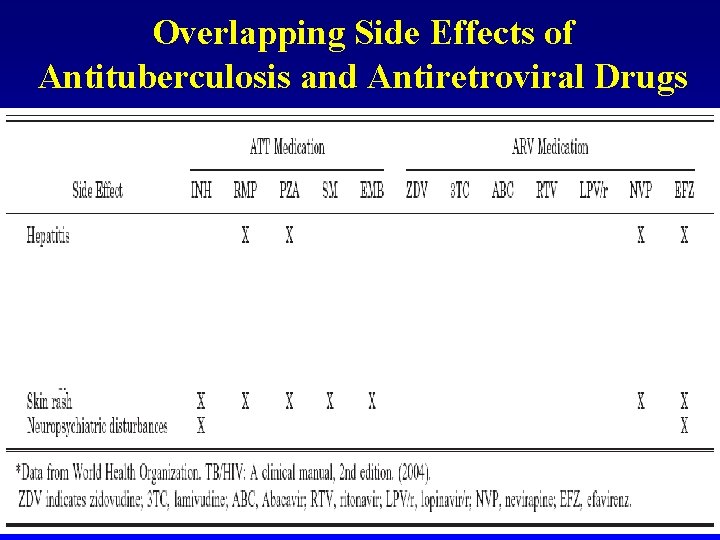
Overlapping Side Effects of Antituberculosis and Antiretroviral Drugs

Overlapping Side Effects of Antituberculosis and Antiretroviral Drugs

IRIS in Thai children • Between May 2002 -2004 (3 hospital) – HIV-infected children enrolled: 153 – ARV regimens: • D 4 T + 3 TC + NVP: 81 • D 4 T + 3 TC + EFV: 72 – Mean Age: 8 years old – Mean CD 4 baseline: 5% – No active OI The Pediatric Infectious Disease Journal • Volume 25, Number 1, January 2006

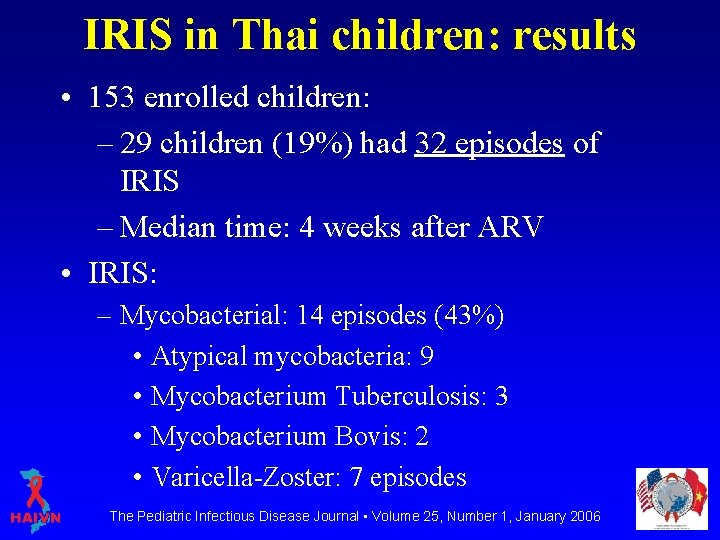
IRIS in Thai children: results • 153 enrolled children: – 29 children (19%) had 32 episodes of IRIS – Median time: 4 weeks after ARV • IRIS: – Mycobacterial: 14 episodes (43%) • Atypical mycobacteria: 9 • Mycobacterium Tuberculosis: 3 • Mycobacterium Bovis: 2 • Varicella-Zoster: 7 episodes The Pediatric Infectious Disease Journal • Volume 25, Number 1, January 2006

IRIS in Thai children: results (cont’) – HSV: 7 episodes – Cryptococcus: 3 – Guillain Barre: 1 The Pediatric Infectious Disease Journal • Volume 25, Number 1, January 2006

IRIS in Thai children: outcomes • One patient interrupted 8 weeks ARV during IRIS • 3 patients died of IRIS or its complications The Pediatric Infectious Disease Journal • Volume 25, Number 1, January 2006

IRIS in infants (South Africa) • • Infants less than 24 months old: 169 ARV: D 4 T + 3 TC + Aluvia Median age: 8 months old IRIS: 34 infants (21%) – Median time: 2 weeks post-ARV – BCG disease: 24/34 (71%) • Local reaction and/or ipsilateral axillary lymph node AIDS 2009, 23: 1097– 1107

IRIS in infants (South Africa) • Tuberculosis: 12 cases (6 co-infected with M. bovis) • Outcomes: – IRIS: 3/34 died (9%) • BCG disease: 2 cases • BCG and TB: 1 case AIDS 2009, 23: 1097– 1107

IRIS Management • Continuation of primary therapy against the pathogen, • Continuation of ARV, • Judicious use of anti-inflammatory agents, • To provide reassurance to the patients: – With appropriate management, IRIS usually does not alter the patients long-term prognosis.

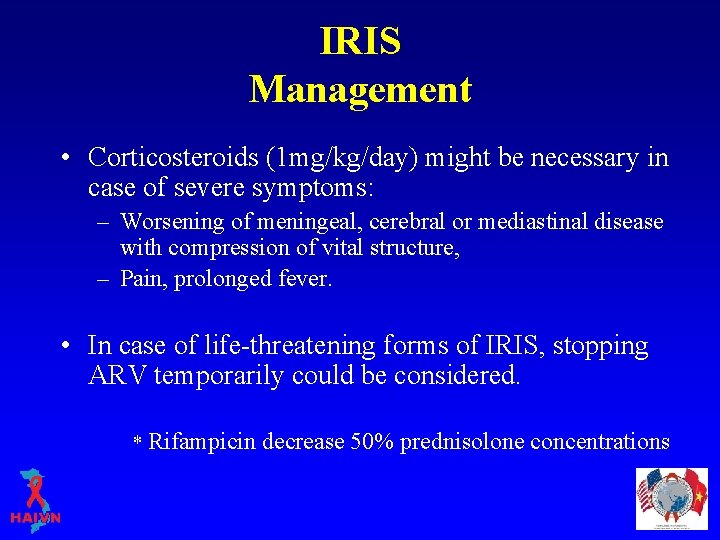
IRIS Management • Corticosteroids (1 mg/kg/day) might be necessary in case of severe symptoms: – Worsening of meningeal, cerebral or mediastinal disease with compression of vital structure, – Pain, prolonged fever. • In case of life-threatening forms of IRIS, stopping ARV temporarily could be considered. * Rifampicin decrease 50% prednisolone concentrations

Managing ARV During IRIS • Continue ARV – Except in life-threatening situations • Adjust for anti-mycobacterial treatment – If rifampin is prescribed: • Switch NVP to EFV • Stop PIs and start EFV or a third NRTI, as appropriate • Super boosted PI

Summary and Key Points • Restoration of immunopathological response to antigens of opportunistic pathogens – subclinical infections – treated infections • Usually presents during the first weeks (2 -12 weeks) of ARV • Less commonly presents after many months of ARV • The most common agent inducing IRIS in Vietnam is TB/BCG 28

Summary and Key Points • IRIS is a diagnosis of exclusion • Treatment consists of antimicrobial + antiinflammatory therapy or anti-inflammatory therapy alone • IRIS is NOT treatment failure: ARV therapy should be continued • The syndrome will be spontaneously resolved after months

Thank you! Questions?
 Immune reconstitution inflammatory syndrome
Immune reconstitution inflammatory syndrome Dr adria rusli
Dr adria rusli Immune reconstitution therapy
Immune reconstitution therapy Primary immune response and secondary immune response
Primary immune response and secondary immune response Haivn
Haivn Risk of blood transfusion
Risk of blood transfusion What is receptive vaginal sex
What is receptive vaginal sex Haivn
Haivn Haivn
Haivn Haivn
Haivn Iris syndrome
Iris syndrome Mucorrhea causes
Mucorrhea causes Inflammatory vs mechanical pain
Inflammatory vs mechanical pain Tromboflibitis
Tromboflibitis Inflammatory breast cancer
Inflammatory breast cancer Treatment of inflammatory breast cancer
Treatment of inflammatory breast cancer Post inflammatory erythema
Post inflammatory erythema Pelvic inflammatory disease men
Pelvic inflammatory disease men Pelvic girdle pain
Pelvic girdle pain Pro and anti inflammatory
Pro and anti inflammatory Inflammatory cells
Inflammatory cells Ulcerative colitis vs chrons
Ulcerative colitis vs chrons Sick
Sick K
K Basilique aemilia reconstitution
Basilique aemilia reconstitution Agora reconstitution
Agora reconstitution Reconstitution calculation formula
Reconstitution calculation formula Reconstitution medication labels
Reconstitution medication labels Reconstitution de bagdad
Reconstitution de bagdad Medmath
Medmath Basilique julia rome
Basilique julia rome Reconstitution du forum romain
Reconstitution du forum romain Herpes zoster
Herpes zoster Delphes reconstitution
Delphes reconstitution